Summary
Background
Carotid intima-media thickness (cIMT) is related to the risk of cardiovascular events in the general population. An association between changes in cIMT and cardiovascular risk is frequently assumed but has rarely been reported. Our aim was to test this association.
Methods
We identified general population studies that assessed cIMT at least twice and followed up participants for myocardial infarction, stroke, or death. The study teams collaborated in an individual participant data meta-analysis. Excluding individuals with previous myocardial infarction or stroke, we assessed the association between cIMT progression and the risk of cardiovascular events (myocardial infarction, stroke, vascular death, or a combination of these) for each study with Cox regression. The log hazard ratios (HRs) per SD difference were pooled by random effects meta-analysis.
Findings
Of 21 eligible studies, 16 with 36 984 participants were included. During a mean follow-up of 7·0 years, 1519 myocardial infarctions, 1339 strokes, and 2028 combined endpoints (myocardial infarction, stroke, vascular death) occurred. Yearly cIMT progression was derived from two ultrasound visits 2–7 years (median 4 years) apart. For mean common carotid artery intima-media thickness progression, the overall HR of the combined endpoint was 0·97 (95% CI 0·94–1·00) when adjusted for age, sex, and mean common carotid artery intima-media thickness, and 0·98 (0·95–1·01) when also adjusted for vascular risk factors. Although we detected no associations with cIMT progression in sensitivity analyses, the mean cIMT of the two ultrasound scans was positively and robustly associated with cardiovascular risk (HR for the combined endpoint 1·16, 95% CI 1·10–1·22, adjusted for age, sex, mean common carotid artery intima-media thickness progression, and vascular risk factors). In three studies including 3439 participants who had four ultrasound scans, cIMT progression did not correlate between occassions (reproducibility correlations between r=−0·06 and r=−0·02).
Interpretation
The association between cIMT progression assessed from two ultrasound scans and cardiovascular risk in the general population remains unproven. No conclusion can be derived for the use of cIMT progression as a surrogate in clinical trials.
Funding
Deutsche Forschungsgemeinschaft.
Introduction
Carotid intima-media thickness (cIMT) is a non-invasive ultrasound biomarker of early atherosclerosis. A positive association exists between it and the risk of sub sequent cardiovascular events in general populations, independent of all major risk factors.1 This relation has promoted the use of cIMT in pathophysiological studies and clinical trials, in which the perception of cIMT has shifted from a secondary endpoint to a surrogate of risk of cardiovascular event. A randomised clinical trial published in 2009 was prematurely stopped on the basis of cIMT results.2
Many studies already include the tacit assumption that relations with cIMT, as seen in the general population or risk cohorts, reflect associations with the risk of cardiovascular events.3–5 Most of these studies use cIMT progression, calculated as an absolute yearly rate of progression. Repeated cIMT measurements are a plausible way to test the effects of interventions on cIMT progression. However, whether change of cIMT affects the risk of cardiovascular events should be systematically investigated. The results of the Multi-Ethnic Study of Atherosclerosis6 show a positive association between cIMT progression and stroke. The association between cIMT progression and the risk of myocardial infarction or mortality in the general population has never been assessed on a large scale. In view of the large variability of cIMT progression, this task requires access to individual participant data from many large cohorts. The aim of the first stage of the PROG-IMT project (individual progression of carotid intima media thickness as a surrogate of vascular risk) is to assemble a large cIMT progression dataset from general populations and to analyse the association of cIMT progression with the risk of cardiovascular events, the results of which we present here. In further stages we will analyse high-risk populations and randomised controlled trials.7
Methods
Study identification and procedures
We comprehensively searched published work for studies that had the following inclusion criteria: longitudinal observational studies, sample of or similar to the general population, well-defined inclusion criteria and recruitment strategy, at least two ultrasound visits with assessment of cIMT, clinical follow-up after the second ultrasound visit recording myocardial infarction, stroke, death, vascular death, or a combination of these, and a minimum of 20 events for at least one endpoint.
We searched PubMed with “intima media” AND (“myocardial infarction” OR ”stroke” OR ”death” OR “mortality”) to find original articles (usually 3000–5000 words) or research reports (usually 1000–1500 words) of relevant studies. We included publications in all languages, published up to Jan 10, 2012. We also manually searched reports referenced in reviews of cIMT. We sent a short screening questionnaire to the authors of potentially relevant reports. If a study fulfilled all inclusion criteria, the study team was invited to participate, contribute a predefined set of variables for individual participants, and collaborate on the project’s objectives.7
The datasets underwent central plausibility checks, in which the cutoff-thresholds to define implausible values were discussed with the investigators and data managers of the individual studies. The data were also harmonised, in which variables were uniformly named, transformed to SI units, and ordinal variables were recoded into binary categories with balanced distributions. Mean common carotid artery intima-media thickness was defined as the average of all mean intimamedia thicknesses of the common carotid artery at one timepoint (including the left and the right common carotid artery, the near and far wall, and all insonation angles). Similarly, maximal common carotid artery intima-media thickness was defined as the average of all maximal common carotid artery intima-media thicknesses. Mean maximal intima-media thickness was defined as the mean of maximal common carotid artery intima-media thickness, maximal intima-media thickness of the carotid bifurcation, and maximal intima-media thickness of the internal carotid artery. From these variables, we calculated the yearly progression rate for two ultrasound scans, and the mean of both scans.
The clinical endpoints (myocardial infarction, stroke, vascular death, and total mortality) were defined as in the individual studies. We included probable or definite myocardial infarction and any stroke (symptoms lasting more than 24 h, including non-traumatic haemorrhage).
