Abstract
Objective
We explored whether the opiate, morphine, affects the actions of maraviroc, as well as a recently synthesized bivalent derivative of maraviroc linked to an opioid antagonist, naltrexone, on HIV-1 entry in primary human glia.
Methods
HIV-1 entry was monitored in glia transiently transfected with an LTR construct containing a luciferase reporter gene under control of a promoter for the HIV-1 transactivator protein Tat. The effect of maraviroc and the bivalent ligand ± morphine on CCR5 surface expression and cytokine release was also explored.
Results
Maraviroc inhibits HIV-1 entry into glial cells, while morphine negates the effects of maraviroc leading to a significant increase in viral entry. We also demonstrate that the maraviroc-containing bivalent ligand better inhibits R5-tropic viral entry in astrocytes than microglia compared to maraviroc when coadministered with morphine. Importantly, the inhibitory effects of the bivalent compound in astrocytes were not compromised by morphine. Exposure to maraviroc decreased the release of pro-inflammatory cytokines and restricted HIV-1-dependent increases in CCR5 expression in both astrocytes and microglia, while exposure to the bivalent had similar effect in astrocytes but not in microglia. CCR5-MOR stoichiometric ratio varied among the two cell types with CCR5 expressed at much higher levels than MOR in microglia, which could explain the effectiveness of the bivalent ligand in astrocytes compared to microglia.
Conclusion
A novel bivalent compound reveals fundamental differences in CCR5-MOR interactions and HIV-1 infectivity among glia, and has unique therapeutic potential in opiate abuse-HIV interactive comorbidity.
Keywords: HIV-1, opioids, bivalent ligand, inflammation, CCR5 antagonists
Introduction
The predominant HIV-1 isolates from the central nervous system (CNS) preferentially use the β-chemokine HIV-1 co-receptor CCR5 (R5-tropic HIV-1 strains) to infect cells [1, 2]. μ-Opioid receptor (MOR) agonists including morphine, methadone, and DAMGO can increase the expression of CCR5 and promote the replication of R5-tropic HIV-1 [3-5], while these increases can be prevented with blockade of MOR by the inhibitors β-funaltrexamine, methylnaltrexone, and naltrexone [6]. Moreover, in addition to opioid drugs, endogenous MOR ligands, which are elevated by inflammation, neuropathic pain, and stress, can also increase HIV-1 replication [7]. The proposed interactions of CCR5 and MOR [3, 8], are thought to result from altered signaling through heterologous desensitization [9] or possibly mediated by direct molecular interactions through protein-protein dimerization/oligomerization [3, 10, 11]. Accordingly, by virtue of their ability to alter CCR5, drug abuse or maintenance therapy could alter the effectiveness of antiretroviral medications targeting CCR5 such as the HIV-1 entry inhibitor, maraviroc, which is used in combined antiretroviral therapy. Therefore, assuming CCR5 forms functional dimers/oligomers with MOR [12, 13] and opioid drugs interact with CCR5 to exacerbate HIV-1 neuropathogenesis [14, 15], it may be advantageous to selectively target CCR5-MOR receptor complexes in opiate-abusing populations.
A novel approach to targeting these protein complexes is accomplished using newly synthesized bivalent ligands to target reputed G-protein-coupled receptor (GPCR) dimers/oligomers. Studies by others have shown that bivalent ligands can be powerful molecular tools to either characterize GPCR protein-protein interactions [16-20], interfere with the function related to such interactions [21-23], or even to treat diseases [12, 24]. Although viral production takes place mainly in brain macrophages and microglial cells, other neural cell types such as the astrocytes may harbor HIV-1 in a more dormant state, contributing to the viral burden in the brain. Numerous evidence suggests that a small subset of astrocytes becomes chronically infected, albeit with very limited viral production and predominant expression of nonstructural HIV-1 components [25]. Moreover, unlike macrophages, astrocytes show unique synergistic increases in CCL5 with morphine in combination with HIV-1 Tat [26-29] and infected astrocytes contribute to HIV-1-associated neuropathogenesis [30], making astrocytes important cellular targets for HIV-1.
Accordingly, we (i) investigated the extent to which morphine effects maraviroc efficacy on HIV-1 entry, (ii) explored the potential inhibitory effects of a bivalent ligand carrying the MOR antagonist, naltrexone, and the CCR5 antagonist, maraviroc, on HIV-1 viral entry, and (iii) examined the impact of morphine on this small-molecule bivalent antagonist to repress viral invasion in primary human glia. Our data shows that actions of the novel bivalent ligand are cell type specific and function more effectively in astrocytes than in microglia, and linkage of the two antagonists may be more effective at blocking viral entry than the combined actions of unlinked maraviroc + naltrexone.
Methods
Reagents
Morphine sulfate was obtained from NIDA and was used at a concentration of 500 nM. Naltrexone (1.5 μM) and maraviroc were purchased from Sigma-Aldrich (St. Louis, MO).
Construction of the MOR and CCR5 heterodimer model
The CXCR4 chemokine receptor (PDB: 3ODU) was chosen as the CCR5 homology modeling template because it exhibits the highest degree of sequence identity with CCR5. MODELLER was used to generate initial CXCR4-based models of CCR5 and the model was further optimized through energy minimization and dynamics simulation. A heterodimer model of the putative CCR5-MOR interaction was then constructed based on the MOR homodimer X-ray crystal structure [31]. The CCR5 homology monomer model was overlapped over one of the monomers in the MOR dimer structure and aligned using homology-based scoring to ensure the best fit and lowest RSMD in the helical domain (3.47 Å).
Cell culture and treatments
Primary human glial cells (ScienCell, Carlsbad, CA) were cultured in 24-well plates and transfected with the plasmid pBlue3′LTR-luc (NIH AIDS Research & Reference Reagent Program) using Lipofectamine 2000 (Invitrogen, Grand Island, NY) followed by infection with HIV-1. After 18-20 h, cells were rinsed twice in 1x PBS then lysed in Cell Culture Lysis Reagent (Promega) and relative Tat protein expression was determined by measuring luciferase activity using the Luciferase Assay System (Promega, Madison, WI; catalog # E1500). Light units were measured using a PHERAstar FS plate reader (BMG Labtech, Cary, NC). Maraviroc and the bivalent compound were initially used at increasing concentrations of 10, 50, 100, and 500 nM, 30-60 min prior to HIV-1 infection to selectively block viral entry. A concentration of 100 nM was chosen since both drugs showed greatest efficacy without loss in cell viability.
