Abstract
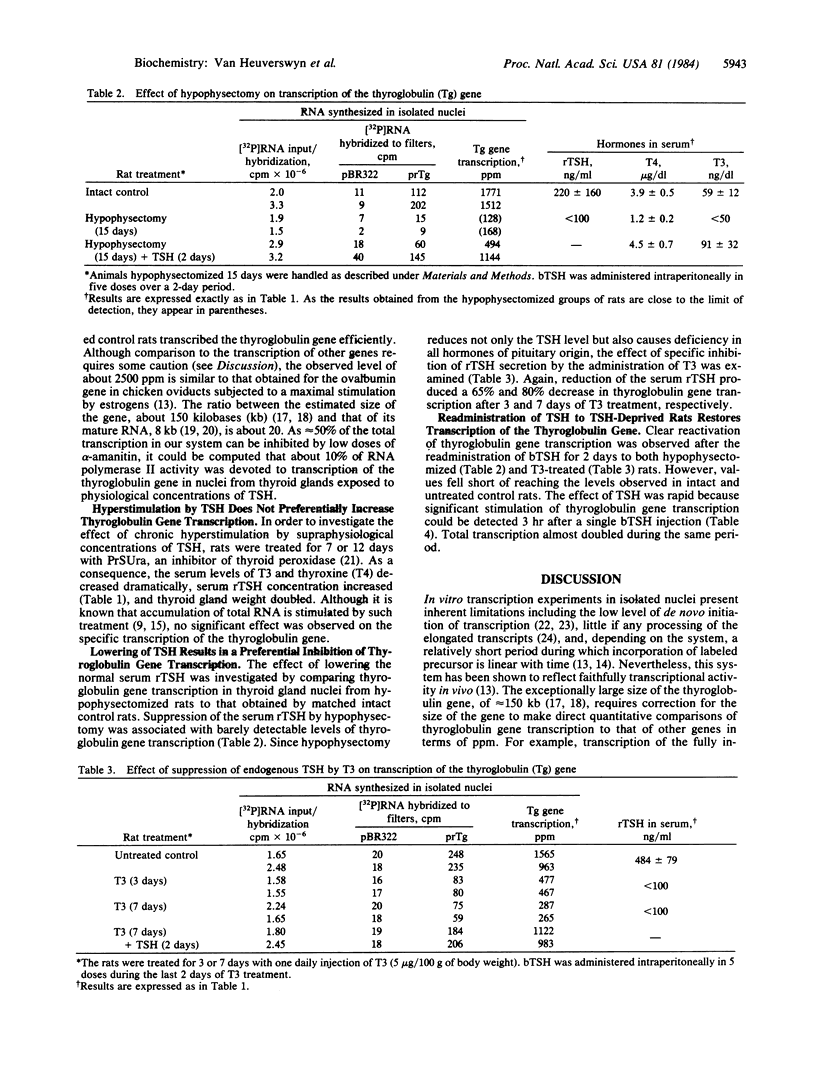
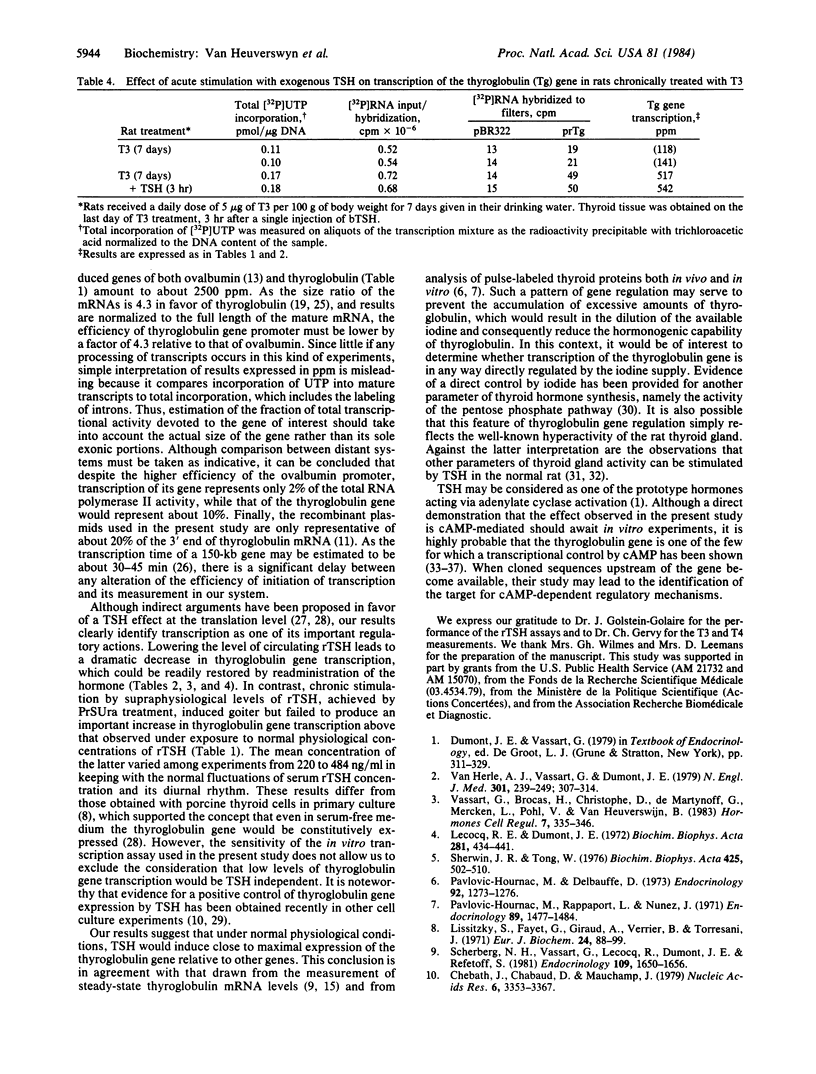
The availability of rat thyroglobulin cDNA clones was exploited to study the regulation of thyroglobulin gene transcription by thyrotropin (TSH). Groups of rats were subjected to treatments leading to reduction or increase in the rat serum TSH (rTSH) levels. Thyroid gland nuclei were isolated, incubated in vitro in the presence of 32P-labeled uridine triphosphate, and thyroglobulin transcripts were quantitated by hybridization to immobilized rat thyroglobulin cDNA clones. Transcription of the thyroglobulin gene was found to be very active in thyroid nuclei from control animals. It represented about 10% of total RNA polymerase II activity. Chronic hyperstimulation of the thyroid glands with endogenous rTSH was achieved in rats treated with the goitrogen propylthiouracil. No significant increase of thyroglobulin gene transcription could be measured in thyroid nuclei from these animals. On the contrary, a dramatic decrease in thyroglobulin gene transcription was observed in those animals in which endogenous rTSH levels had been suppressed by hypophysectomy or by the administration of triiodothyronine. Injection of exogenous bovine TSH in such animals readily restored transcriptional activity of the gene. Our results identify transcription as an important regulatory step involved in TSH action. They suggest that normal TSH levels induce close to maximal expression of the thyroglobulin gene but that continuous presence of TSH is required in order to maintain the gene in an activated state.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale E. G., Hartley J. L., Granner D. K. N6,O2'-dibutyryl cycle AMP and glucose regulate the amount of messenger RNA coding for hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1982 Feb 25;257(4):2022–2028. [PubMed] [Google Scholar]
- Brocas H., Christophe D., Van Heuverswijn B., Scherberg N., Vassart G. Molecular cloning of Pst I fragments from rat double stranded thyroglobulin complementary DNA. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1785–1792. doi: 10.1016/0006-291x(80)91381-9. [DOI] [PubMed] [Google Scholar]
