Abstract
In the Cre-loxp system, expression level and activity of Cre recombinase in a Cre deletor line are critical because these determine not only the cell specificity of gene knockout (KO), but also the efficiency of Cre-mediated excision in a specific cell lineage. Although the spatiotemporal expression pattern of a Cre transgene is usually defined upon the generation of the mouse line, the Cre excision efficiency in a specific targeted cell lineage is rarely evaluated and often assumed to be 100%. Incomplete excision can lead to highly variable phenotypes due to mosaicism (i.e. co-existence of cells with the flox or the recombined flox allele) and this problem has long been overlooked. Here, we report that Stra8-iCre, a transgenic allele expressing codon-improved Cre recombinase (iCre) under the control of the male germ cell-specific Stra8 promoter, could efficiently delete one Mov10l1 flox allele in spermatogenic cells, whereas the excision was incomplete when two Mov10l1 flox alleles were present. The incomplete Cre-mediated excision led to a testicular phenotype that was much less severe than that in the true conditional KO (100% inactivation) mice. Our findings suggest that it is essential to determine the efficiency of Cre excision when Cre-loxp system is used for deleting genes in a specific cell lineage and the Cre; genelox / Δ genotype should be used to evaluate phenotypes instead of Cre; genelox/lox due to the fact that the latter usually bears incomplete deletion of the flox allele(s).
Keywords: conditional gene knockout, Cre, loxp, piRNA, testis, germ line, phenotype, mosaicism
Gene knockout (KO) technologies have greatly facilitated biomedical research by allowing researchers to determine the physiological roles of genes during development and in adult physiology (Gama Sosa et al., 2010; Guan et al., 2010; Yan, 2009). However, many genes are indispensible for embryonic development and global inactivation of these genes leads to embryonic lethality, thus precluding further investigation of their functions in adulthood (Aoki and Taketo, 2008; Miller, 2011). To overcome this problem, the Cre-loxp system has been widely utilized for inactivating genes in a spatiotemporally regulated manner (Aoki and Taketo, 2008; Speck and Iruela-Arispe, 2009). Generally, this system requires two key components: one is the Cre recombinase, the expression of which is driven by a cell lineage- or tissue-specific promoter; the other is a gene flanked by two loxp sequences generated by gene targeting (Pluck, 1996). Numerous male germline-specific Cre transgenic mouse lines have been generated and utilized for producing conditional KO mouse lines (Tamowski et al., 2010), including TNAP-Cre, Blimp1-Cre, Vasa-Cre, TSPY-Cre, Kit-Cre, Sycp1-Cre, Pgk2-Cre, Hsp2-Cre, Eno2-Cre, Ngn3-Cre, Prm1-Cre (Chung et al., 2004; Gallardo et al., 2007a; Hammond and Matin, 2009; Inselman et al., 2010; Kido and Lau, 2005; Vidal et al., 1998). These mice were designed to have Cre expression specifically in male germ cell lineage in developing (e.g. embryonic, fetal and/or prepubertal) and adult testes, and the actual spatiotemporal expression pattern of Cre is mostly evaluated either by crossing these Cre lines with conditional LacZ or GFP reporter mouse lines (Gallardo et al., 2007b; Novak et al., 2000), or based upon detection of Cre mRNA/protein using in situ hybridization or immunohistochemistry. However, the actual Cre excision efficiency in the targeted cell lineage is rarely evaluated at genomic levels, and incomplete Cre-mediated excision may be responsible for many of the studies reporting either that some of those Cre lines failed to completely delete floxed genes in the testicular germ cells (Kimura et al., 2003; Lei et al., 2010; Rasoulpour and Boekelheide, 2006), or a lack of phenotype in the targeted cell types in which Cre expression is detected. Moreover, discrepancies in phenotypes have often been observed among exactly the same Cre-loxp cKO lines (Hayashi et al., 2008; Maatouk et al., 2008), leading to confusion in functional interpretation of these genes. We have unexpectedly observed that several of the Cre mouse lines that we have used displayed abundant Cre expression specifically in the male germ cell lineage, but the efficiency of Cre-mediated excision rarely reached 100%, rendering a variable proportion of Cre-expressing cells still retaining the functional flox allele(s). Here we use the Stra8-iCre; Mov10l1lox/lox line as an example to demonstrate this hidden problem. We observed a drastic phenotypic difference between Stra8-iCre; Mov10l1lox/lox and Stra8-iCre; Mov10l1lox/Δ mice, and the partial Cre-mediated excision appeared to be the cause.
Stra8 (Stimulated by retinoic acid 8) is a germline-specific gene exclusively expressed in spermatogonial stem cells (SSCs) in fetal testes, undifferentiated (i.e. SSCs and prospermatogonia) and differentiated (type A, intermediate and type B) spermatogonia in postnatal testes (Anderson et al., 2008; Hogarth et al., 2011). Stra8-iCre transgenic mice, generated by inserting a 1.4kb Stra8 promoter region upstream of the iCre-coding sequence, were intended to mimic the endogenous expression of Stra8 in the spermatogenic cell lineage (Sadate-Ngatchou et al., 2008). By crossing with Tg(ACTB-Bgeo/GFP)21Lbe (Z/EG) reporter females (Novak et al., 2000), a previous study has shown that Cre activity can be first detected in prospermatogonia in postnatal day 3 (P3) testes, and Cre expression continues until preleptotene spermatocyte stage (Sadate-Ngatchou et al., 2008).