HIV-1 infection of glial cells
Primary human glial cells were infected by incubation with the neurotropic HIV-1 strain SF162 originally isolated by Dr. Jay A. Levy [32]. A concentration of HIV-1 p24 50 pg/106 cells [33] was used, and a culture lacking virus served as a negative control. Viral stocks were quantified by assaying for HIV-1 p24 (Alliance p24 Antigen ELISA Kit; Advanced Bioscience, Kensington, MD).
Flow cytometry
MOR and CCR5 immunoreactivity was detected by direct immunofluorescence with flow cytometric analysis as previously described [34]. Cells were incubated with primary MOR antibody (Novus Biologicals, LLC; Littleton, CO, USA; catalog number NBP1-31180) followed by a secondary antibody conjugated to allophycocyanin (BioLegend, Inc.; catalog # 408001) and Alexa Fluor® 488 conjugated anti-mouse CD195 (CCR5) antibody (BioLegend, Inc.; catalog # 107008) in permeabilization buffer at a 1:500 dilution. Fluorescence was measured from 10,000-gated cells per treatment in each experiment using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA).
Cytokine release
The protein levels of the cytokines TNF-α, IL-6, IL-1β and the chemokine RANTES were measured by ELISA at 18-20 h post treatment (R&D Systems, Minneapolis, MN).
Cytotoxicity assay
Live and dead cells were assessed after 24 h treatment by measuring acridine orange/propidium iodide dual fluorescence using a Cellometer Vision CBA (Nexcelom Bioscience LLC, Lawrence, MA).
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) techniques (SYSTAT 11.0 for Windows, SYSTAT Inc., Chicago, IL) followed by Duncan’s post-hoc analyses. An alpha level of p < 0.05 was considered significant.
Results
A bivalent ligand targeting the putative MOR-CCR5 dimer
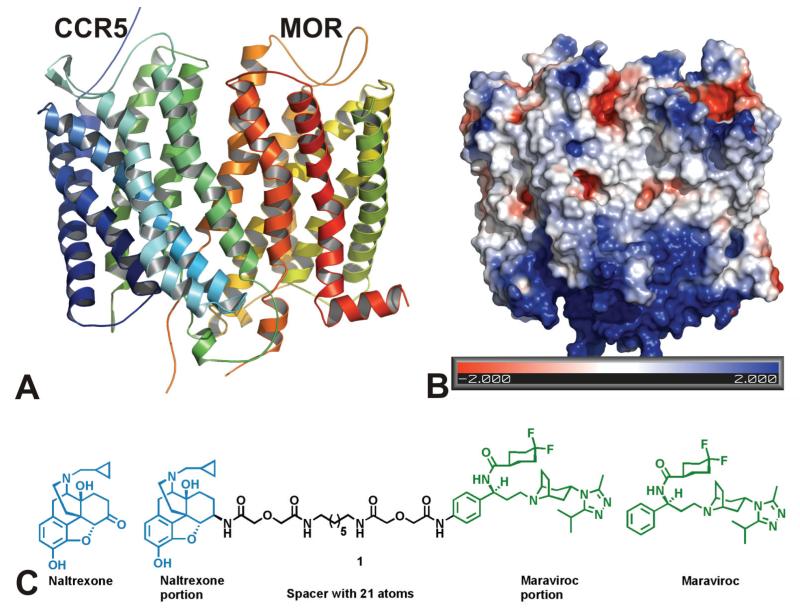
Accumulating in vitro studies have demonstrated that CCR5 and MOR may crosstalk with each other by undergoing dimerization [9-11, 35, 36]. As shown in Fig. 1A, these two receptors may interact favorably through interfacing of transmembrane helices V and VI to form a heterodimer. A bivalent ligand was designed and synthesized as proof-of-concept to study this putative dimerization process and its potential role in viral inhibition (Fig. 1B) [12]. Naltexone, a MOR antagonist, has been successfully applied to investigate the dimerization of opioid receptors [37, 38] and is used clinically to treat opiate addiction and alcoholism [39, 40]. Maraviroc is the only FDA approved CCR5 antagonist to treat HIV-1 infection. Both naltrexone and maraviroc show high affinity and reasonable selectivity toward MOR and CCR5, respectively.
Figure 1. (A) Construction of a MOR-CCR5 dimer model.
Left: The ribbons colored in blue and green represent CCR5, and the ribbons colored in red and yellow represent MOR. Each ribbon was given an arbitrary color in order to distinguish each individual helix from the other. Right: Representation of the Poisson-Boltzmann electrostatic potentials at the surface of the heterodimer using the APBS plugin by PYMOL. Red represents acidic residues (−2 kBT/e); blue represents basic residues (+2 kBT/e); white represents uncharged residues. The figure indicates that the majority of the interactions between the two receptors are likely to be hydrophobic. (B) Construction of a chemical probe that interacts with both the MOR and CCR5 receptors simultaneously.
The locus on each pharmacophore for tethering two pharmacophores through a spacer affects the binding affinities of the resultant bivalent ligands [41, 42]. The consensus of several studies is that a spacer, 16 to 22 atoms in length, is most beneficial for targeting GPCR dimers and that a 21 atom spacer is ideal when both pharmacophores are antagonists at their respective receptors [43, 44]. The rationale for design of such spacers (containing one alkyldiamine moiety and two diglycolic units) is to keep a favorable balance between hydrophobicity and hydrophilicity, as well as to possess reasonable rigidity, high stability and low toxicity. The chemical synthesis, binding affinity characterization, and the preliminary effects on HIV infectivity of this novel ligand has been reported recently [24, 45].
