Abstract
The protective barrier, lubricant and clearance functions of mucus are intimately coupled to its microstructure and bulk rheology. Mucus gels consist of a network of mucin biopolymers along with lipids, salts and other proteins, and exhibit similar biochemical and physical properties across diverse mucosal surfaces. Nevertheless, mucus is exposed to a broad range of pH throughout the human body. Protein functions are typically sensitive to small changes in pH, and prior investigations using reconstituted, purified mucin gels suggested mucus transitions from a low viscosity liquid at neutral pH to a highly viscoelastic solid at low pH. We sought to determine whether those observations hold for fresh, minimally-perturbed human mucus ex vivo, by using different-sized muco-inert nanoparticles to probe microstructure, and cone-and-plate rheometry to measure bulk rheology. We demonstrate that both the microstructure and bulk rheology of fresh, undiluted and minimally perturbed cervicovaginal mucus exhibit relatively minor changes from pH 1–2 to 8–9, in marked contrast with the pH sensitivity of purified mucin gels. Our work also suggests additional components in mucus secretions, typically eliminated during mucin purification and reconstitution, may play an important role in maintaining the protective properties of mucus.
Keywords: biophysics, glycoprotein, microscopy, mucins, multiple particle tracking, nanotechnology
INTRODUCTION
Humans secrete liters of mucus each day to protect mucosal surfaces, including those of the airways, gastrointestinal tract, female reproductive tract and surface of the eye.1, 2 Mucus, composed primarily of long and heavily glycosylated macromolecules called mucins, prevents trauma due to large shear forces, such as during the transit of food and feces through the gastrointestinal tract, rapid blinking or copulation. Mucus is also critical to blocking infections by trapping pathogens and other foreign particles via its dense microstructure, and then rapidly clearing them via ciliary, peristaltic, and other clearance mechanisms that depend critically on mucus bulk rheology.1, 3, 4 Clearance of mucus is impaired when mucus secretions are too thick, as in patients with cystic fibrosis5 or chronic obstructive pulmonary disease,6 while protection against infection is compromised when secretions are too thin, as in women with bacterial vaginosis.7 Mucus rheology is widely thought to depend critically on its microstructure, since classical theories of the mechanics of entangled polymer gels consistently describe an empirical relationship between changes in bulk rheology and changes in the network microstructure and pore size.8–10
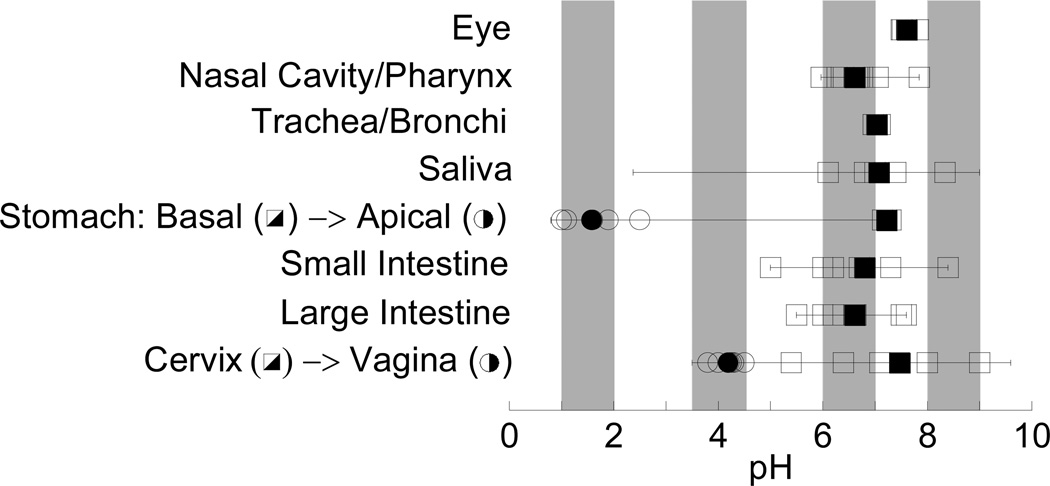
Across the different mucosal surfaces of the human body, mucus pH ranges from as low as ~1 in the gastric lumen up to ~9 in the endocervix (Figure 1 and Table S1 in Supporting Information). Rheological studies of the effect of pH on mucus have relied primarily on reconstituted solutions of purified pig gastric mucins. Substantial quantities of minimally perturbed and healthy human mucus are difficult to obtain; pig gastric mucus is readily available, and purified mucin solutions offer the opportunity to precisely control solution composition. Previous observations that such reconstituted purified mucin solutions undergo large bulk rheological changes with pH11–13 suggest that the microstructure of native mucus gels is also highly pH sensitive. For example, as the pH of a reconstituted pig gastric mucin solution was decreased from pH 6 to 4, the bulk viscous and elastic moduli was reported to increase by as much as 1,000-fold, with a further ~10-fold increase from pH 4 to 2.12 This ~10,000-fold increase in bulk viscoelasticity from pH 6 to 2 corresponds theoretically to a 6- to 100-fold decrease in average pore size, depending on whether mucus is modeled as a flexible, semiflexible, or stiff polymer network.8–10 Such large changes in bulk rheology and pore size would significantly alter the protective actions of mucus. Indeed, the high viscosity and collapsed state of the mucin network in pig gastric mucin gel at pH 2 was thought to help prevent HCl penetration from the stomach lumen and, thereby, protect the gastric epithelium, compared to the loose mesh network structure of mucins at pH 5–7.11, 14 It has also been suggested, based on studies with similarly purified and reconstituted mucus gels, that gelation of mucins at low pH may decrease particle mobility,15 while local alkalization of gastric mucus by H. pylori may reduce mucus viscoelasticity and allow the bacteria to penetrate mucus and establish infection.16
Figure 1.