- Chabaud O., Chebath J., Giraud A., Mauchamp J. Modulation by thyrotropin of thyroglobulin synthesis in cultured thyroid cells : correlations with polysome profile and cytoplasmic thyroglobulin mRNA content. Biochem Biophys Res Commun. 1980 Mar 13;93(1):118–126. doi: 10.1016/s0006-291x(80)80254-3. [DOI] [PubMed] [Google Scholar]
- Chambon P. Eukaryotic nuclear RNA polymerases. Annu Rev Biochem. 1975;44:613–638. doi: 10.1146/annurev.bi.44.070175.003145. [DOI] [PubMed] [Google Scholar]
- Chebath J., Chabaud O., Bergé-Lefranc J. L., Cartouzou G., Lissitzky S. Molecular weight of the thyroglobulin messenger RNA of sheep thyroid gland. Biochem Biophys Res Commun. 1977 Nov 7;79(1):267–273. doi: 10.1016/0006-291x(77)90090-0. [DOI] [PubMed] [Google Scholar]
- Chebath J., Chabaud O., Mauchamp J. Modulation of thyroglobulin messenger RNA level by thyrotropin in cultured thyroid cells. Nucleic Acids Res. 1979 Jul 25;6(10):3353–3367. doi: 10.1093/nar/6.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe D., Pohl V., Van Heuverswijn B., De Martynoff G., Brocas H., Dumont J. E., Pasteels J. L., Vassart G. Isolation and characterization of a fragment of rat thyroglobulin gene. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1166–1175. doi: 10.1016/0006-291x(82)91092-0. [DOI] [PubMed] [Google Scholar]
- Davies E., Dumont J. E., Vassart G. Thyrotropin-stimulated recruitment of free monoribosomes on to membrane-bound thyroglobulin-synthesizing polyribosomes. Biochem J. 1978 May 15;172(2):227–231. doi: 10.1042/bj1720227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lauro R., Obici S., Acquaviva A. M., Alvino C. G. Construction of recombinant plasmids containing rat thyroglobulin mRNA sequences. Gene. 1982 Jul-Aug;19(1):117–125. doi: 10.1016/0378-1119(82)90196-2. [DOI] [PubMed] [Google Scholar]
- Fayet G., Hovsépian S., Dickson J. G., Lissitzky S. Reorganization of porcine thyroid cells into functional follicles in a chemically defined, serum- and thyrotropin-free medium. J Cell Biol. 1982 May;93(2):479–488. doi: 10.1083/jcb.93.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Soreq H., Aviv H. Initiation of RNA synthesis in isolated nuclei. Eur J Biochem. 1977 Jul 15;77(2):393–400. doi: 10.1111/j.1432-1033.1977.tb11679.x. [DOI] [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecocq R. E., Dumont J. E. Stimulation by thyrotropin of amino acid incorporation into proteins in dog thyroid slices in vitro. Biochim Biophys Acta. 1972 Oct 27;281(3):434–441. doi: 10.1016/0005-2787(72)90459-5. [DOI] [PubMed] [Google Scholar]