Antiviral effect of maraviroc and the bivalent ligand in human astroglia
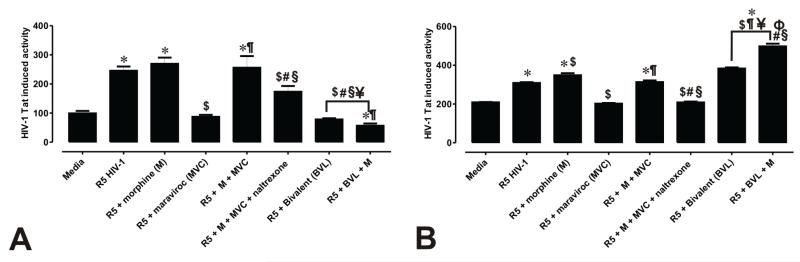
HIV-1SF162 infectivity of human glia was determined based on the relative amount of Tat protein expressed by the virus using a luciferase-based reporter assay, while levels of tat gene expression were determined by qRT-PCR (Supplementary Figure 1). Reporters under the control of the long terminal repeat (LTR) viral promoter are a robust means of measuring HIV-1 infection and uniquely activated by HIV-1 Tat. Infected cells were assayed at 18-20 h to allow sufficient time for viral infection. In addition, we wanted to pick a time after morphine-MOR had internalized (~17 h) and when virus had completed a full cycle of replication (~24 h) to assess the efficacy of viral entry inhibition and the impact of morphine on the replicative capacity of the virus. After incubation with R5-tropic HIV-1SF162 alone or in combination with morphine, relative Tat expression was significantly increased in human astrocytes (Fig. 2). In fact, 2.5- and 2.7-fold increases in Tat expression were observed after infection with HIV-1 alone and in combination with morphine, respectively. As expected, the HIV-1 entry inhibitor maraviroc prevented viral entry and caused a 2.8-fold decrease in Tat expression when compared to exposure to virus alone. By contrast, morphine in combination with maraviroc completely abolished the antiviral effect of maraviroc, and caused a significant increase in viral entry with a 2.6-fold increase in the amount of Tat expressed. Unlike maraviroc, the CCR5 antagonist TAK-779 was unaffected by morphine and limited HIV entry in both astroglia and microglia (Supplementary Figure 2). This unanticipated finding, prompted us to ask whether the MOR antagonist naltrexone would counteract the effect of morphine on maraviroc, and indeed, Tat expression was significantly reduced (Fig. 2A).
Figure 2. Maraviroc and a bivalent CCR5-MOR antagonist differentially inhibit HIV-1 entry into human glia and display cell-specific interactions with morphine.
HIV-1 infection was monitored by transfecting astrocytes (A) and microglia (B) with a pBlue3′LTR-luc reporter sensitive to Tat expression and examining luciferase activity. Values are luminescence intensity ± SEM from 3-5 independent experiments at 18 h post-infection (*p < 0.005 vs. un-infected cells; $p < 0.05 vs. R5 HIV-1; #p <0.05 vs. R5 + morphine (M); ¶p < 0.05 vs. R5 + maraviroc (MVC); §p < 0.05 vs. R5 + M + MVC; ¥p<0.05 vs. R5 + M + MVC + naltrexone).
The antiviral effect of the newly synthesized bivalent ligand compared to its component parts. We also determined whether morphine influenced the effect of the bivalent ligand on HIV-1 viral entry. Addition of the bivalent ligand was extremely effective in inhibiting viral entry and caused a 4.4-fold decrease in Tat expression when compared to R5 HIV-1 alone, and a 2.0-fold decrease when compared to R5 virus plus maraviroc. Moreover, morphine did not influence the infection rate of virus in the presence of the bivalent compound, which still produced a significant decrease in Tat expression that was 2.9-fold lower when compared to R5 plus maraviroc and 4.4-fold lower when compared to maraviroc with naltrexone plus morphine. Similar studies were conducted in microglia (Fig. 2B), and while we observed an antiviral effect with maraviroc that was significantly impaired by morphine, the bivalent ligand did not prevent infectivity in microglia (Fig 2B). To further demonstrate that HIV-1 entry into astroglial cells is inhibited by the bivalent compound, we inoculated astrocytes with R5-tropic HIV-1Bal tagged with Vpr-GFP and visualized GFP-tagged virions by confocal microscopy in the absence or presence of the bivalent ligand (Supplementary Figure 3).