Reported pH values at various mucosal surfaces in the human body: eye, respiratory tract, gastrointestinal tract and female genital tract. Open symbols indicate normal values reported for healthy individuals; closed symbols indicate the average of these values. Bars indicate the entire range of pH possible at the mucosal surface (including under disease conditions); note that the range for salivary pH is based on consumption of various foods, for example citric acid. Shaded regions represent pH ranges in which the macro- and nanoscale properties of human cervicovaginal mucus (CVM) were characterized here. Please see Table S1 and Supporting Information for references.
Nevertheless, proper physiological functioning of mucus gels likely requires that they operate within a defined range of viscoelasticity and pore sizes, which implies mucus should retain those properties across the wide range of pH to which it is exposed in the human body. Many neutral or slightly alkaline mucus secretions, including intestinal and endocervical mucus, are known to be highly viscoelastic.4 Furthermore, pH-neutral gastric mucus at the epithelial surface must be highly viscoelastic in order for the mucus layer to remain firmly adherent to the epithelium (a liquid-like solution, similar to reconstituted purified pig gastric mucins at pH 6, would not be able to anchor a solid-like gel, such as that of reconstituted purified mucins at pH 2). Thus, we raise an alternative hypothesis, that the microstructure/pore size and bulk rheology of native mucus gels are in fact relatively pH tolerant due to interactions between mucins and other components present in physiological mucus. In contrast, reconstituted gels of purified mucins, such as those studied previously, may fail to capture the rheological and structural properties of native mucus due to removal of these components and changes in the physicochemical properties of mucins with purification.17 Indeed, studies of pig gastrointestinal mucus revealed differences in the transport of nanoparticles within native pig gastrointestinal mucus vs. a reconstituted purified pig gastric mucin preparation, suggesting distinct structural properties of mucus vs. mucin solution.18
To determine the extent to which pH alters native mucus microstructure and bulk rheology, we used muco-inert nanoparticles of various sizes to probe the microstructure, and cone-and-plate rheometry to observe the bulk rheology, of freshly obtained human cervicovaginal mucus (CVM). CVM is derived from slightly alkaline endocervical mucus that enters the vagina and becomes acidified, normally to pH ~4, by lactic acid-secreting lactobacilli.19 In addition to secreted MUC5B mucin from endocervical mucus, CVM also contains MUC1 and other cell-surface mucins shed by vaginal epithelial cells.20 CVM is thus similar in mucin composition to secretions found in many human mucosal tissues.4 Atomic force and electron microscopic observations of mucus are subject to numerous artifacts due to dehydration and/or fixation steps, and have produced a wide range of estimates on similar mucus secretions, ranging from average pore sizes as small as ~30 nm to 10,000 nm or even larger.21–24 In contrast, the mobility of different sized nanoprobes may be used to infer pore sizes in freshly obtained human mucus with minimal perturbation. A key requirement of this approach is that the probe particles not form adhesive interactions with mucus. Conventional nanoparticles adhere strongly to the mucus mesh, and are thus unsuitable as probes of mucus microstructure. To make muco-inert probe particles, we coated fluorescent nanoparticles with a dense layer of low molecular weight polyethylene glycol; these particles (termed mucus-penetrating particles or MPP) do not adhere to a variety of human mucus secretions.3, 25–27 The diffusion rates of MPP of various sizes can be used to characterize mucus pore size via an obstruction scaling model, which describes the extent to which the diffusion of a non-interacting solute is restricted by the surrounding medium.28
MATERIALS AND METHODS
Preparation and characterization of mucus-penetrating particles (MPP)
Yellow-green (Ex/Em 505/515 nm) or red (Ex/Em 580/605 nm) fluorescent carboxyl-modified polystyrene particles sized 200, 500 and 1000 nm (Molecular Probes, Eugene, OR, USA) were covalently modified with low M.W. (2–3.4 kDa), amine-functionalized polyethylene glycol (PEG; Nektar Therapeutics, San Carlos, CA, USA) by a carboxyl-amine reaction, similar to that previously described.25 PEG is a hydrophilic and uncharged polymer that, at high surface density and low MW, can effectively shield the hydrophobic polystyrene core from adhesive interactions with mucins, while also minimizing interpenetrating network effects and hydrogen bonding between PEG chains and mucins.25, 29 Particle size and ξ-potential (Table S2) were determined by dynamic light scattering and laser Doppler anemometry, respectively, using a Zetasizer Nano ZS90 (Malvern Instruments, Southborough, MA, USA) according to instrument instructions. Size measurements were performed at 25°C at a scattering angle of 90°. Samples for size and ξ-potential measurements were prepared in saline adjusted to different pH or treated with reagents to the same final concentrations as those tested in particle tracking studies. The ξ-potentials of all MPP were near-neutral under pH neutral conditions compared to the highly negative charge of uncoated particles, indicating dense PEG surface coverage on MPP.