To verify the spatiotemporal expression of Stra8-iCre, we first crossed Stra8-iCre males with Rosa26mTmGtg/tg reporter (Muzumdar et al., 2007) females, a double fluorescent Cre reporter transgenic line in which cells without Cre activity express membrane-tagged tomato red fluorescence protein (mT) and cells with Cre activity express membrane-tagged eGFP (mG) due to Cre excision of the floxed STOP cassette. Cryosections of developing Stra8-iCre; Rosa26mTmG+/tg testes were prepared for imaging analyses (Figure 1). In these testes, mG-positive (green) cells represent those with successful Cre excision of the floxed STOP cassette, whereas Cre-negative cells remain red due to constitutive expression of mT. Previously we have detected iCre activity first in postnatal day 3 (P3) testes (Wu et al., 2012). Similar to P3 testes, only a small proportion of spermatogonia were green at P4 and P8, suggesting either iCre was not expressed or the Cre excision did not occur in those non-green cells. At P14, all meiotic cells displayed strong mG signals within the seminiferous tubules, suggesting iCre-mediated excision had peaked in spermatocytes. After P21, the highest levels of mG expression were detected in round spermatids and pachytene spermatocytes, whereas mG expression in spermatogonia and pre-pachytene spermatocytes was barely detectable (Fig. 1). The expression patterns of mG suggest that Stra8-iCre expression starts in a proportion of spermatogonia around P3 or P4 and continues to increase with male germ cell development from spermatogonia to spermatocytes and then spermatids. However, the efficiency of Cre-mediated excision was much lower than 100% in spermatogonia, leading to a significant proportion of spermatogonia without mG expression either during testicular development or in adult testes. The iCre-mediated excision reaches full efficiency only in pachytene spermatocytes and spermatids in adult testes, whereas levels of iCre decrease to the minimum in spermatogonia in the adult testes (Fig. 1). Therefore, the Stra8-iCre line is not appropriate for inactivating floxed genes in spermatogonia and a lack of effects/phenotype in spermatogonia when this Cre line is used may not necessarily suggest that this particular gene does not have an essential role in spermatogonial stage because of the partial Cre-mediated excision in spermatogonia.
Figure 1. Visualization of Stra8-iCre expression and iCre activity in developing testes by crossing the Stra8-iCre line with the Rosa26mTmGtg/tg reporter transgenic mouse line.
Confocal microscopic analyses of testicular cryosections from Stra8-iCre; mTmG+/tg mice at postnatal day 4 (P4), P8, P14, P21, P60, respectively. Representative fluorescent images counterstained with DAPI at various ages are shown and specific membrane-tagged eGFP (mG) signals are only observed within the seminiferous tubules. At P4 and P8, some germ cells exhibit strong mG signals (arrows), while the rest display weak membrane-tagged tomato red (mT) signals (arrowheads). At P14, germ cells with stronger mG expression are spermatocytes. Levels of mG signals on spermatogonia are much lower compared to those on spermatocytes and spermatids in P21 and P60 testes. Scale bar=50μm.