Expression levels of CCR5 and MOR among human astrocytes and microglia
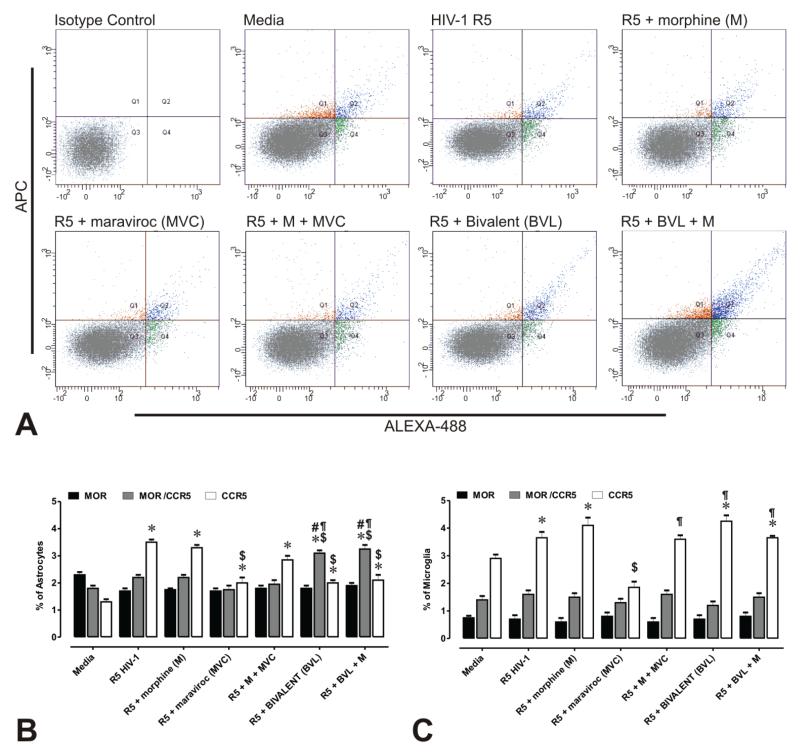
We quantitatively measured the expression levels of CCR5 and MOR in glia by qRT-PCR. Importantly, the stoichiometric ratio of CCR5:MOR differed greatly in microglia (with higher levels of CCR5 versus MOR), while CCR5 and MOR were expressed at equivalent levels in astroglia (Supplementary Figure 4). The effect of maraviroc and the bivalent compound on CCR5 and MOR immunoreactivity were analyzed in virally infected glia by flow cytometry. Differences in forward scatter and side scatter intensities suggested that astroglia are heterogeneous in the expression of CCR5, with a small percentage of the total population possessing CCR5, MOR, or combined CCR5 and MOR (Fig. 3A,B). The proportion of microglia possessing MOR antigenicity was even less than astroglia (Fig. 3C). Irrespective of baseline levels of expression, the proportion of MOR+ astroglia or microglia remained constant regardless of treatment. Alternatively, the percentage of CCR5+ astroglia and microglia was significantly increased after exposure to HIV-1 R5 ± morphine (Fig. 3A-C), while exposure of infected astrocytes or microglia to maraviroc caused a significant decrease in CCR5+ cells compared to infection alone. Interestingly, exposure to the bivalent ligand ± morphine caused a 1.5-fold decrease in CCR5 expression levels in astrocytes compared to R5 HIV-1 alone, while increasing CCR5+ microglia. Moreover, exposure to the bivalent ligand caused a 1.8-fold increase in the proportion of CCR5 and MOR immunopositive astrocytes when compared to HIV-1 alone. Treatment with the bivalent ligand upregulated both CCR5 and MOR in astrocytes, but not microglia. The proportion of microglia co-expressing CCR5 and MOR was low and remained unaffected by HIV or drug exposure. Collectively, although our data shows that microglia and astroglia both express MOR and CCR5, the stoichiometric ratio of CCR5:MOR differs significantly in each cell type. Concurrent increases in the expression of CCR5 and MOR may also contribute to the selective ability of the bivalent ligand to preferentially inhibit viral entry in astrocytes.
Figure 3. Flow cytometric analysis of CCR5 and MOR immunofluorescence in primary human glia.
(A) Scatterplots of CCR5 (Alexa 488-green) and MOR (APC-red) immunofluorescence intensity associated with astrocytes ± R5 HIV-1 and/or drug treatments. Auto-fluorescence was compensated by setting the detector voltage to the minimum level that discriminates between auto-fluorescence and specific immunofluorescence in both negative and positive controls. Isotype control antibodies were used to define settings in histogram plot analyses. Values in each histogram indicate the mean percentages ± SEM of CCR5 and/or MOR immunopositive astrocytes (B) or microglia (C). Differences in forward scatter and side scatter intensities suggest that CCR5 and MOR are not uniformly expressed by astroglia or microglia. Moreover, co-expression (as assessed by immunofluorescence) is only present in a small proportion of astrocytes and even a smaller percentage of microglia. Importantly, unlike astrocytes, a greater proportion of microglia possessed CCR5 immunoreactivity alone— suggesting heterodimer formation only occurs in a subset of CCR5+ microglia. Note the proportion of CCR5+ astrocytes increases while CCR5+ microglia increases in following exposure to the bivalent ligand—suggesting fundamental differences in the actions of the bivalent and regulation of CCR5 in each glial type. Approximately 20,000 events were analyzed per treatment condition in each experiment and size discrimination was used as a crude method for viability determination. Graphs represent the data obtained from three independent experiments (*p < 0.005 vs. un-infected cells; $p < 0.05 vs. R5 HIV-1; #p <0.05 vs. R5 + morphine (M); ¶p < 0.05 vs. R5 + maraviroc (MVC); §p < 0.05 vs. R5 + M + MCV).
The bivalent ligand variably effects the release of pro-inflammatory cytokines in astroglia and microglia
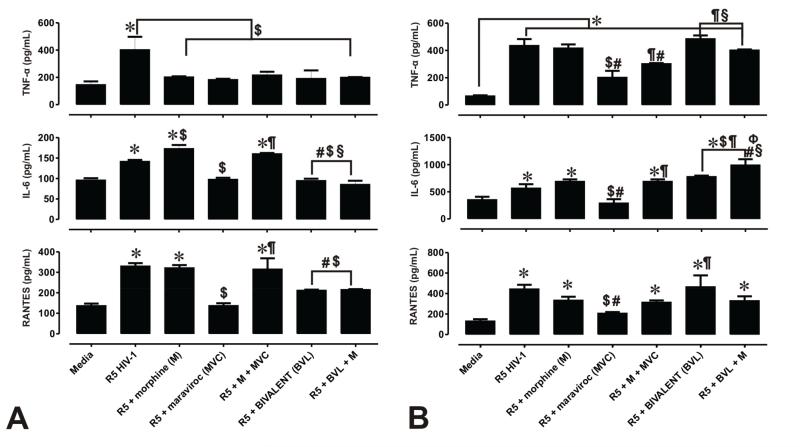
In an effort to determine whether viral entry inhibitors reduce the inflammatory effect associated with viral infection, HIV-1-induced pro-inflammatory cytokines were measured in HIV-1 infected astrocytes treated with maraviroc and the bivalent ligand. After 18-20 h exposure, supernatant was removed and analyzed by ELISA to quantitatively assess the release of the cytokines TNF-α, IL-1β and IL-6 and the chemokine RANTES. Release of IL-1β was below detection levels and not reported. Exposure to HIV-1 caused significant, 1.5- to 3.5-fold increases in the release of TNF-α, IL-6 and RANTES compared with media alone (Fig. 4A,B), while HIV-1 and morphine co-exposure significantly increased IL-6 beyond amounts seen with HIV-1 alone (Fig. 4A). Exposure to maraviroc decreased cytokine release in HIV-1 exposed cells, while the combination of maraviroc with morphine in HIV-1 infected astrocytes caused a significant increase in IL-6 and RANTES (Fig. 4A), and a significant increase in TNF-α and IL-6 in microglia (Fig. 4B), when compared to cells treated with R5 HIV-1 + maraviroc. Likewise, exposure to the bivalent ligand ± morphine caused a significant decrease in TNF-α, IL-6 and RANTES release in astrocytes, but had no inhibitory effect in microglia compared to HIV-1 by itself. Thus, besides inhibiting viral entry, CCR5 antagonists have variable anti-inflammatory effects in astrocytes and microglia. Interestingly, the bivalent ligand was more effective than maraviroc in inhibiting viral entry in astrocytes, the compound was less effective at abolishing the HIV-1-induced inflammation.
