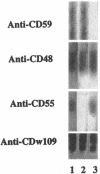
Abstract
Glycosylphosphatidylinositol (GPI)-anchored proteins are nonmembrane spanning cell surface proteins that have been demonstrated to be signal transduction molecules. Because these proteins do not extend into the cytoplasm, the mechanism by which cross-linking of these molecules leads to intracellular signal transduction events is obscure. Previous analysis has indicated that these proteins are associated with src family member tyrosine kinases; however, the role this interaction plays in the generation of intracellular signals is not clear. Here we show that GPI-anchored proteins are associated with alpha subunits of heterotrimeric GTP binding proteins (G proteins) in both human and murine lymphocytes. When the GPI-anchored proteins CD59, CD48, and Thy-1 were immunoprecipitated from various cell lines or freshly isolated lymphocytes, all were found to be associated with a 41-kDa phosphoprotein that we have identified, by using specific antisera, as a mixture of tyrosine phosphorylated G protein alpha subunits: a small amount of Gialpha1, and substantial amounts of Gialpha2 and Gialpha3. GTP binding assays performed with immunoprecipitations of CD59 indicated that there was GTP-binding activity associated with this molecule. Thus, we have shown by both immunochemical and functional criteria that GPI-anchored proteins are physically associated with G proteins. These experiments suggest a potential role of G proteins in the transduction of signals generated by GPI-anchored molecules expressed on lymphocytes of both mouse and human.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgeier A., Offermanns S., Van Sande J., Spicher K., Schultz G., Dumont J. E. The human thyrotropin receptor activates G-proteins Gs and Gq/11. J Biol Chem. 1994 May 13;269(19):13733–13735. [PubMed] [Google Scholar]
- Bohuslav J., Horejsí V., Hansmann C., Stöckl J., Weidle U. H., Majdic O., Bartke I., Knapp W., Stockinger H. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med. 1995 Apr 1;181(4):1381–1390. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown D. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr Opin Immunol. 1993 Jun;5(3):349–354. doi: 10.1016/0952-7915(93)90052-t. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Fong H. K., Simon M. I., Gilman A. G. Gz, a guanine nucleotide-binding protein with unique biochemical properties. J Biol Chem. 1990 Feb 5;265(4):2383–2390. [PubMed] [Google Scholar]
- Casey P. J. Lipid modifications of G proteins. Curr Opin Cell Biol. 1994 Apr;6(2):219–225. doi: 10.1016/0955-0674(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Chabre O., Conklin B. R., Brandon S., Bourne H. R., Limbird L. E. Coupling of the alpha 2A-adrenergic receptor to multiple G-proteins. A simple approach for estimating receptor-G-protein coupling efficiency in a transient expression system. J Biol Chem. 1994 Feb 25;269(8):5730–5734. [PubMed] [Google Scholar]
- Chang W. J., Ying Y. S., Rothberg K. G., Hooper N. M., Turner A. J., Gambliel H. A., De Gunzburg J., Mumby S. M., Gilman A. G., Anderson R. G. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994 Jul;126(1):127–138. doi: 10.1083/jcb.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun M., Liyanage U. K., Lisanti M. P., Lodish H. F. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinek T., Horejsí V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992 Oct 1;149(7):2262–2270. [PubMed] [Google Scholar]
- Davis L. S., Patel S. S., Atkinson J. P., Lipsky P. E. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J Immunol. 1988 Oct 1;141(7):2246–2252. [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Fra A. M., Williamson E., Simons K., Parton R. G. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra A. M., Williamson E., Simons K., Parton R. G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994 Dec 9;269(49):30745–30748. [PubMed] [Google Scholar]
- Garnett D., Barclay A. N., Carmo A. M., Beyers A. D. The association of the protein tyrosine kinases p56lck and p60fyn with the glycosyl phosphatidylinositol-anchored proteins Thy-1 and CD48 in rat thymocytes is dependent on the state of cellular activation. Eur J Immunol. 1993 Oct;23(10):2540–2544. doi: 10.1002/eji.1830231024. [DOI] [PubMed] [Google Scholar]
- Groux H., Huet S., Aubrit F., Tran H. C., Boumsell L., Bernard A. A 19-kDa human erythrocyte molecule H19 is involved in rosettes, present on nucleated cells, and required for T cell activation. Comparison of the roles of H19 and LFA-3 molecules in T cell activation. J Immunol. 1989 May 1;142(9):3013–3020. [PubMed] [Google Scholar]
- Gudermann T., Birnbaumer M., Birnbaumer L. Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2+ mobilization. Studies with the cloned murine luteinizing hormone receptor expressed in L cells. J Biol Chem. 1992 Mar 5;267(7):4479–4488. [PubMed] [Google Scholar]
- Hanada K., Nishijima M., Akamatsu Y., Pagano R. E. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. J Biol Chem. 1995 Mar 17;270(11):6254–6260. doi: 10.1074/jbc.270.11.6254. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Solomon K., Hom R. C., Soman G., Bergelson J. M., Bhan A. K., Finberg R. W. Cellular expression of a GPI-linked T cell activation protein. Cell Immunol. 1994 Jul;156(2):357–370. doi: 10.1006/cimm.1994.1181. [DOI] [PubMed] [Google Scholar]