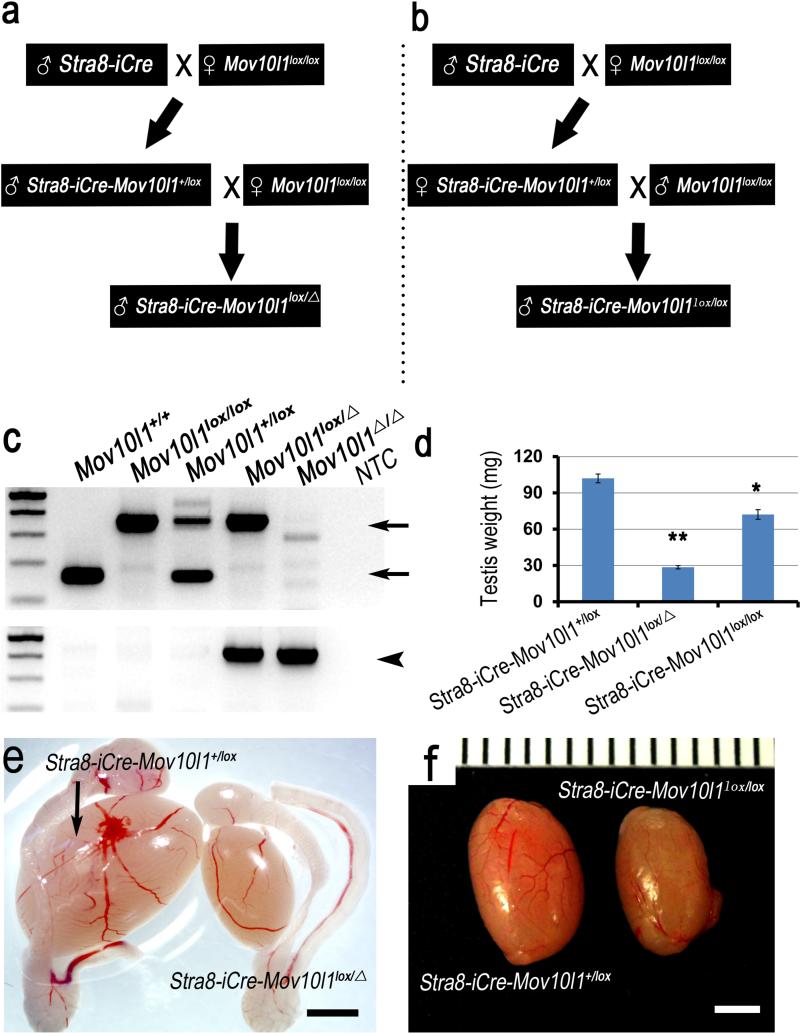
By crossing Stra8-iCre males with Mov10l1lox/lox females, we generated Stra8-iCre; Mov10l1+/lox mice. Because of Stra8 expression in spermatogenic cell during spermatogenesis, sperm from Stra8-iCre; Mov10l1+/lox male are genotypically Stra8-iCre; Mov10l1+/Δ. We then crossed Stra8-iCre; Mov10l1+/lox males with Mov10lox/lox females. The resultant Stra8-iCre; Mov10l1+/Δ males contained one flox allele and one recombined flox allele (i.e. KO or delete allele, Δ) in all of their spermatogenic cells (Figure 2a). Meanwhile, by crossing Stra8-iCre; Mov10l1+/lox females with Mov10l1lox/lox males, we obtained Stra8-iCre; Mov10l1lox/lox males that have two Mov10l1 flox alleles in all of their spermatogenic cells (Figure 2b). Mov10l1 encodes a putative RNA helicase essential for the biogenesis of PIWI-interacting RNAs (piRNAs) in the murine germline (Frost et al., 2010; Zheng et al., 2010). Global inactivation of Mov10l1 leads to a phenotype exclusively confined to the testis characterized by spermatogenic arrest in leptotene to zygotene transition and male infertility. Wild type, flox and recombined (Δ) alleles were genotyped by PCR using two sets of primers (Figure 2c). The first set of primers (FW1+Rev) detected the wild type and floxed allele bands, whereas a second set (FW2+Rev) was used to validate the recombined Mov10l1 flox allele (Δ). Interestingly, testes collected from P60 Stra8-iCre; Mov10l1lox/Δ males were much smaller in size compared to those of Stra8-iCre; Mov10l1+/lox littermates (controls) (Figure 2e). Unexpectedly, testes derived from P60 Stra8-iCre; Mov10l1lox/lox males showed a slight decrease in both weight and size compared to those of Stra8-iCre; Mov10l1+/lox littermates (Figure 2f). A significant difference in testis weight was also observed between Stra8-iCre; Mov10l1 lox/Δ and Stra8-iCre; Mov10l1lox/lox males (Figure 2d).
Figure 2. Generation and phenotype of Stra8-iCre; Mov10l1+/lox (control), Stra8-iCre; Mov10l1lox/lox (mosaic) and Stra8-iCre; Mov10l1lox/Δ (true cKO) mice.
(a) Male Stra8-iCre; Mov10l1+/lox mice were crossed with Mov10l1lox/lox females to produce Stra8-iCre;Mov10l1lox/Δ male mice. (b) Stra8-iCre; Mov10l1lox/lox males were generated by mating female Stra8-iCre; Mov10l1+/lox with Mov10l1lox/lox males. (c) Representative PCR gel images showing the different genotypes as indicated. Upper panel: wild type and flox allele bands were detected using the first set of primers (FW1 and Rev) at ~160bp and 400bp, respectively (arrows), and both bands were absent after Stra8-iCre-mediated excision of Mov10l1 flox allele (Δ); Lower panel: a ~500bp band indicates the recombined floxed allele (arrowheads). (d) Comparison of the testis weight at postnatal day 60 among the Stra8-iCre; Mov10l1+/lox, Stra8-iCre; Mov10l1Δ/lox and Stra8-iCre; Mov10l1lox/lox males (*: P<0.05; **: P<0.01). (e) Gross morphology and testis size at postnatal day 60 in Stra8-iCre; Mov10l1+/lox and Stra8-iCre; Mov10l1lox/Δ male mice. (f) Gross morphology and testis size at postnatal day 60 in Stra8-iCre; Mov10l1+/lox and Stra8-iCre;Mov10l1lox/lox male mice (Scale bar=2mm).