Figure 4. Maraviroc and a bivalent CCR5 antagonist differentially inhibit the release of inflammatory cytokines from primary human glia.
Protein levels of TNF-α, IL-6, IL-1β and RANTES were measured by ELISA from supernatants of HIV-1 infected astroglia (A) and microglia (B). Values are based on standard curves ± SEM of 3 independent experiments. (*p < 0.005 vs. un-infected cells; $p < 0.05 vs. R5 HIV-1; #p <0.05 vs. R5 + morphine (M); ¶p < 0.05 vs. R5 + maraviroc (MVC); §p < 0.05 vs. R5 + M + MVC; Фp < 0.05 vs. bivalent (BVL) + M).
Effect of experimental treatments on cell viability
To determine whether HIV-1 ± morphine exposure affected cell viability in the presence or absence of maraviroc or the bivalent compound, a cell death assay was performed at 24 h after treatment. While we observed a slight decrease in astrocytes’ viability after exposure to maraviroc, it was not significantly different when compared to uninfected cells. HIV-1 ± morphine with or without maraviroc or the bivalent ligand did not significantly affect the survival of astrocytes and microglia (Fig. 5A,B).
Figure 5. HIV-1 ± morphine, maraviroc, and/or a bivalent CCR5 antagonist did not affect the viability of primary human glia.
Cell viability of astrocytes (A) and microglia (B) were assessed after 24 h treatment by measurement of both live and dead cells using fluorescence labeling. Data are percent viable cells ± SEM from 3 independent experiments.
Discussion
The neural pathways involved in opioid enhancement of HIV-1 infection suggest possible targets for therapeutic intervention in opioid abuse-accelerated AIDS [46-48]. Furthermore, the use of drug abuse medications (e.g., methadone, buprenorphine, naloxone, and naltrexone) to prevent HIV infection [49, 50] in combination with antiretroviral medications such as efavirenz, atazanavir, and maraviroc heightens the potential for drug-drug interactions [51-55]. It is therefore important to develop new therapeutic strategies to target the consequences of opioid abuse and HIV-1 comorbidity. In this report we observed that (i) maraviroc inhibits HIV-1 entry into astrocytes, and (ii) co-exposure with morphine negates the effects of maraviroc leading to a significant increase in viral entry. We also demonstrate that (iii) the newly synthesized bivalent ligand carrying both a MOR and CCR5 antagonist has a more potent inhibitory effect on R5-tropic viral entry in astrocytes compared to maraviroc alone, and (iv) the inhibitory effects of the bivalent compound were not compromised by morphine. Accordingly, the bivalent ligand might be uniquely beneficial as an adjunctive therapy with maraviroc in individuals with high levels of opiate exposure. A strategy involving bivalent ligands may be particularly advantageous for blocking possible CCR5 heterodimeric pairings that promote HIV infectivity or are beneficial in blocking CD4-independent modes of HIV entry where heterodimers substitute for CD4 in stabilizing gp41-GPCR co-receptor binding.
Possible explanations for why the bivalent ligand blocked HIV entry in astrocytes, but not microglia, include (i) the differential affinity of maraviroc versus the bivalent ligand for CCR5 and (ii) inherent differences in the pattern and levels of expression of CCR5, CD4, and/or MOR among astrocytes and microglia. Because the stoichiometric ratio of CCR5 and MOR expression levels are relatively equal in astrocytes versus receptor expressions in microglia, and because the maraviroc moiety of the bivalent ligand has a lower affinity for CCR5 than unmodified maraviroc [45], higher concentrations of bivalent ligand may be required to inhibit HIV-1 entry in microglia compared to astrocytes. In addition, the presence of CD4 on microglia, but not astrocytes, will anchor gp120/gp41 thereby increasing the likelihood of gp120/gp41-CCR5 interactions [56]. Besides CCR5, human microglia were found to express very low relative levels of MOR compared to astrocytes suggesting there may be far fewer CCR5-MOR heterodimers available for the bivalent ligand to bind in microglia. Lastly, differences in MOR splice variants expressed by astrocytes and microglia [57] (as well as underlying differences in signaling) could also contribute to this discrepancy.
Although the exact mechanism by which morphine overrides maraviroc’s action is not known, it is well accepted that activation of MOR leads to up-regulation of CCR5 in human host cells and facilitates HIV-1 infection and replication [5, 58-60]. These observations agree with our flow cytometry data (Fig. 3), in that CCR5 protein levels were significantly elevated in astrocytes after viral exposure alone or in combination with morphine. Exposure to maraviroc followed by viral infection caused a decrease in receptor levels, while co-exposure with morphine reversed the maraviroc effect and caused an increase in CCR5 expression. The presence of morphine may increase CCR5 expression levels beyond the capacity of 100 nM maraviroc to occupy CCR5 [6], To attempt to clarify this observation, we used 500 nM of maraviroc, but detected increased toxicity to the cells. A 150 mg dose of maraviroc results in 7.5 ± 1.3 ng/ml in cerebrospinal fluid (CSF) at 4 h [61]. Since patients can receive 300 mg b.i.d. or “intensification” doses of 600 mg b.i.d. [62], the 100 nM maraviroc concentration in our studies (52.9 ng/ml) is likely to approximate levels seen in CSF clinically. The morphine concentration used are likely to mimic those seen with chronic drug abuse. Levels of morphine at or well exceeding 500 nM are assumed to transiently occur after heroin injection in the CNS of opiate addicts [63].