Statistical analysis
To assess the risk of the first cardiovascular event, we excluded all individuals who had a stroke or myocardial infarction before the second cIMT scan. For each study, we fitted Cox regression models for each endpoint: myocardial infarction, stroke, death, and the combined endpoint (myocardial infarction, stroke, or vascular death). In studies for which vascular death was not assessed, we included total mortality. Each model estimated the hazard ratio (HR) of the cIMT progression variable per study-specific SD. Model 1 adjusted for age and sex; model 2 also adjusted for the mean cIMT of the first and the second scan. Model 3 included variables from model 2 and also adjusted for ethnic origin and socioeconomic status, and model 4 included variables from model 3 plus the mean and the progression of vascular risk factors (systolic blood pressure, antihypertensive treatment, total cholesterol, lipid-lowering treatment, creatinine concentration, haemoglobin concentration, smoking, and diabetes). We pooled the log HR estimates of the different studies by random effects meta-analysis8 and displayed them in forest plots. Heterogeneity was assessed with the I2 statistic.9
We used multiple imputation for missing values with ten imputed datasets per study.10 Ultrasound data, conventional risk factors, and endpoint data were used in the imputation together,11 but endpoint data were not imputed. Risk factor variables with more than 20% of values missing were neither imputed nor used in the analyses. As a result, of 194 risk factor variables in 17 cohorts, eight variables in five cohorts were lost: six variables were affected in only one of two visits (baseline or follow-up), two variables were dropped for both visits. cIMT values were imputed and used if the individual variable had more than 80% valid values or if the cIMT variables of one carotid segment at one visit had at least one valid value in more than 95% of participants, which was the case in all cohorts. The main analyses were repeated with non-imputed datasets in sensitivity analyses.
To corroborate our analyses, we did several sensitivity analyses. In addition to HR per one SD difference of cIMT progression, we estimated HR per 0·1 mm difference of cIMT progression. Because the cIMT progression variables had a non-normal distribution with wide tails, we repeated the analyses with a normalising transformation, preserving the ranks, to address potential effects of outliers. The proportional hazard assumption was assessed with an interaction term between cIMT progression and follow-up time from the second cIMT to event. To account for differential effects of age, we investigated the effect of an interaction term of age and cIMT progression. To account for potential sex differences, we repeated the analyses stratified by sex. A potential dose-response effect was assessed by analysis of cIMT and progression in quintiles.
In studies that did more than two ultrasound scans, individual cIMT progression was reassessed on the basis of three (or more) measurements by linear regression, excluding individuals who had had stroke or myocardial infarction before the last scan. These progression estimates were compared with those relying on two measurements and, when endpoints were recorded after the third scan, Cox regression models were repeated. For studies with four ultrasound visits, the reproducibility of assessment of cIMT progression was estimated by comparison of the first-to-second progression and third-to-fourth progression. Study selection bias was assessed by funnel plots.12 At the study level, we used meta-regression to investigate the associations between cIMT reproducibility or year of first ultrasound examination, and log HR of cIMT progression.13 The principal analysis and much of the sensitivity analyses used a previously published predefined analysis plan.7 All analyses were done with Stata/IC (version 11.1) or SPSS (version 19).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. MWL and SGT had full access to all the data in the study and MWL had final responsibility for the decision to submit for publication.
Results
The publication search yielded 1649 reports. 22 cohorts fulfilled the inclusion criteria (appendix p 11); 16 of which provided individual participant data and were included (table 1). Six study groups declined to participate (appendix p 1). Included cohorts had 58 407 participants and 625 593 person-years of follow-up, studies not included had 30 351 participants and 254 130 person-years. Thus, data included are 66% of data available worldwide in terms of number of participants, and 71% in terms of person-years of follow-up. Comparison of the characterstics of the studies included (table 1) and not included (appendix p 1) provides no indication of selection bias. After exclusion of individuals with previous events and events before the second ultrasound, and counting only the follow-up time after the second ultrasound scan (appendix p 2), the cohorts included 36 984 individuals with 257 067 person-years of follow-up. On average, people included were younger and had lower risk factors than were those who were excluded. 1519 myocardial infarctions, 1339 strokes, and 4268 deaths occurred, and 2028 participants reached the combined endpoint (myocardial infarction, stroke, or vascular death).
Table 1.
Included studies
| Countries | Total number of individuals (n) | Participants after exclusion (n)* | Ethnic origins (n, %)† | Endpoints | Age at baseline (years) | Men (n, %)† | Scan interval‡ (mean, years) | Follow-up after second scan (mean, years) | Segments | Measurements | Intima- media thickness definition | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atherosclerosis and Insulin Resistance14 | Sweden | 391 | 297 | White (297, 100·0%) | Myocardial infarction, stroke, death, vascular death | 57–58 | 297 (100·0%) | 3·2 | 5·6 | CCA, BIF | Far wall, left and right | Mean, maximal |
| Atherosclerosis Risk in Communities Study15§ | USA | 14 289 | 12 221 | White (9448, 77·3%), African American (2773, 22·7%) | Myocardial infarction, stroke, death | 45–64 | 5217 (42·7%) | 2·9 | 8·2 | CCA, BIF, ICA | Near and far wall, left and right, three insonation angles (CCA) | Mean, maximal |
| Bogalusa Heart Study16 | USA | 1399 | 558 | White (395, 70·8%), African American (163, 29·2%) | Death, vascular death | 24–43 | 241 (43·2%) | 2·3 | 4·5 | CCA, BIF, ICA | Far wall, left and right | Maximal |