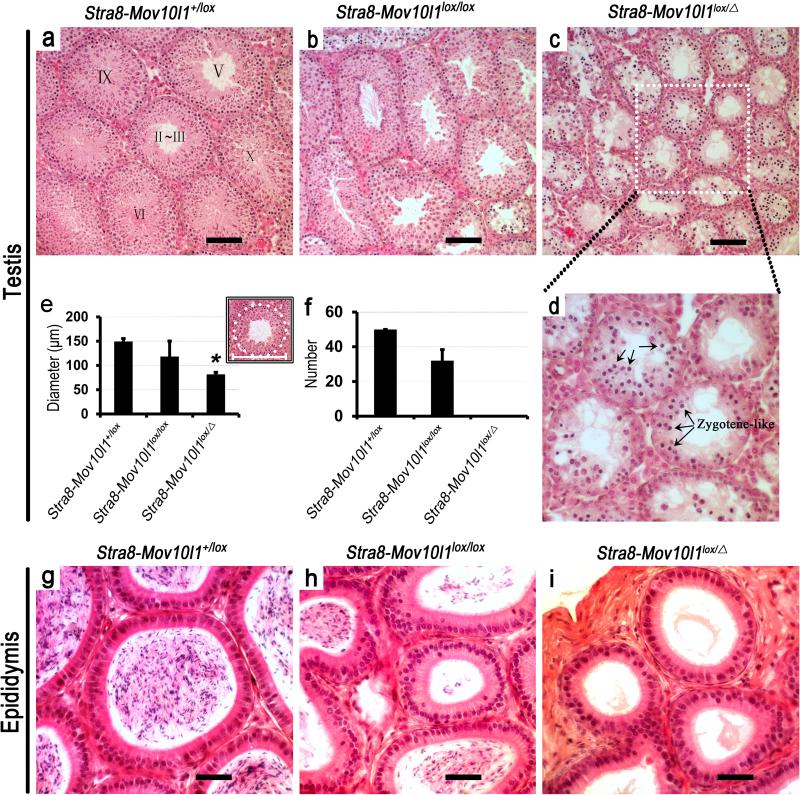
To further define the phenotypic differences at histological levels, we analyzed hematoxylin-eosin (HE)-stained, paraffin-embedded cross sections of Stra8-iCre; Mov10l1 lox / Δ and Stra8-iCre; Mov10l1lox/lox testes (Figure 3). Control (Stra8-iCre; Mov10l1+/lox) male testes display normal spermatogenesis (Figure 3a), whereas in Stra8-iCre;Mov10l1 lox / Δ males, the most advanced stages of spermatogenic cells within the seminiferous epithelium were those zygotene-like spermatocytes (Figure 3d, arrows), and all tubules exhibited early meiotic arrest at leptotene to zygotene transition without exception (Figure 3c,d), which is consistent with previous reports on global Mov10l1 knockout mice (Mov10l1-/-) (Frost et al., 2010; Zheng et al., 2010). Interestingly, Stra8-iCre; Mov10l1lox/lox testes displayed no clear-cut spermatogenic arrest in the seminiferous epithelium (Figure 3b), as seen in Stra8-iCre; Mov10l1 lox / Δ testes (Figure 3c). As a reflection of spermatogenic disruptions, diameters of tubules in Stra8-iCre; Mov10l1 lox / Δ testes were much smaller than those of control (Stra8-iCre; Mov10l1+/lox) testes (Figure 3e). Histologically normal seminiferous tubules at various stages of the epithelial cycles were often observed in Stra8-iCre; Mov10l1lox/lox testes but completely absent in Stra8-iCre; Mov10l1 lox / Δ males (Figure 3f). Upon examining the cauda epididymis, we discovered a complete absence of spermatozoa in Stra8-iCre; Mov10l1 lox / Δ cauda epididymis, whereas numerous spermatozoa were present in Stra8-iCre; Mov10l1lox/lox cauda epididymis (Figure 3h) although the total number was drstically reduced compared to controls (Stra8-iCre; Mov10l1+/lox) (Figure 3g).
Figure 3. Testicular and epididymal histology of Stra8-iCre; Mov10l1+/lox (control), Stra8-iCre; Mov10l1lox/lox (mosaic) and Stra8-iCre; Mov10l1lox/Δ (true cKO) mice.
All testis and epididymis samples were collected from postnatal day 60 males. (a, b, c) Micrographs of hematoxylin-eosin (HE)-stained testicular cross-sections of mice with the three genotypes. (d) Magnified view of framed area in panel c. (e) Diameter of seminiferous tubules in the testes of the three genotypes. 50 round or close to round tubules in the cross sections as illustrated in the inset were calculated for statistical analyses (n=50, *: P<0.01). (f) Percentage of seminiferous tubules displaying a typical spermatogenic stage (a total of 12 stages in the wild type mouse) among HE stained cross sections derived from mice with the three genotypes (n=50). Note that no cross sections showing any of the 12 typical stages of the seminiferous epithelial cycle are present in Stra8-iCre; Mov10l1 lox/Δ males. (g, h, i) HE-stained cauda epididymal cross-sections from mice of the three genotypes. Note that mature sperm are completely absent in the Stra8-iCre; Mov10l1 lox/Δ epididymis, whereas abundant mature sperm are present in the epididymides with the other two genotypes. Scale bar=50μm.