The “dimerization-oligomerization” concept for GPCRs has been widely accepted following revealing research of several groups on metabotropic GABAB receptors [64, 65], and further established through X-ray crystallographic modeling [66]. The dimerization/oligomerization of GPCRs poses a differentiated pharmacology from that of the monomers [67]. Thus, opiates potentially affect HIV-1 entry and infectivity via direct molecular interactions between MOR and CCR5, and/or through convergent downstream signaling [5, 10]. Accordingly, bivalent ligands have been used as tools to explore the underlying biology and pharmacology of GPCR dimerization/oligomerization, as well as to develop novel therapeutic agents to target potential disease mechanisms involving GPCR heteromeric interactions [20].
MOR undergoes extensive alternative splicing [68], and astrocytes express increased numbers of MOR splice variants compared to neurons and microglia [57]. We also characterized effects in astrocytes because the bivalent ligand was created based on modeling CCR5 with the canonical MOR variant MOR-1 [31]. MOR-1 is highly expressed in human astrocytes, but may not be expressed in other CNS cell types (including microglia and neurons) [57]. The concentration of 100 nM was used for maraviroc and the bivalent ligand, because, as mentioned above, 500 nM concentration of maraviroc showed toxicity. Thus, as the bivalent ligand requires the presence of both MOR and CCR5 for maximal binding interactions, the expression of increased numbers of MOR variants by astrocytes may require less ligand for inhibition of viral entry into this cell type. Also of importance is the affinity of the naltrexone moiety of the bivalent ligand for different MOR splice variants as well as the affinity of different MOR variants for heterodimerization with CCR5 [10]. Solving the crystal structure of human MOR [31] facilitated our ability to model the likelihood of different MOR splice variant-CCR5 combinations. Improvements in modeling are likely to lead to tailored strategies for inhibition of viral entry into specific cell types expressing a particular MOR (or CCR5 or alternative HIV-1 co-receptor) variant.
The newly synthesized ligand described herein can interact simultaneously with MOR and CCR5 receptors. Besides the novelty of this chemical property, this probe was never tested before for its potential as an antiviral drug. Interestingly, because of its specificity to the inhibition of viral entry into astrocytes, the bivalent compound may be of particular importance to decrease viral reservoirs in astrocytes, which could lead to less damage to the surrounding neurons from chronic infection. Emerging findings indicate that opiate abuse alone can increase premature Alzheimer-like neurodegenerative changes including hyperphosphorylated tau [69]. The latest cART era evidence indicates that preferential heroin abuse exacerbates HIV-associated neurocognitive disorders (HAND) [70-72] and peripheral neuropathies [73]. While some clinical studies report minimal or no neurocognitive differences between HIV ± opiate abuse [74, 75], these studies fail to consider critical genetic, pharmacokinetic, and pharmacodynamic differences among opiate abusers [76-78]. Here we show that an opiate can limit the antiretroviral effects of maraviroc, while the coordinated blockade of MOR and CCR5, using this novel bivalent compound, has considerable potential for development as a therapeutic strategy or as a molecular tool to study HIV-GPCR co-receptor interactions.
Supplementary Material
Acknowledgements
This work was funded by NIH grants DA026744 (NEH), DA007027 and DA033898 (SMD), DA018633, DA027374, and DA034231 (KFH), DA024022 (YZ). Flow cytometry was supported, in part, by NIH National Cancer Institute Cancer Center Support Grant P30 CA016059. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
The authors have no conflict of interest to report.
NEH designed the study and NEH and SMD conducted the experiments. SMD generated the HIV-1BaL Vpr-GFP virus and CKA and YZ designed and conducted the molecular modeling studies. NEH, SMD, KFH and YZ analyzed and interpreted the data. NEH, EMP and SMD drafted the manuscript and CKA, YZ and KFH did critical revision of the manuscript for important intellectual content. All authors approved the final article.
Reference List
- 1.Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7(10):e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Perez MP, O’Connell O, Lin R, Sullivan WM, Bell J, Simmonds P, et al. Independent evolution of macrophage-tropism and increased charge between HIV-1 R5 envelopes present in brain and immune tissue. Retrovirology. 2012;9:20. doi: 10.1186/1742-4690-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP. Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol. 2002;169(7):3589–3599. doi: 10.4049/jimmunol.169.7.3589. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki S, Carlos MP, Chuang LF, Torres JV, Doi RH, Chuang RY. Methadone induces CCR5 and promotes AIDS virus infection. FEBS Lett. 2002;519(1-3):173–177. doi: 10.1016/s0014-5793(02)02746-1. [DOI] [PubMed] [Google Scholar]
- 5.Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309(1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 6.Ho WZ, Guo CJ, Yuan CS, Douglas SD, Moss J. Methylnaltrexone antagonizes opioid-mediated enhancement of HIV infection of human blood mononuclear phagocytes. J Pharmacol Exp Ther. 2003;307(3):1158–1162. doi: 10.1124/jpet.103.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical mu-opioid receptor [In Process Citation] Neuropharmacology. 1999;38(2):273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 8.El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF. CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates. J Neuroimmunol. 2006;178(1-2):9–16. doi: 10.1016/j.jneuroim.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song C, Rahim RT, Davey PC, Bednar F, Bardi G, Zhang L, et al. Protein kinase Czeta mediates micro-opioid receptor-induced cross-desensitization of chemokine receptor CCR5. J Biol Chem. 2011;286(23):20354–20365. doi: 10.1074/jbc.M110.177303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483(2-3):175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280(2):192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Y, Arnatt CK, Li G, Haney KM, Ding D, Jacob JC, et al. Design and synthesis of a bivalent ligand to explore the putative heterodimerization of the mu opioid receptor and the chemokine receptor CCR5. Org Biomol Chem. 2012;10(13):2633–2646. doi: 10.1039/c2ob06801j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280(2):192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- 14.El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF. CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 Tat-exposed mice. J Neuroimmune Pharmacol. 2008;3(4):275–285. doi: 10.1007/s11481-008-9127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, et al. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53(2):132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47(12):2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 17.Mathews JL, Peng X, Xiong W, Zhang A, Negus SS, Neumeyer JL, et al. Characterization of a novel bivalent morphinan possessing kappa agonist and micro agonist/antagonist properties. J Pharmacol Exp Ther. 2005;315(2):821–827. doi: 10.1124/jpet.105.084343. [DOI] [PubMed] [Google Scholar]