| Bruneck Study17 | Austria, Italy | 821 | 633 | White (633, 100·0%) | Myocardial infarction, stroke, death, vascular death | 45–84 | 299 (47·2%) | 5·0 | 9·1 | CCA, ICA | Near and far wall, left and right | Mean¶, maximal |
| Cardiovascular Health Study,18 cohort 1|| | USA | 5201 | 3551 | White (3382, 95·2%), African American (153, 4·3%), other (16, 0·5%) | Myocardial infarction, stroke, death, vascular death | 65–95 | 1380 (38·9%) | 2·9 | 9·9 | CCA, ICA | Near and far wall, left and right, three insonation angles (ICA) | Mean, maximal |
| Cardiovascular Health Study,18 cohort 2|| | USA | 687 | 297 | African American (296, 99·7%), other (1, 0·3%) | Myocardial infarction, stroke, death, vascular death | 64–86 | 98 (33·0%) | 5·9 | 5·6 | CCA, ICA | Near and far wall, left and right, three insonation angles (ICA) | Mean, maximal |
| Carotid Atherosclerosis Progression Study19 | Germany | 6972 | 3284 | White (3284, 100·0%) | Myocardial infarction, stroke, death | 19–87 | 1591 (48·4%) | 3·2 | 5·3 | CCA, BIF, ICA | Far wall, left and right | Mean |
| Edinburgh Artery Study20 | UK | 1605 | 613 | White (613, 1000%) | Myocardial infarction, stroke, death, vascular death | 60–80 | 291 (47·5%) | 6·6 | 5·7 | CCA | Far wall, left and right | Mean, maximal |
| Etude sur le vieillissement artériel21 | France | 1040 | 922 | White (922, 100·0%) | Vascular death, death | 59–71 | 367 (39·8%) | 2·0 | 14·1 | CCA | Far wall, left and right | Mean |
| Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg22 | Germany | 3908 | 2534 | White (2534, 100·0%)§ | Myocardial infarction, stroke, death | 53–94 | 985 (38·9%) | 2·1 | 4·0 | CCA | Far wall, left and right | Mean |
| Kuopio Ischemic Heart Disease Study23 | Finland | 1399 | 849 | White (849, 100·0%) | Myocardial infarction, stroke, death, vascular death | 42–61 | 849 (100·0%) | 4·1 | 15·4 | CCA | Far wall, left and right | Mean, maximal |
| Northern Manhattan Study/The Oral Infections and Vascular Disease Epidemiology Study24 | USA | 784** | 653 | Hispanic (403, 61·7%), white (250, 38·3%) | Myocardial infarction,†† stroke, death, vascular death | 48–94 | 257 (39·4%) | 3·6 | 3·0 | CCA, BIF, ICA | Near and far wall, left and right | Mean, maximal |
| Progression of Lesions in the Intima of the Carotid25 | Italy | 1782 | 1538 | White (1538, 100·0%) | Myocardial infarction, stroke, death, vascular death | 15–82 | 607 (39·5%) | 2·2 | 4·1 | CCA | Far wall, left and right | Mean, maximal |
| Prospective Investigation of the Vasculature in Uppsala Seniors26 | Sweden | 1017 | 680 | White (680, 100·0%) | Death | 70 | 313 (46·0%) | 5·1 | 1·9 | CCA | Far wall, left and right | Mean, maximal |
| Rotterdam Study27 | Netherlands | 7983 | 2611 | White (2549, 98·7%), other (62, 1·3%) | Myocardial infarction, stroke, death | 55–95 | 991 (38·0%) | 6·5 | 5·8 | CCA | Near and far wall, left and right | Mean, maximal |
| Study of Health in Pomerania28 | Germany | 4308 | 1751 | White (1751, 100·0%) | Myocardial infarction, stroke, death | 44–80 | 874 (49·9%) | 5·2 | 5·9 | CCA | Far wall, left and right | Mean |
| Tromsø Study29 | Norway | 4821 | 3992 | Norwegian (3615, 98·1%), other (377, 1·9%) | Myocardial infarction, stroke, death, vascular death | 25–79 | 1823 (45·7%) | 6·3 | 4·4 | CCA, BIF | Near and far wall, right side | Mean, maximal |
CCA=common carotid artery. BIF=carotid bifurcation. ICA=internal carotid artery.
Reasons for exclusion were myocardial infarction, stroke, or death before the second ultrasound visit, or fewer than two ultrasound scans.
After exclusion.
Time between first and second ultrasound scan.
Declined to participate, public-use dataset included.
Excluded from mean common carotid artery intima-media thickness analyses because it had not assessed mean carotid intima-media thickness at two ultrasound scans.
The Cardiovascular Health Study consists of two cohorts, one of white participants and one of African American participants that was begun 3 years later, when the first follow-up visit of the white cohort was due. They were treated as different cohorts in all subsequent analyses.
A small sample was included because of the need to await adjudication of outcome events by the study neurologists and cardiologists at the time of analyses. No inference should be made about conclusions regarding the full sample.
No myocardial infarctions happened after exclusion of the events that occurred before the second scan.
Most participants were white, although other ethnic origins were also well represented (table 1). The sampling and endpoint identification procedures were of a high standard, although differences did exist (appendix p 3). The different cohorts and their study protocols had multiple potential sources of heterogeneity, including different age ranges (table 1), ultrasound protocols (table 1, appendix pp 4, 12), and endpoint definitions (appendix pp 5–6). Although the definition of other segments differed, the region designated “common carotid artery” was relatively consistent (appendix p 12). One study restricted the measurements to one side, and six included near and far wall measurements of cIMT. Ten studies used semi-automated edge-detection algorithms.
The mean estimates of cIMT progression ranged from 0·001 to 0·030 mm per year for mean common carotid artery intima-media thickness, from 0·001 to 0·065 mm per year for maximal common carotid artery intima-media thickness, and from 0·000 to 0·023 mm per year for mean maximal intima-media thickness (appendix pp 7–8). Overall, intima-media thickness (mean of baseline and follow-up) had only a very weak correlation with yearly intima-media thickness progression (r ranged from −0·38 to 0·25). The average reproducibility of cIMT (correlations between two examinations) ranged from r=0·27 to r=0·84.
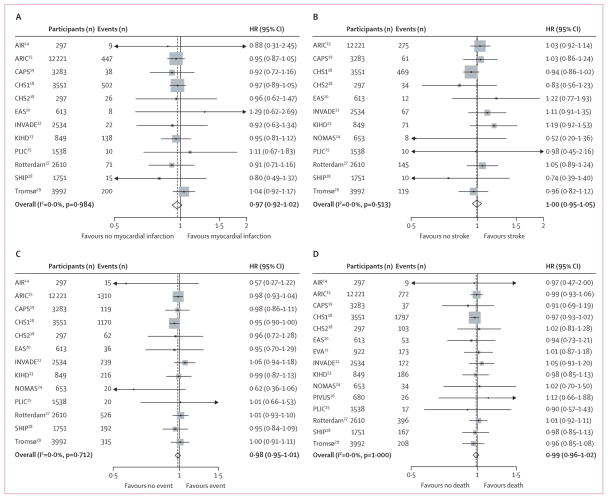
Figure 1 shows the association between mean common carotid artery intima-media thickness progression and the four endpoints in the fully adjusted model (model 4). The overall estimated HR per one SD increase in mean common carotid intima-media thickness progression for the combined endpoint was 0·97 (95% CI 0·94–1·00) when adjusted for age, sex, and mean common carotid artery intima-media thickness, and 0·98 (0·95–1·01) when also adjusted for vascular risk factors. We observed no heterogeneity in the HRs between studies.