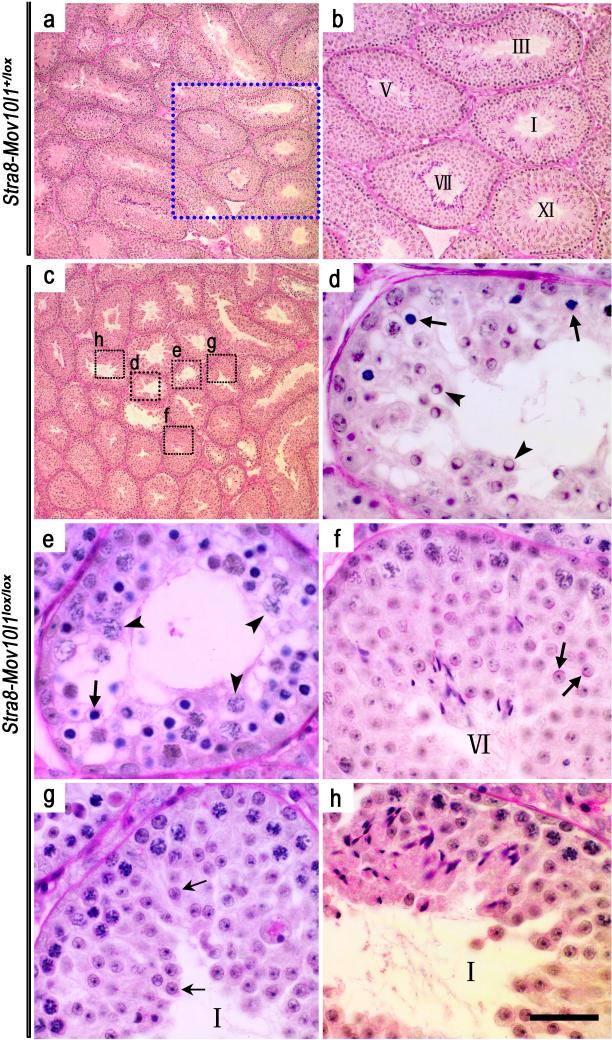
To further evaluate spermatogenic disruptions, periodic acid-Schiff (PAS) stained testicular sections were examined (Figure 4). Control (Stra8-iCre; Mov10l1+/lox) testes (Figure 4a,b) contained tubules of all 12 spermatogenic stages, whereas cross sections of Stra8-iCre; Mov10l1lox/lox testes exhibited a much more heterogeneous histology (Figure 4c-h). Aberrant spermatogenesis appeared to be arrested at late pachytene spermatocyte to round spermatid stages (Figure 4d,e). Numerous Sertoli cell vacuoles were observed, suggesting active spermatogenic cell depletion (Kyronlahti et al., 2011). While morphologically normal-looking stages VI and I seminiferous tubules were present (Figure 4f,h), a stage I tubule displayed a distinct spermiogenic arrest at the elongation step (steps 9-10) (Figure 4f,h). Thus, testes of the Stra8-iCre; Mov10l1lox/lox males displayed a much more heterogeneous spermatogenic disruptions (Figure 4c~h) compared to those of the Stra8-iCre; Mov10l1Δ/lox males, in which spermatogenesis is uniformly arrested at leptotene to zygotene transition (Figure 3c,d). These results suggest that iCre is more competent to cleave one rather than two flox alleles because the only difference between Stra8-iCre; Mov10l1 lox / Δ and Stra8-iCre; Mov10l1lox/lox males was one extra flox allele. Interestingly, we observed similar phenomenon in several other Cre-flox lines in which either Stra8-iCre or other Cre lines (e.g. Ddx4-Cre or Blimp1-Cre) was used as the deletor line (data not shown), suggesting that for most of the Cre lines partial Cre-mediated excision is a common problem when Cre has to deal with two flox alleles (lox/lox).
Figure 4. Spermatogenic disruptions in Stra8-iCre; Mov10lox/lox (mosaic) mice.
(a) Normal spermatogenesis in Stra8-iCre; Mov10+/lox testes at postnatal day 60. (b) Enlarged image of the framed area in panel a, showing spermatogenic stages (marked with Roman numerals) of the seminiferous epithelial cycle in Stra8-iCre; Mov10+/lox testes at postnatal day 60. All sections were stained using the periodic acid-Schiff's (PAS) reagent that allows accurate staging based upon the shape of the developing acrosome in spermatids. (c-h) Highly variable spermatogenic disruptions in Stra8-iCre; Mov10lox/lox seminiferous tubules. Panels d-h are magnified images corresponding to the framed area in panel c. (d) Arrows and arrowheads indicate abnormal zygotene spermatocytes and round spermatids, respectively; (e) Arrows and arrowheads demonstrate zygotene-like and pachytene spermatocytes, respectively; Sporadic vacuoles are present, which are generally located in the cytoplasm of Sertoli cells and are indicative of active germ cell depletion. (f) A morphologically normal tubule cross-section containing round spermatids (step 6) (arrows). (g) A seminiferous tubule contains no elongating/elongated spermatids, suggesting a block in the elongation step of spermiogenesis. Arrows indicate the arrested step 4 spermatids. (h) A relatively morphologically normal seminiferous tubule. Scale bar=20μm.