- Harnett M., Rigley K. The role of G-proteins versus protein tyrosine kinases in the regulation of lymphocyte activation. Immunol Today. 1992 Dec;13(12):482–486. doi: 10.1016/0167-5699(92)90022-Y. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Pitcher J. A., Luttrell D. K., Linder M. E., Kurose H., Parsons S. J., Caron M. G., Lefkowitz R. J. Tyrosine phosphorylation of G protein alpha subunits by pp60c-src. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5720–5724. doi: 10.1073/pnas.89.13.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Aminopeptidase P is anchored by a glycosyl-phosphatidylinositol moiety. FEBS Lett. 1988 Mar 14;229(2):340–344. doi: 10.1016/0014-5793(88)81152-9. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Nakafuku M., Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Scherübl H., Hescheler J., Schultz G., Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993 Feb 5;259(5096):832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- Kniep B., Cinek T., Angelisová P., Horejsí V. Association of the GPI-anchored leucocyte surface glycoproteins with ganglioside GM3. Biochem Biophys Res Commun. 1994 Sep 15;203(2):1069–1075. doi: 10.1006/bbrc.1994.2291. [DOI] [PubMed] [Google Scholar]
- Korty P. E., Brando C., Shevach E. M. CD59 functions as a signal-transducing molecule for human T cell activation. J Immunol. 1991 Jun 15;146(12):4092–4098. [PubMed] [Google Scholar]
- Li S., Okamoto T., Chun M., Sargiacomo M., Casanova J. E., Hansen S. H., Nishimoto I., Lisanti M. P. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995 Jun 30;270(26):15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y. H., Cook R. F., Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994 Jul;126(1):111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Johansen F., Olweus J., Symington F. W., Arli A., Thompson J. S., Vilella R., Skubitz K., Horejsi V. Activation of human monocytes and granulocytes by monoclonal antibodies to glycosylphosphatidylinositol-anchored antigens. Eur J Immunol. 1993 Nov;23(11):2782–2791. doi: 10.1002/eji.1830231110. [DOI] [PubMed] [Google Scholar]
- Mayor S., Rothberg K. G., Maxfield F. R. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994 Jun 24;264(5167):1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kleuss C., Gilman A. G. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995 Jan 27;80(2):249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Neubig R. R. Membrane organization in G-protein mechanisms. FASEB J. 1994 Sep;8(12):939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- Pimplikar S. W., Simons K. Regulation of apical transport in epithelial cells by a Gs class of heterotrimeric G protein. Nature. 1993 Apr 1;362(6419):456–458. doi: 10.1038/362456a0. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994 Feb 11;76(3):411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Crise B., Rose J. K. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994 Aug;14(8):5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., McIntosh D. P., Dvorak A. M., Liu J., Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995 Sep 8;269(5229):1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Schroeder R., London E., Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Dietzen D. J., Kwong J., Link D. C., Lublin D. M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994 Jul;126(2):353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Gauen L. K., Kwong J., Shaw A. S., Lublin D. M. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993 Oct;13(10):6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Kwong J., Fujita T., Olszowy M. W., Shaw A. S., Lublin D. M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol. 1992 Dec 1;149(11):3535–3541. [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Stahl A., Mueller B. M. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol. 1995 Apr;129(2):335–344. doi: 10.1083/jcb.129.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Corcoran M. L., Horak E. M., Wahl L. M., Bolen J. B., Horak I. D. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993 Oct 5;268(28):20725–20728. [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Telfer J. C., Rudd C. E. A 32-kD GTP-binding protein associated with the CD4-p56lck and CD8-p56lck T cell receptor complexes. Science. 1991 Oct 18;254(5030):439–441. doi: 10.1126/science.1925604. [DOI] [PubMed] [Google Scholar]
- Thomas P. M., Samelson L. E. The glycophosphatidylinositol-anchored Thy-1 molecule interacts with the p60fyn protein tyrosine kinase in T cells. J Biol Chem. 1992 Jun 15;267(17):12317–12322. [PubMed] [Google Scholar]
- Thompson L. F., Ruedi J. M., Glass A., Low M. G., Lucas A. H. Antibodies to 5'-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J Immunol. 1989 Sep 15;143(6):1815–1821. [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Daley J., Rock K. L. Stimulation of T cells via the TAP molecule, a member in a family of activating proteins encoded in the Ly-6 locus. J Immunol. 1987 Jan 1;138(1):91–97. [PubMed] [Google Scholar]