- Lissitzky S., Fayet G., Giraud A., Verrier B., Torresani J. Thyrotrophin-induced aggregation and reorganization into follicles of isolated porcine-thyroid cells. 1. Mechanism of action of thyrotrophin and metabolic properties. Eur J Biochem. 1971 Dec 22;24(1):88–99. doi: 10.1111/j.1432-1033.1971.tb19658.x. [DOI] [PubMed] [Google Scholar]
- Malan P. G., Strang J., Tong W. TSH initiation of hormone secretion by rat thyroid lobes in vitro. Endocrinology. 1974 Aug;95(2):397–405. doi: 10.1210/endo-95-2-397. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. Rna synthesis in isolated nuclei processing of adenovirus serotype 2 late messenger rna precursors. J Mol Biol. 1982 Aug 25;159(4):581–599. doi: 10.1016/0022-2836(82)90102-4. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Miles M. F., Hung P., Jungmann R. A. Cyclic AMP regulation of lactate dehydrogenase. Quantitation of lactate dehydrogenase M-subunit messenger RNA in isoproterenol-and N6,O2'-dibutyryl cyclic AMP-stimulated rat C6 glioma cells by hybridization analysis using a cloned cDNA probe. J Biol Chem. 1981 Dec 10;256(23):12545–12552. [PubMed] [Google Scholar]
- Pavlovic-Hournac M., Delbauffe D. Action of TSH on the in vivo incorporation of labeled amino acid into thyroglobulin and other thyroidal proteins. Endocrinology. 1973 Apr;92(4):1273–1276. doi: 10.1210/endo-92-4-1273. [DOI] [PubMed] [Google Scholar]
- Pavlovic-Hournac M., Rappaport L., Nunez J. Incorporation of labeled amino acid into protein by thyroid glands from hypophysectomized rats. I. In vitro studies. Endocrinology. 1971 Dec;89(6):1477–1484. doi: 10.1210/endo-89-6-1477. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Roeder R. G. Ribosomal RNA synthesis in isolated nuclei. J Mol Biol. 1972 Jun 28;67(3):433–441. doi: 10.1016/0022-2836(72)90461-5. [DOI] [PubMed] [Google Scholar]
- Scherberg N. H. Isolation of thyroglobulin messenger RNA from rats: increased yield in propylthiouracil-induced hyperplasia. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1415–1423. doi: 10.1016/0006-291x(78)91161-0. [DOI] [PubMed] [Google Scholar]