Figure 1. Hazard ratios (HRs) per one SD increase in mean common carotid intima-media thickness progression for four endpoints.
HRs are for risk of myocardial infarction (A), stroke (B), the combined endpoint (C), and death (D). HRs adjusted for vascular risk factors (model 4, see text). Weights are from random effects analysis. AIR=Atherosclerosis and Insulin Resistance study. ARIC=Atherosclerosis Risk in Communities Study. CAPS=Carotid Atherosclerosis Progression Study. CHS=Cardiovascular Health Study. EAS=Edinburgh Artery Study. INVADE=Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg. KIHD=Kuopio Ischaemic Heart Disease Study. PLIC=Progression of Lesions in the Intima of the Carotid. SHIP=Study of Health in Pomerania. Rotterdam=Rotterdam Study. Tromsø=Tromsø Study.
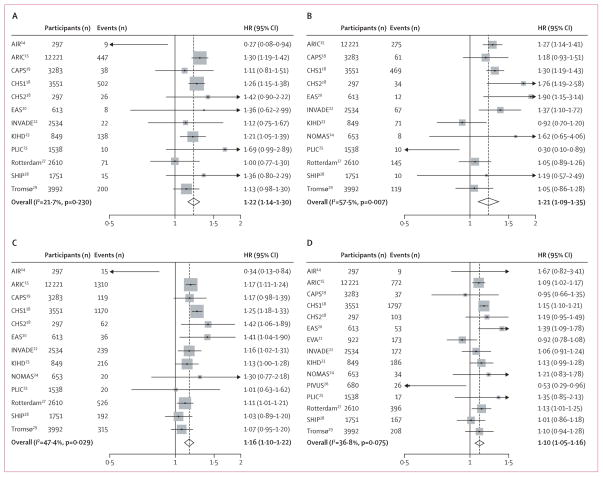
Figure 2 shows the same analyses for the mean common carotid artery intima-media thickness. The HRs per one SD increase for the combined endpoint were 1·24 (1·16–1·32) when adjusted for age, sex, and mean common carotid artery intima-media thickness progression, and 1·16 (1·10–1·22) when also adjusted for vascular risk factors. Some heterogeneity was evident when the mean cIMT HRs were combined.
Figure 2. Hazard ratios (HRs) per one SD increase in mean common carotid intima-media thickness for four endpoints.
HRs are for risk of myocardial infarction (A), stroke (B), the combined endpoint (C), and death (D). HRs adjusted for vascular risk factors (model 4, see text). Weights are from random effects analysis. AIR=Atherosclerosis and Insulin Resistance study. ARIC=Atherosclerosis Risk in Communities Study. CAPS=Carotid Atherosclerosis Progression Study. CHS=Cardiovascular Health Study. EAS=Edinburgh Artery Study. INVADE=Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg. KIHD=Kuopio Ischaemic Heart Disease Study. PLIC=Progression of Lesions in the Intima of the Carotid. SHIP=Study of Health in Pomerania. Rotterdam=Rotterdam Study. Tromsø=Tromsø Study.
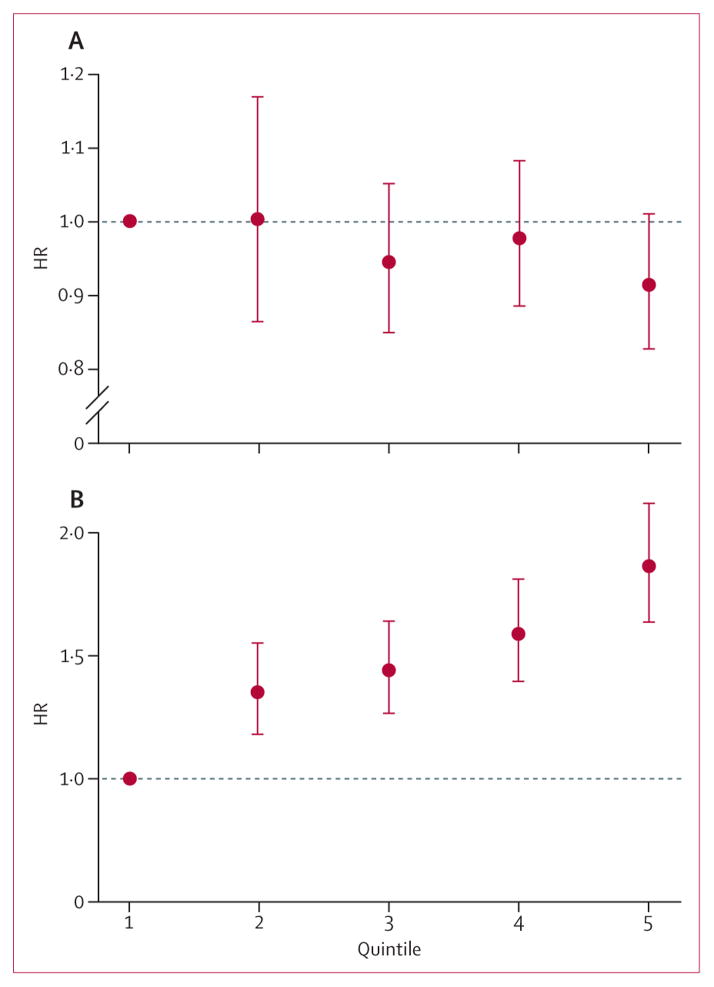
Table 2 shows the results of the primary analyses (for the results of the sensitivity analyses see appendix p 9). Irrespective of the definition of cIMT (mean common carotid artery intima-media thickness, maxi mal common carotid artery intima-media thickness, mean maximal intima-media thickness), the endpoint, and adjustment, no significant association existed between cIMT progression and any endpoints. The association of cIMT (mean of baseline and follow-up) with the endpoints was significant and positive. These associations were attenuated after adjustment for vascular risk factors, as expected. Some analyses showed significant heterogeneity in the HRs across studies. The calculation of the HRs per 0·1 mm instead of one SD, the use of nonimputed data, or the use of a normalising trans formation of the cIMT progression distribution did not qualitatively change any of the results (appendix p 9). When cIMT progression was categorised in quintiles (figure 3A), no significant association existed with the combined endpoint, by contrast with mean cIMT (figure 3B). In analyses stratified by sex, no evidence existed of an association between cIMT progression and the endpoints for either sex (appendix p 9). An interaction term of age and cIMT progression was not significant, showing no effects of age. The main results from studies including plaques in the cIMT measurement did not differ from studies avoiding plaques (appendix p 9). No evidence existed of non-proportional hazards over time for cIMT progression or for mean cIMT. Finally, the principal analysis for stroke was repeated including published estimates from the Multi-Ethnic Study of Atherosclerosis,6 which pro vided much the same overall results (appendix p 9).