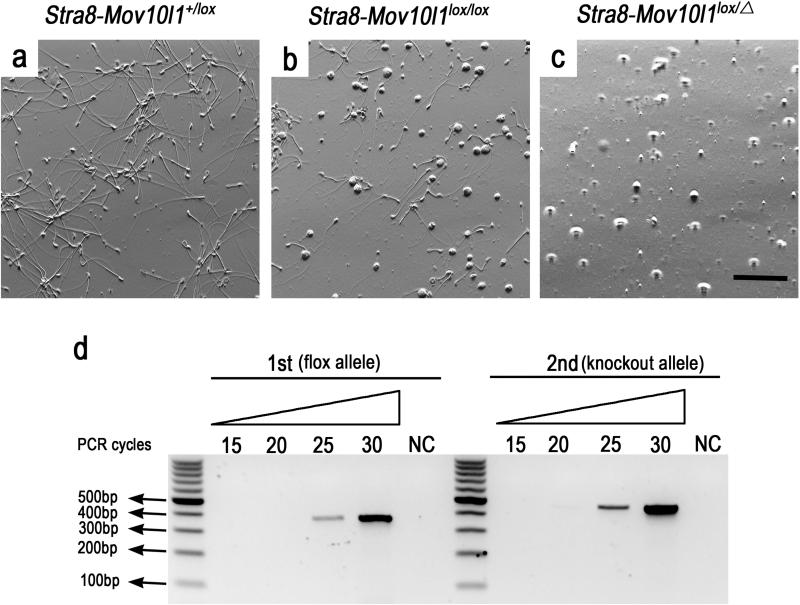
If the Cre excision efficiency were 100%, mice with the two genotypes, Stra8-iCre; mov10l1 lox/Δ and Stra8-iCre; mov10l1lox/lox, should have displayed identical or at least similar phenotype. The phenotypic differences must have resulted from persistence of the flox allele in Stra8-iCre; Mov10l1lox/lox spermatogenic cells due to partial Cre-mediated excision. To verify this, sperm were collected from the cauda epididymis of Stra8-iCre; Mov10l1lox/lox males. Abundant sperm were observed in the epididymis of control (Stra8-iCre; Mov10l1+/lox) male mice (Figure 5a), whereas no mature sperm were present in the cauda epididymis of Stra8-iCre; Mov10l1 lox/Δ mice (Figure 5c). In the cauda epididymis of Stra8-iCre; Mov10l1lox/lox mice, both morphologically normal and abnormal sperm, as well as depleted round spermatids are present (Figure 5b). Those sperm must have developed from those earlier spermatogenic cells (e.g. spermatogonia, spermatocytes or spermatids) in which one or two flox alleles were not completely deleted through Cre-mediated excision. To confirm this, sperm were collected from Stra8-iCre; Mov10l1lox/lox cauda epididymis followed by DNA extraction and PCR-based genotyping. Indeed, both knockout (i.e. recombined flox allele) and flox alleles were steadily detected in sperm from Stra8-iCre; Mov10l1lox/lox cauda epididymis (Figure 5d). A semi-quantitative PCR analysis estimated the excision efficiency was ~70%. These results equivocally demonstrate that sperm carrying un-recombined flox allele were indeed present, confirming the partial Cre-mediated excision in Stra8-iCre; Mov10l1lox/lox mice.
Figure 5. Partial Cre-mediated excision in spermatogenic cells of Stra8-iCre; Mov10lox/lox testes as evidenced by detection of the Mov10l1 flox allele in cauda epididymal sperm.
(a, b, c) Phase contract microscopic images of cauda epididymal contents in mice of the three genotypes indicated (Scale bar=100μm). (d) Semi-qPCR analyses of relative levels of the flox and the recombined flox (Δ) alleles in sperm of Stra8-iCre; Mov10lox/lox testes. Two sets of primers were used and the first one (FW1 + Rev) was used to detect the flox allele, whereas the other (FW2+Rev) was employed for determining the recombined flox (knockout, Δ) allele.