- Scherberg N. H., Vassart G., Lecocq R., Dumont J. E., Refetoff S. Modulation of thyroglobulin messenger RNA accumulation in the rat thyroid. Endocrinology. 1981 Nov;109(5):1650–1656. doi: 10.1210/endo-109-5-1650. [DOI] [PubMed] [Google Scholar]
- Scherberg N., Refetoff S. Iodination-deiodination. A radiochemical method for detection of structure and changes in structure in RNA. Biochim Biophys Acta. 1977 Mar 18;475(2):337–351. doi: 10.1016/0005-2787(77)90024-7. [DOI] [PubMed] [Google Scholar]
- Schulz-Harder B., Tata J. R. Inhibition of nuclear ribonuclease activity during transcription in vitro by aurintricarboxylic acid and vanadyl riboncleoside complexes. Biochem Biophys Res Commun. 1982 Feb 11;104(3):903–910. doi: 10.1016/0006-291x(82)91334-1. [DOI] [PubMed] [Google Scholar]
- Sherwin J. R., Tong W. Stimulatory actions of thyrotropin and dibutyryl cyclic AMP on transcription and translation in the regulation of thyroidal protein synthesis. Biochim Biophys Acta. 1976 Apr 2;425(4):502–510. doi: 10.1016/0005-2787(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Van Herle A. J., Vassart G., Dumont J. E. Control of thyroglobulin synthesis and secretion. (First of two parts). N Engl J Med. 1979 Aug 2;301(5):239–249. doi: 10.1056/NEJM197908023010504. [DOI] [PubMed] [Google Scholar]
- Vassart G., Verstreken L., Dinsart C. Molecular weight of thyroglobulin 33 S messenger RNA as determined by polyacrylamide gel electrophoresis in the presence of formamide. FEBS Lett. 1977 Jul 1;79(1):15–18. doi: 10.1016/0014-5793(77)80340-2. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Tsang A. S., Mahbubani H. A change in the rate of transcription of a eukaryotic gene in response to cyclic AMP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7171–7175. doi: 10.1073/pnas.77.12.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte A., McKenzie J. M. Regulation of the rat thyrotropin receptor in vitro. Endocrinology. 1981 Jan;108(1):305–309. doi: 10.1210/endo-108-1-305. [DOI] [PubMed] [Google Scholar]
- van Ommen G. J., Arnberg A. C., Baas F., Brocas H., Sterk A., Tegelaers W. H., Vassart G., de Vijlder J. J. The human thyroglobulin gene contains two 15-17 kb introns near its 3'-end. Nucleic Acids Res. 1983 Apr 25;11(8):2273–2285. doi: 10.1093/nar/11.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]