Table 2.
Overall hazard ratios for each endpoint per one SD increase in mean common carotid artery intima-media thickness in four models
| Yearly carotid intima-media
thickness progression |
Mean carotid intima-media
thickness of scans 1 and 2 |
|||
|---|---|---|---|---|
| Overall HR (95% CI) | I2 (p)* | Overall HR (95% CI) | I2 (p)* | |
|
Myocardial
infarction†
| ||||
| Model 1 | 0·99 (0·94–1·04) | 0·0% (0·9216) | .. | .. |
| Model 2 | 0·96 (0·92–1·01) | 0·0% (0·9857) | 1·30 (1·19–1·43) | 57·5% (0·0067) |
| Model 3 | 0·96 (0·92–1·01) | 0·0% (0·9831) | 1·30 (1·19–1·43) | 56·9% (0·0076) |
| Model 4 | 0·97 (0·92–1·02) | 0·0% (0·9841) | 1·22 (1·14–1·30) | 21·6% (0·2302) |
|
| ||||
|
Stroke‡
| ||||
| Model 1 | 1·01 (0·96–1·07) | 0·0% (0·7598) | .. | .. |
| Model 2 | 0·99 (0·95–1·04) | 0·0% (0·5969) | 1·32 (1·19–1·48) | 67·0% (0·0005) |
| Model 3 | 0·99 (0·95–1·05) | 0·0% (0·5933) | 1·32 (1·20–1·45) | 54·1% (0·0128) |
| Model 4 | 1·00 (0·95–1·05) | 0·0% (0·5134) | 1·21 (1·09–1·35) | 57·5% (0·0068) |
|
| ||||
|
Combined§
| ||||
| Model 1 | 0·99 (0·96–1·03) | 16·0% (0·2832) | .. | .. |
| Model 2 | 0·97 (0·94–1·00) | 0·0% (0·5583) | 1·24 (1·16–1·32) | 70·5% (<0·0001) |
| Model 3 | 0·97 (0·94–1·00) | 0·0% (0·5588) | 1·23 (1·17–1·31) | 60·3% (0·0026) |
| Model 4 | 0·98 (0·95–1·01) | 0·0% (0·7114) | 1·16 (1·10–1·22) | 47·4% (0·0294) |
|
| ||||
|
Death¶
| ||||
| Model 1 | 1·00 (0·97–1·03) | 0·0% (0·5991) | .. | .. |
| Model 2 | 0·98 (0·95–1·01) | 0·0% (0·9886) | 1·15 (1·08–1·22) | 60·7% (0·0012) |
| Model 3 | 0·98 (0·95–1·01) | 0·0% (0·9859) | 1·15 (1·09–1·21) | 44·5% (0·0325) |
| Model 4 | 0·99 (0·96–1·02) | 0·0% (0·9996) | 1·10 (1·05–1·16) | 36·8% (0·0754) |
p value of test for heterogeneity.
Studies included: Atherosclerosis and Insulin Resistance study; Atherosclerosis Risk in Communities Study; Carotid Atherosclerosis Progression Study; Cardiovascular Health Study, cohorts 1 and 2; Edinburgh Artery Study; Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg; Kuopio Ischemic Heart Disease Study; Progression of Lesions in the Intima of the Carotid; Rotterdam; Study of Health in Pomerania; Tromsø Study.
Studies included: Atherosclerosis Risk in Communities Study; Carotid Atherosclerosis Progression Study; Cardiovascular Health Study, cohorts 1 and 2; Edinburgh Artery Study; Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg; Kuopio Ischemic Heart Disease Study; Northern Manhattan Study/Infections and Vascular Disease Epidemiology Study; Progression of Lesions in the Intima of the Carotid; Rotterdam Study; Study of Health in Pomerania; Tromsø Study.
Studies included: Atherosclerosis and Insulin Resistance study; Atherosclerosis Risk in Communities Study; Carotid Atherosclerosis Progression Study; Cardiovascular Health Study, cohorts 1 and 2; Edinburgh Artery Study; Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg; Kuopio Ischemic Heart Disease Study; Northern Manhattan Study/Infections and Vascular Disease Epidemiology Study; Progression of Lesions in the Intima of the Carotid; Rotterdam Study; Tromsø Study.
Studies included: Atherosclerosis and Insulin Resistance study; Atherosclerosis Risk in Communities Study; Carotid Atherosclerosis Progression Study; Cardiovascular Health Study, cohorts 1 and 2; Edinburgh Artery Study; Etude sur le vieillissement artériel; Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg; Kuopio Ischemic Heart Disease Study; Northern Manhattan Study/Infections and Vascular Disease Epidemiology Study; Prospective Investigation of the Vasculature in Uppsala Seniors; Progression of Lesions in the Intima of the Carotid; Rotterdam Study; Study of Health in Pomerania; Tromsø Study.