In conclusion, our data demonstrate that Stra8-iCre is not capable of excising two flox alleles with 100% efficiency. In the presence of two flox alleles (lox/lox), the partial Cre-mediated excision tends to lead to mosaicism in the targeted cell lineage. However, it can excise one flox allele with 100% efficiency in pachytene spermatocytes and spermatids. For this reason, Stra8-iCre; genelox/Δ mice represents more of conditional knockouts, whereas Stra8-iCre; genelox/lox mice are more like mosaic cKOs with a proportion of the target cells retaining the flox allele. This may explain why some previous studies (Kimura et al., 2003; Lei et al., 2010; Rassoulzadegan et al., 2002) report inefficient excision of the floxed genomic DNA when some germline-specific Cre transgenic lines are used. These and other studies may have included data from Cre; genelox/lox mice, which would display much less severe and highly variable phenotypes compared to Cre; genelox/Δ mice. We believe that this phenomenon is not restricted to only the Stra8-iCre line reported here, as we previously found insufficiencies of several other germline Cre lines in excising two flox alleles (data not shown). Interestingly, the problem of insufficient Cre excision appears to be common in Cre transgenic lines generated by introducing a short transgenic construct or a large bacterial artificial chromosome (BAC) fragment containing the Cre gene into the genome through the classic pronuclear injection method. It is commonly believed that Cre lines generated through targeted insertion of a Cre gene into a locus immediately downstream of the promoter of an endogenous gene, e.g. Rosa26 locus, tend to display better excision efficiency. This notion, however, remains to be validated. To date, it's still unclear why iCre in the Stra8-iCre line couldn't efficiently excise two flox alleles despite their abundant expression. Nevertheless, the present study strongly suggests that it is essential to generate Cre;gene lox/Δ mice rather than Cre;genelox/lox mice for phenotypic characterization because they are closer to “true” conditional knockout mice (100% excision efficiency).
Methods and Materials
Animals
Stra8-iCre, Rosa26mTmGtg/tg, and Mov10l1lox/lox mice were purchased from the Jackson Laboratory and maintained in a temperature- and humidity-controlled room with free access to food and water. Animal use was approved by Institutional Animal Use and Care Committee (IAUCC) of the University of Nevada, Reno. The compound conditional knockout mice (Stra8-iCre; Mov10l1lox/Δ or Stra8-iCre; mTmG+/tg) are available to the research community upon request.
Mouse genotyping and sperm DNA extraction
The genotyping primers used were as follows: Stra8-iCre: Forward: GTG CAA GCT GAA CAA CAG GA; Reverse: AGG GAC ACA GCA TTG GAG TC; mov10l1: FW1: TACCCCAGCTGAGAGGTCAC; FW2: CACTGGTGATTCAGGGGACT; Rev: TCCCAGAAGGCCTTACACAC. PCR was performed as described previously with annealing temperature at 60°C for Stra8-iCre and 56°C for Mov10l1, respectively (Frost et al., 2010). For sperm isolation, cauda epididymides were dissected and sperm were carefully released into the HTF medium (100μl/per cauda) (Irvine Scientific, Cat#: 90125) using two sharp tweezers. Sperm DNA extraction was performed using a commercial kit (Illustra™ genomicPrep Mini Spin, Cat#: 28-9042-75).
Histological analyses of the testis
Testis paraffin block preparation, hematoxylin-eosin (HE) and periodic acid-Schiff's (PAS) staining were all performed as described (Wu et al., 2012).
Fluorescent imaging
Testicular cryosections were prepared as described (Bao et al., 2010) for confocal imaging. Briefly, developing and adult testes were dissected and immediately fixed in 4% PFA solution at room temperature for 3~5 hours with gently shaking, followed by dehydration with serial sucrose solutions at room temperature: 10% sucrose for 1hr; 15% for 1hr; and 20% for 3hrs. After OCT embedding, the samples were cut into 10μm cryosections. Slides were finally counterstained with DAPI (1μg/ml) and images were acquired using an Olympus FV1000 confocal microscope system. Samples were protected from light as much as possible during the whole operation process to prevent loss of fluorescence.