- 18.Nowak I. Probing the membrane targeting C1 subdomains of PKC with bivalent ligands. Curr Top Med Chem. 2007;7(4):355–362. doi: 10.2174/156802607779941260. [DOI] [PubMed] [Google Scholar]
- 19.Decker M, Lehmann J. Agonistic and antagonistic bivalent ligands for serotonin and dopamine receptors including their transporters. Curr Top Med Chem. 2007;7(4):347–353. doi: 10.2174/156802607779941297. [DOI] [PubMed] [Google Scholar]
- 20.Berque-Bestel I, Lezoualc’h F, Jockers R. Bivalent ligands as specific pharmacological tools for G protein-coupled receptor dimers. Curr Drug Discov Technol. 2008;5(4):312–318. doi: 10.2174/157016308786733591. [DOI] [PubMed] [Google Scholar]
- 21.Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Pharmacological properties of bivalent ligands containing butorphan linked to nalbuphine, naltrexone, and naloxone at mu, delta, and kappa opioid receptors. J Med Chem. 2007;50(9):2254–2258. doi: 10.1021/jm061327z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol. 2007;566(1-3):75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Mathew SC, Ghosh N, By Y, Berthault A, Virolleaud MA, Carrega L, et al. Design, synthesis and biological evaluation of a bivalent micro opiate and adenosine A1 receptor antagonist. Bioorg Med Chem Lett. 2009;19(23):6736–6739. doi: 10.1016/j.bmcl.2009.09.112. [DOI] [PubMed] [Google Scholar]
- 24.Li F, Liu J, Jas GS, Zhang J, Qin G, Xing J, et al. Synthesis and evaluation of a near-infrared fluorescent non-peptidic bivalent integrin alpha(v)beta(3) antagonist for cancer imaging. Bioconjug Chem. 2010;21(2):270–278. doi: 10.1021/bc900313d. [DOI] [PubMed] [Google Scholar]
- 25.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13(1):1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 26.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increase in intracellular Ca2+, and the release of MCP-1, RANTES and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF. CCL5/RANTES Gene Deletion Attenuates Opioid-Induced Increases in Glial CCL2/MCP-1 Immunoreactivity and Activation in HIV-1 Tat-Exposed Mice. J Neuroimmune Pharmacol. 2008 doi: 10.1007/s11481-008-9127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, et al. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca2+]i, NF-kB trafficking and transcription. PLosONE. 2008;3:1–14. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, et al. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53(2):132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111(2):194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng-Mayer C, Levy JA. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Ann Neurol. 1988;23(Suppl):58–61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- 33.El-Hage N, Dever SM, Fitting S, Ahmed T, Hauser KF. HIV-1 coinfection and morphine coexposure severely dysregulate hepatitis C virus-induced hepatic proinflammatory cytokine release and free radical production: increased pathogenesis coincides with uncoordinated host defenses. J Virol. 2011;85(22):11601–11614. doi: 10.1128/JVI.05239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Hage N, Dever SM, Fitting S, Ahmed T, Hauser KF. HIV-1 coinfection and morphine coexposure severely dysregulate hepatitis C virus-induced hepatic proinflammatory cytokine release and free radical production: increased pathogenesis coincides with uncoordinated host defenses. J Virol. 2011;85(22):11601–11614. doi: 10.1128/JVI.05239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, et al. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J Leukoc Biol. 2003;74(6):1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- 36.Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, et al. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99(16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portoghese PS, Larson DL, Yim CB, Sayre LM, Ronsisvalle G, Lipkowski AW, et al. Stereostructure-activity relationship of opioid agonist and antagonist bivalent ligands. Evidence for bridging between vicinal opioid receptors. J Med Chem. 1985;28(9):1140–1141. doi: 10.1021/jm00147a002. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Yekkirala A, Tang Y, Portoghese PS. A bivalent ligand (KMN-21) antagonist for mu/kappa heterodimeric opioid receptors. Bioorg Med Chem Lett. 2009;19(24):6978–6980. doi: 10.1016/j.bmcl.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comer SD, Sullivan MA, Hulse GK. Sustained-release naltrexone: novel treatment for opioid dependence. Expert Opin Investig Drugs. 2007;16(8):1285–1294. doi: 10.1517/13543784.16.8.1285. [DOI] [PubMed] [Google Scholar]
- 40.Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation. Health Technol Assess. 2007;11(6):iii–85. doi: 10.3310/hta11060. [DOI] [PubMed] [Google Scholar]
- 41.Neumeyer JL, Zhang A, Xiong W, Gu XH, Hilbert JE, Knapp BI, et al. Design and synthesis of novel dimeric morphinan ligands for kappa and micro opioid receptors. J Med Chem. 2003;46(24):5162–5170. doi: 10.1021/jm030139v. [DOI] [PubMed] [Google Scholar]
- 42.Rook Y, Schmidtke KU, Gaube F, Schepmann D, Wunsch B, Heilmann J, et al. Bivalent beta-carbolines as potential multitarget anti-Alzheimer agents. J Med Chem. 2010;53(9):3611–3617. doi: 10.1021/jm1000024. [DOI] [PubMed] [Google Scholar]