Figure 3. Overall hazard ratio (HR) of the combined endpoint by quintile.
Data shown for mean common carotid artery intima-media thickness progression (A) and mean common carotid artery intima-media thickness (B), relative to the lowest quintile. Bars are 95% CIs. HRs are adjusted for vascular risk factors (model 4, see text). Included studies: Atherosclerosis and Insulin Resistance study, Atherosclerosis Risk in Communities Study, Carotid Atherosclerosis Progression Study, Cardiovascular Health Study cohorts 1 and 2, Edinburgh Artery Study, Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg, Kuopio Ischaemic Heart Disease Study, Nothern Manhattan Study/The Oral Infections and Vascular Disease Epidemiology Study, Progression of Lesions in the Intima of the Carotid, Rotterdam Study, and Tromsø Study.
From the studies with more than two ultrasound visits, we recalculated the yearly cIMT progression rate including three or four cIMTs and compared them with those assessed from two ultrasound scans (appendix p 10). The SD of the estimates of cIMT progression decreased when three or four measurements were included. On the basis of reassessed cIMT progression estimates and only including clinical events after the third ultrasound scan, the HR for cIMT progression was recalculated in four cohorts with available clinical follow-up after the third ultrasound visit. The HR estimates from two ultrasound visits and from three ultrasound visits had only small differences in inconsistent directions (appendix p 13). The reproducibility correlations of cIMT progression for the cohorts with four ultrasound visits were −0·02 for Atherosclerosis Risk in Communities,15 −0·04 for Interventionsprojekt zerebro vaskuläre Erkrankungen und Demenz im Landkreis Ebersberg,22 and −0·06 for the Kuopio Ischaemic Heart Disease study (appendix pp 7–8);23 all were near zero.
Omission of two studies indicative of selection bias (appendix p 14) did not change the overall results. A meta-regression analysis did not suggest any effect of cIMT reproducibility or year of first ultrasound on the HRs for cIMT progression (appendix p 15).
Discussion
We have collated 71% of the data from general population cohort studies available worldwide, and have been able to undertake comprehensive and standardised analysis on the basis of individual participant records. We found no evidence of an association between individual cIMT progression and the risk of subsequent cardiovascular events, irrespective of definition of cIMT, endpoint, and adjustment.
By contrast with these results, the Multi-Ethnic Study of Atherosclerosis6 had a significant and positive association between yearly mean common carotid artery intima-media thickness progression and risk of stroke. Combination of Multi-Ethnic Study of Atherosclerosis results— based on 42 strokes—with the data for 1339 strokes from our 16 studies provided a non-significant association (HR 1·02, 95% CI 0·96–1·09). An effect dependent on ethnic origin seems highly unlikely, because the three most common ethnic origins in the Multi-Ethnic Study of Atherosclerosis were also present in our cohorts, and the fourth (Chinese) had only one stroke event. The possibility of a spurious finding in the Multi-Ethnic Study of Atherosclerosis should not be excluded.
By contrast with our consistent null result for cIMT progression, a positive, robust, and statistically significant association exists between mean cIMT and subsequent clinical endpoints. What are the possible methodological or biological explanations?
Differences between study procedures, ultrasound protocols, endpoint definitions, or durations of ultrasound and clinical follow-up could affect the progression estimates and their precision. However, the definition of common carotid artery intima-media thickness used in the primary and most secondary analyses was much the same in most studies (appendix p 12). The endpoint procedures and definitions differed only slightly, and most studies used expert adjudications to assess events. We found no evidence of statistical heterogeneity between the cIMT progression HRs. The differences in the rates of events could be explained by different characteristics of the populations, including their age distributions.
All included studies took several steps to minimise measurement errors (appendix p 4). Nevertheless, cIMT progression as assessed from two ultrasound scans several years apart does not seem to be a reliable measure, irrespective of how modern and accurate the cIMT measurements were. This reduced reliability seems to be a more plausible methodological explanation for our negative result than is heterogeneity between studies.
Biological factors could explain the absence of relation between cIMT progression and clinical endpoints. Atherosclerosis is a lifelong process that progresses slowly at a young age, and could accelerate with accumulation of risk factors.30 Slow progression of cIMT in healthy populations is difficult to detect. In intermediate stages, the diffuse thickening of the intimamedia complex can become superimposed by focal plaques at vessel sites with the highest cIMT.31 The diffuse (cIMT) and focal (plaque) manifestations of atherosclerosis could have different associations with risk factors.32–34 The final occurrence of clinical endpoints could be more strongly related to plaque formation than to cIMT progression.35
Participation in a longitudinal population study might change an individual’s behaviour, an effect known as the Hawthorne effect.36 Lifestyle changes could have had complex effects—on cIMT progression, stabilisation of plaques, and improved survival—that are difficult to adjust for, diluting the association between cIMT change and clinical events. However, such behavioural effects are more plausible in high-risk populations than in the general population. Changing behaviour by motivational carotid ultrasound has not been substantiated for smoking cessation.37 Moreover, only six of 16 studies informed participants of their cIMTs, which makes the Hawthorne effect unlikely.
The ethnic origins of participants were typical for the locations of the cohorts, so our results are only generalisable to the USA and Europe. Survivor bias was inevitably introduced by the need to exclude individuals with previous cardiovascular events and fewer than two ultrasound scans.
In conclusion, the association between individual cIMT progression and cardiovascular risk in the general population is still unproven, despite the strong association between single cIMT measurement and cardiovascular disease,1,38 as shown again in this study. We strongly advocate further validations and improvements of ultrasound protocols. Although efforts have been made to develop standardised ultrasound protocols for single and repeated cIMT assessments,39 methodological issues have only begun to be addressed.40–43
In population studies, ultrasound scans are typically repeated 2–5 years apart. More frequent cIMT measurements could increase the precision of the assessment of cIMT progression. If ultrasound protocols and study designs to minimise measurement errors are combined and carefully validated, cIMT progression in population studies could become a more reproducible biomarker.