ACKNOWLEDGEMENTS
The work was financially supported by NIH grants (HD060858 and HD071736) to W.Y., and the authors thank the University of Nevada Genetic Engineering Center (funded in part by NIH grant P20-RR18751) for animal care.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
LITERATURE CITED
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Taketo MM. Tissue-specific transgenic, conditional knockout and knock-in mice of genes in the canonical Wnt signaling pathway. Methods Mol Biol. 2008;468:307–331. doi: 10.1007/978-1-59745-249-6_24. [DOI] [PubMed] [Google Scholar]
- Bao J, Zhang J, Zheng H, Xu C, Yan W. UBQLN1 interacts with SPEM1 and participates in spermiogenesis. Mol Cell Endocrinol. 2010;327:89–97. doi: 10.1016/j.mce.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YF, Lee-Chang JS, Imam JS, Buddavarapu KC, Subaran SS, Sinha-Hikim AP, Gorospe M, Rao MK. Interaction between microRNAs and actin-associated protein Arpc5 regulates translational suppression during male germ cell differentiation. Proc Natl Acad Sci U S A. 2012;109:5750–5755. doi: 10.1073/pnas.1117837109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Cuzin F, Rassoulzadegan M, Wolgemuth DJ. Primary spermatocyte-specific Cre recombinase activity in transgenic mice. Transgenic Res. 2004;13:289–294. doi: 10.1023/b:trag.0000034716.73957.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R, Olson EN. MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc Natl Acad Sci U S A. 2010;107:11847–11852. doi: 10.1073/pnas.1007158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, Castrillon DH. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis. 2007a;45:413–417. doi: 10.1002/dvg.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, Castrillon DH. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis. 2007b;45:413–417. doi: 10.1002/dvg.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama Sosa MA, De Gasperi R, Elder GA. Animal transgenesis: an overview. Brain Struct Funct. 2010;214:91–109. doi: 10.1007/s00429-009-0230-8. [DOI] [PubMed] [Google Scholar]
- Guan C, Ye C, Yang X, Gao J. A review of current large-scale mouse knockout efforts. Genesis. 2010;48:73–85. doi: 10.1002/dvg.20594. [DOI] [PubMed] [Google Scholar]
- Hammond SS, Matin A. Tools for the genetic analysis of germ cells. Genesis. 2009;47:617–627. doi: 10.1002/dvg.20539. [DOI] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D, Small C, Amory JK, Griswold MD. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol Reprod. 2011;84:957–965. doi: 10.1095/biolreprod.110.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselman AL, Nakamura N, Brown PR, Willis WD, Goulding EH, Eddy EM. Heat shock protein 2 promoter drives Cre expression in spermatocytes of transgenic mice. Genesis. 2010;48:114–120. doi: 10.1002/dvg.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido T, Lau YF. A Cre gene directed by a human TSPY promoter is specific for germ cells and neurons. Genesis. 2005;42:263–275. doi: 10.1002/gene.20147. [DOI] [PubMed] [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–1700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- Korhonen HM, Meikar O, Yadav RP, Papaioannou MD, Romero Y, Da Ros M, Herrera PL, Toppari J, Nef S, Kotaja N. Dicer is required for haploid male germ cell differentiation in mice. PLoS One. 2011;6:e24821. doi: 10.1371/journal.pone.0024821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyronlahti A, Euler R, Bielinska M, Schoeller EL, Moley KH, Toppari J, Heikinheimo M, Wilson DB. GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol Cell Endocrinol. 2011;333:85–95. doi: 10.1016/j.mce.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Lin J, Li X, Li S, Zhou H, Araki Y, Lan ZJ. Postnatal male germ-cell expression of cre recombinase in Tex101-iCre transgenic mice. Genesis. 2010;48:717–722. doi: 10.1002/dvg.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatouk DM, Loveland KL, McManus MT, Moore K, Harfe BD. Dicer1 is required for differentiation of the mouse male germline. Biol Reprod. 2008;79:696–703. doi: 10.1095/biolreprod.108.067827. [DOI] [PubMed] [Google Scholar]
- Miller RL. Transgenic mice: beyond the knockout. Am J Physiol Renal Physiol. 2011;300:F291–300. doi: 10.1152/ajprenal.00082.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Pluck A. Conditional mutagenesis in mice: the Cre/loxP recombination system. Int J Exp Pathol. 1996;77:269–278. [PMC free article] [PubMed] [Google Scholar]
- Rasoulpour RJ, Boekelheide K. The Sycp1-Cre transgenic mouse and male germ cell inhibition of NF-kappa b. J Androl. 2006;27:729–733. doi: 10.2164/jandrol.106.000950. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M, Magliano M, Cuzin F. Transvection effects involving DNA methylation during meiosis in the mouse. EMBO J. 2002;21:440–450. doi: 10.1093/emboj/21.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis. 2008;46:738–742. doi: 10.1002/dvg.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck NA, Iruela-Arispe ML. Conditional Cre/LoxP strategies for the study of hematopoietic stem cell formation. Blood Cells Mol Dis. 2009;43:6–11. doi: 10.1016/j.bcmd.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamowski S, Aston KI, Carrell DT. The use of transgenic mouse models in the study of male infertility. Syst Biol Reprod Med. 2010;56:260–273. doi: 10.3109/19396368.2010.485244. [DOI] [PubMed] [Google Scholar]
- Vidal F, Sage J, Cuzin F, Rassoulzadegan M. Cre expression in primary spermatocytes: a tool for genetic engineering of the germ line. Mol Reprod Dev. 1998;51:274–280. doi: 10.1002/(SICI)1098-2795(199811)51:3<274::AID-MRD6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wu Q, Song R, Ortogero N, Zheng H, Evanoff R, Small CL, Griswold MD, Namekawa SH, Royo H, Turner JM, Yan W. The RNase III enzyme DROSHA is essential for microRNA production and spermatogenesis. J Biol Chem. 2012;287:25173–25190. doi: 10.1074/jbc.M112.362053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W. Male infertility caused by spermiogenic defects: lessons from gene knockouts. Mol Cell Endocrinol. 2009;306:24–32. doi: 10.1016/j.mce.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaughlin KJ, Stark A, Sachidanandam R, Pillai RS, Wang PJ. Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl Acad Sci U S A. 2010;107:11841–11846. doi: 10.1073/pnas.1003953107. [DOI] [PMC free article] [PubMed] [Google Scholar]