- 43.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102(52):19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A bivalent ligand (KDAN-18) containing delta-antagonist and kappa-agonist pharmacophores bridges delta2 and kappa1 opioid receptor phenotypes. J Med Chem. 2005;48(6):1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- 45.Yuan Y, Arnatt CK, El-Hage N, Dever SM, Jacob JC, Selley DE, et al. A bivalent ligand targeting the putative mu opioid receptor and chemokine receptor CCR5 heterodimer: binding affinity versus functional activities. Med Chem Commun. 2013;4:847–851. doi: 10.1039/C3MD00080J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hauser KF, El-Hage N, Buch S, Berger JR, Tyor RWeal. Molecular Targets of Opiate Drug abuse in NeuroAids. Neurotoxicity Research. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- 48.Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Curr HIV Res. 2012;10(5):435–452. doi: 10.2174/157016212802138779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metzger DS, Woody GE, O’Brien CP. Drug treatment as HIV prevention: a research update. J Acquir Immune Defic Syndr. 2010;55(Suppl 1):S32–S36. doi: 10.1097/QAI.0b013e3181f9c10b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uhlmann S, Milloy MJ, Kerr T, Zhang R, Guillemi S, Marsh D, et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105(5):907–913. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCance-Katz EF, Moody DE, Morse GD, Ma Q, Rainey PM. Lack of clinically significant drug interactions between nevirapine and buprenorphine. Am J Addict. 2010;19(1):30–37. doi: 10.1111/j.1521-0391.2009.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruce RD, Altice FL, Gourevitch MN, Friedland GH. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: implications and management for clinical practice. J Acquir Immune Defic Syndr. 2006;41(5):563–572. doi: 10.1097/01.qai.0000219769.89679.ec. [DOI] [PubMed] [Google Scholar]
- 53.Khalsa JH, Elkashef A. Drug interactions between antiretroviral medications and medications used in the treatment of drug addiction: research needs. Am J Addict. 2010;19(1):96–100. doi: 10.1111/j.1521-0391.2009.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batkis MF, Treisman GJ, Angelino AF. Integrated opioid use disorder and HIV treatment: rationale, clinical guidelines for addiction treatment, and review of interactions of antiretroviral agents and opioid agonist therapies. AIDS Patient Care STDS. 2010;24(1):15–22. doi: 10.1089/apc.2009.0242. [DOI] [PubMed] [Google Scholar]
- 55.Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, Kazatchkine M, Sidibe M, Strathdee SA. Time to act: a call for comprehensive responses to HIV in people who use drugs. Lancet. 2010;376(9740):551–563. doi: 10.1016/S0140-6736(10)60928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazmierski WM, Kenakin TP, Gudmundsson KS. Peptide, peptidomimetic and small-molecule drug discovery targeting HIV-1 host-cell attachment and entry through gp120, gp41, CCR5 and CXCR4. Chem Biol Drug Des. 2006;67(1):13–26. doi: 10.1111/j.1747-0285.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 57.Dever SM, Xu R, Fitting S, Knapp PE, Hauser KF. Differential expression and HIV-1 regulation of mu-opioid receptor splice variants across human central nervous system cell types. J Neurovirol. 2012;18(3):181–190. doi: 10.1007/s13365-012-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY. Morphine induces gene expression of CCR5 in human CEMx174 lymphocytes. J Biol Chem. 2000;275(40):31305–31310. doi: 10.1074/jbc.M001269200. [DOI] [PubMed] [Google Scholar]
- 59.Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50(6):435–442. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis. 2002;185(1):118–122. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garvey L, Nelson M, Latch N, Erlwein OW, Allsop JM, Mitchell A, et al. CNS effects of a CCR5 inhibitor in HIV-infected subjects: a pharmacokinetic and cerebral metabolite study. J Antimicrob Chemother. 2012;67(1):206–212. doi: 10.1093/jac/dkr427. [DOI] [PubMed] [Google Scholar]
- 62.Hunt PW, Shulman N, Hayes TL, Dahl V, Somsouk M, Funderburg NT, et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized trial. Blood. 2013 doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, et al. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102(3):555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC. Role of heteromer formation in GABAB receptor function. Science. 1999;283(5398):74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 65.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84(3):835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 66.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318(5854):1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22(10):532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- 68.Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24(11):736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- 69.Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133(Pt 12):3685–3698. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- 70.Cohen RA. The Changing Face of HIV-Associated Cognitive and Neuropsychiatric Disturbance. In: Paul RH, Sacktor NC, Valcour V, Tashima KT, editors. HIV and the Brain: New Challenges in the Modern Era. Humana Press Inc.; New York: 2009. pp. 133–186. [Google Scholar]
- 71.Meyer J, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, et al. HIV and Recent Illicit Drug Use Interact to Affect Verbal Memory in Women. J Acquir Immune Defic Syndr. 2013;63(1):67–76. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58(2):154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson-Papp J, Gelman BB, Grant I, Singer E, Gensler G, Morgello S. Substance abuse increases the risk of neuropathy in an HIV-infected cohort. Muscle Nerve. 2012;45(4):471–476. doi: 10.1002/mus.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Royal W, III, Updike M, Selnes OA, Proctor TV, Nance-Sproson L, Solomon L, et al. HIV-1 infection and nervous system abnormalities among a cohort of intravenous drug users. Neurology. 1991;41(12):1905–1910. doi: 10.1212/wnl.41.12.1905. [DOI] [PubMed] [Google Scholar]
- 75.Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83(1-2):77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- 76.Proudnikov D, Randesi M, Orna L, Crystal H, Dorn M, Ott J, et al. Association of polymorphisms of the mu opioid receptor gene with the severity of HIV infection and response to HIV treatment. J Infect Dis. 2012;205(11):1745–1756. doi: 10.1093/infdis/jis264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dever SM, Xu R, Fitting S, Knapp PE, Hauser KF. Differential expression and HIV-1 regulation of μ-opioid receptor splice variants across human central nervous system cell types. J Neurovirol. 2012;18(3):181–190. doi: 10.1007/s13365-012-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Curr HIV Res. 2012;10(5):435–452. doi: 10.2174/157016212802138779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.