Our results do not permit conclusions to be made about the surrogacy of cIMT progression in randomised controlled trials, which involve important differences in ultrasound assessment and population characteristics. This issue will be addressed in stage three of the PROGIMT study.
Supplementary Material
Acknowledgments
We used a restricted access dataset of the Atherosclerosis Risk In Communities (ARIC) Study. The ARIC Study was supported by National Heart, Lung and Blood Institute (Bethesda, MD, USA) in collaboration with the ARIC study investigators. This Article does not necessarily convey the opinions or views of the ARIC Study or the National Heart, Lung and Blood Institute. The Bruneck study was supported by the Pustertaler Verein zur Praevention von Herz- und Hirngefaesserkrankungen, Gesundheitsbezirk Bruneck, and the Assessorat fuer Gesundheit (Province of Bolzano, Italy). The Carotid Atherosclerosis Progression Study was supported by the Stiftung Deutsche Schlaganfall-Hilfe. The Cardiovascular Health Study research reported in this article was supported by contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 to N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke (Bethesda, MD, USA). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (Bethesda, MD, USA). A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Etude sur le vieillissement artériel was organised with an agreement between INSERM and Merck, Sharp, and Dohme-Chibret. The Northern Manhattan Study/The Oral Infections and Vascular Disease Epidemiology Study is funded by the National Institute of Neurological Disorders and Stroke grant R37 NS 029993 and the Oral Infections, Carotid Atherosclerosis, and Stroke study by the National Institute of Dental and Craniofacial Research (Bethesda, MD, USA) grant R01 DE 13094. The Interventionsprojekt zerebrovaskuläre Erkrankungen und Demenz im Landkreis Ebersberg study was supported by AOK Bayern. The Rotterdam Study was supported by the Netherlands Foundation for Scientific Research, ZonMw, Vici 918-76-619. The Study of Health in Pomerania is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (BMBF 01ZZ9603 and 01ZZ0103), the Ministry of Cultural Affairs, and the Social Ministry of the Federal State of Mecklenburg-West Pomerania. The PROG-IMT project was funded by the Deutsche Forschungsgemeinschaft (DFG Lo 1569/2-1).
Footnotes
Contributors
MLW designed the study, searched the published work, collected, analysed, and interpreted data, and wrote the Article. MK collected data. LG analysed and interpreted data. KZ collected, analysed, and interpreted data and wrote the Article. MLB interpreted data and wrote the Article. SGT designed the study, collected and interpreted data, and wrote the Article. The other authors collected and interpreted data and wrote the Article.
Collaborators for this report
USA David Yanez, Michal Juraska (University of Washington, Seattle, WA); Sathanur R Srinivasan, Gerald S Berenson (Tulane University, New Orleans, LA); Ralph L Sacco (University of Miami, FL).
Netherlands Jacqueline C M Witteman, Monique M B Breteler, Albert Hofman (Erasmus Medical Center, Rotterdam).
Norway Stein H Johnsen, Eva Stensland (University of Tromsø, Tromsø); Stefan Agewall (Oslo University Hospital, Oslo).
Germany Matthias Sitzer, Helmuth Steinmetz (J W Goethe-University, Frankfurt); Marcus Dörr, Ulf Schminke (Greifswald University Clinic, Greifswald); Holger Poppert, Horst Bickel (University of Technology, Munich). Finalnd Jussi Kauhanen, Kimmo Ronkainen (University of Eastern Finland, Kuopio). France Jean Philippe Empana (University Paris Descartes, Paris); Pierre Ducimetiere (University Paris-Sud XI).
Italy Giuseppe D Norata (University of Milan, Milan); Liliana Grigore (IRCSS, Milan). UK Jackie Price, Gerry Fowkes (University of Edinburgh, Edinburgh). Austria Johann Willeit (Medical University Innsbruck). Sweden Lena Bokemark (Uppsala University, Uppsala); Björn Fagerberg (Gothenburg University, Gothenburg). For a full list of the members of the PROG-IMT study group see appendix pp 16–18.
Conflicts of interest
Michiel Bots has received grants from AstraZeneca, Dutch Heart Foundation, Organon, Pfizer, Servier, the Netherlands Organisation for Health Research and Development, and TNO-Zeist, and consultancy fees from AstraZeneca, Boeringher, Organon, Pfizer, Servier, Schering-Plough, and Unilever. He runs the Vascular Imaging Center in Utrecht, a core laboratory for cIMT measurements in national and international observational and intervention studies. All other authors declare that they have no conflicts of interest.
References
- 1.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–22. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 3.Künzli N, Jerrett M, Garcia-Esteban R, et al. Ambient air pollution and the progression of atherosclerosis in adults. PLoS One. 2010;5:e9096. doi: 10.1371/journal.pone.0009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–38. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki Y, Katakami N, Furukado S, et al. Long-term effects of pioglitazone on carotid atherosclerosis in Japanese patients with type 2 diabetes without a recent history of macrovascular morbidity. J Atheroscler Thromb. 2010;17:1132–40. doi: 10.5551/jat.4663. [DOI] [PubMed] [Google Scholar]
- 6.Polak JF, Pencina MJ, O‘Leary DH, D‘Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke. 2011;42:3017–21. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenz MW, Bickel H, Bots ML, et al. PROG-IMT Study Group. Individual progression of carotid intima media thickness as a surrogate for vascular risk (PROG-IMT): rationale and design of a meta-analysis project. Am Heart J. 2010;159:730–36. doi: 10.1016/j.ahj.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. (Wiley Series in Probability and Statistics). [Google Scholar]
- 11.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 13.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 14.Wallenfeldt K, Bokemark L, Wikstrand J, Hulthe J, Fagerberg B. Apolipoprotein B/apolipoprotein A-I in relation to the metabolic syndrome and change in carotid artery intima-media thickness during 3 years in middle-aged men. Stroke. 2004;35:2248–52. doi: 10.1161/01.STR.0000140629.65145.3c. [DOI] [PubMed] [Google Scholar]
- 15.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–76. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 17.Kiechl S, Egger G, Mayr M, et al. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 2001;103:1064–70. doi: 10.1161/01.cir.103.8.1064. [DOI] [PubMed] [Google Scholar]
- 18.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 20.Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–81. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 21.Bonithon-Kopp C, Touboul PJ, Berr C, et al. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries. The Vascular Aging (EVA) Study. Arterioscler Thromb Vasc Biol. 1996;16:310–16. doi: 10.1161/01.atv.16.2.310. [DOI] [PubMed] [Google Scholar]
- 22.Sander D, Kukla C, Klingelhöfer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3-year follow-up study. Circulation. 2000;102:1536–41. doi: 10.1161/01.cir.102.13.1536. [DOI] [PubMed] [Google Scholar]
- 23.Lynch J, Kaplan GA, Salonen R, Salonen JT. Socioeconomic status and progression of carotid atherosclerosis. Prospective evidence from the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol. 1997;17:513–19. doi: 10.1161/01.atv.17.3.513. [DOI] [PubMed] [Google Scholar]
- 24.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70:1200–07. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norata GD, Garlaschelli K, Ongari M, Raselli S, Grigore L, Catapano AL. Effects of fractalkine receptor variants on common carotid artery intima-media thickness. Stroke. 2006;37:1558–61. doi: 10.1161/01.STR.0000221803.16897.22. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Macias KA, Lind L, Naessen T. Thicker carotid intima layer and thinner media layer in subjects with cardiovascular diseases. An investigation using noninvasive high-frequency ultrasound. Atherosclerosis. 2006;189:393–400. doi: 10.1016/j.atherosclerosis.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Iglesias del Sol A, Bots ML, Grobbee DE, Hofman A, Witteman JC. Carotid intima-media thickness at different sites: relation to incident myocardial infarction; the Rotterdam Study. Eur Heart J. 2002;23:934–40. doi: 10.1053/euhj.2001.2965. [DOI] [PubMed] [Google Scholar]
- 28.von Sarnowski B, Lüdemann J, Völzke H, Dörr M, Kessler C, Schminke U. Common carotid intima-media thickness and Framingham risk score predict incident carotid atherosclerotic plaque formation: longitudinal results from the study of health in Pomerania. Stroke. 2010;41:2375–77. doi: 10.1161/STROKEAHA.110.593244. [DOI] [PubMed] [Google Scholar]
- 29.Stensland-Bugge E, Bønaa KH, Joakimsen O, Njølstad I. Sex differences in the relationship of risk factors to subclinical carotid atherosclerosis measured 15 years later: the Tromsø study. Stroke. 2000;31:574–81. doi: 10.1161/01.str.31.3.574. [DOI] [PubMed] [Google Scholar]
- 30.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–78. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 31.Kiechl S, Willeit J. The natural course of atherosclerosis: part I: incidence and progression. Arterioscler Thromb Vasc Biol. 1999;19:1484–90. doi: 10.1161/01.atv.19.6.1484. [DOI] [PubMed] [Google Scholar]
- 32.Fagerberg B, Wikstrand J, Berglund G, Samuelsson O, Agewall S. Mortality rates in treated hypertensive men with additional risk factors are high but can be reduced. A randomized intervention study. Am J Hypertens. 1998;11:14–22. doi: 10.1016/s0895-7061(97)00363-4. [DOI] [PubMed] [Google Scholar]
- 33.Agewall S, Fagerberg B, Schmidt C, Wendelhag I, Wikstrand J. Multiple risk factor intervention in high risk hypertensive men. Comparison of ultrasound intima-media thickness and clinical outcome during six years of follow-up. J Intern Med. 2001;249:305–14. doi: 10.1046/j.1365-2796.2001.00818.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt C, Fagerberg B, Wikstrand J, Hulthe J RIS Study Group. Multiple risk factor intervention reduces cardiovascular risk in hypertensive patients with echolucent plaques in the carotid artery. J Intern Med. 2003;253:430–38. doi: 10.1046/j.1365-2796.2003.01129.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11:21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 36.Landsberger HA. Cornell studies in industrial and labor relations. Vol. 9. Ithaca: Cornell University; 1958. Hawthorne revisited; p. 119. [Google Scholar]
- 37.Rodondi N, Collet TH, Nanchen D, et al. Impact of carotid plaque screening on smoking cessation and other cardiovascular risk factors: a randomized controlled trial. Arch Intern Med. 2012;172:344–52. doi: 10.1001/archinternmed.2011.1326. [DOI] [PubMed] [Google Scholar]
- 38.Simon A, Gariepy J, Chironi G, Megnien JL, Levenson J. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20:159–69. doi: 10.1097/00004872-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 40.Dogan S, Duivenvoorden R, Grobbee DE, et al. Radiance 1 and 2 Study Groups. Completeness of carotid intima media thickness measurements depends on body composition: the RADIANCE 1 and 2 trials. J Atheroscler Thromb. 2010;17:526–35. doi: 10.5551/jat.3269. [DOI] [PubMed] [Google Scholar]
- 41.Peters SA, Palmer MK, Grobbee DE, et al. METEOR Study Group. Effect of number of ultrasound examinations on the assessment of carotid intima-media thickness changes over time: the example of the METEOR study. J Hypertens. 2011;29:1145–54. doi: 10.1097/HJH.0b013e328345d85e. [DOI] [PubMed] [Google Scholar]
- 42.Polak JF, Pencina MJ, Herrington D, O‘Leary DH. Associations of edge-detected and manual-traced common carotid intima-media thickness measurements with Framingham risk factors: the multi-ethnic study of atherosclerosis. Stroke. 2011;42:1912–16. doi: 10.1161/STROKEAHA.110.603449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters SA, den Ruijter HM, Palmer MK, et al. on behalf of the METEOR Study Group. Manual or semi-automated edge detection of the maximal far wall common carotid intima-media thickness: a direct comparison. J Intern Med. 2012;271:247–56. doi: 10.1111/j.1365-2796.2011.02422.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.