Human cervicovaginal mucus (CVM) collection
In contrast to other organs, such as the lungs, stomach or intestines, the vagina does not secrete mucus. Instead, the cervix continuously secretes mucus, which enters the vagina, becomes acidified by vaginal lactobacilli, and undergoes other biochemical changes caused by the vaginal micro-flora. We have thus adopted the term “cervicovaginal” mucus or CVM to reflect this unique physiology. CVM samples were obtained from women of reproductive age (ranging from 18 to 27 years old) with healthy vaginal micro-flora. Donors stated they had not used vaginal products nor participated in unprotected intercourse within 3 days prior to donating. Undiluted CVM, averaging 0.3 g per sample, was collected using a self-sampling menstrual collection device following protocols approved by the IRB of the Johns Hopkins University, as described previously.25, 30 The device, coated with CVM, is placed in a 50 mL tube, and CVM gently collected at the bottom of the tube by centrifugation for 1 min at 200 ×g. Collected mucus was stored in the 50 mL collection tube at 4 °C until used for microscopy or bulk rheology measurements the same day (typically within 1–3 hrs). CVM microstructure and bulk rheology do not change appreciably over at least a day of storage at 4 °C (data not shown). The samples were collected at random times throughout the menstrual cycle, but none were obtained during ovulation based on the absence of spinnbarkeit by visual inspection. Samples that were non-uniform in color or consistency were discarded.
Measurement and adjustment of mucus pH
The pH of CVM samples was measured using an Ultra-M micro pH electrode (Lazar Research Laboratories, Inc., Los Angeles, CA, USA) connected to a digital pH meter (Jenco Instruments, San Diego, CA, USA). The pH electrode and meter were calibrated to standard pH 4 and pH 7 buffer solutions (Fischer Scientific, Pittsburgh, PA, USA) prior to each use. Measurements were performed according to instrument instructions. Solutions of 3 or 5 N sodium hydroxide and 1 N hydrochloric acid (J.T. Baker, Phillipsburg, NJ, USA) were used to adjust the pH of physiological CVM (pH ~4) to the pH 1–2, 6–7, or 8–9 range, similar to a previously described procedure.31 The total volume of acid or base solutions needed to achieve the desired pH value constituted on average ~4% v/v and at most ~6% v/v of the mucus samples. Mucus pH was measured both before and after particle tracking to confirm the stability of pH levels during tracking. The average pH values across individual samples were 3.87 ± 0.11 (physiological pH), 1.55 ± 0.17 (target pH 1–2), 6.43 ± 0.17 (target pH 6–7), and 8.45 ± 0.35 (target pH 8–9).
Treatment of CVM with various reagents
To determine how different components of mucus contribute to its pH-independent behavior, we treated CVM samples with Nonoxynol-9 (N9, a nonionic detergent), ethylene glycol tetraacetic acid (EGTA, a calcium chelator), or tris(2-carboxyethyl)phosphine (TCEP, a disulfide reducing agent). Each reagent was added to mucus at 1% v/v and incubated for 2 hr at 37 °C prior to use for multiple particle tracking. Reagent concentrations were chosen as follows:
N9 – 10% w/v N9 was added to mucus at 1% v/v to give a final concentration of 0.1% w/v, similar to previous studies.28, 32
EGTA – The concentration of Ca2+ ions in human cervical mucus has been measured to be on average ~3 mM, and at most 5 mM.33, 34 EGTA (~0.8 M) was added to mucus at 1% v/v to give a final concentration of ~8 mM, well in excess of the Ca2+ concentration.
TCEP – The concentration of mucins in human cervical mucus has been reported to be 1.5% w/w, of which protein constitutes 20% w/w with ~30 mol cysteine residues per 1000 mol of amino acid;35 assuming an average amino acid molecular weight of 120 Da, the effective concentration of cysteines in mucus is thus ~0.75 mM. Based on biochemical characterization of mucins from cystic fibrosis sputum,36 disulfide bonds accessible to reducing agents represent only 35% of the total of disulfide bonds and thiol groups (disulfide bonds as a whole represent about 45% of the total of disulfide bonds and thiol groups). Thus, the effective concentration of accessible disulfide bonds is ~0.2 mM. TCEP (1M) was added to mucus at 1% v/v to give a final concentration of 10 mM, well in excess of the estimated number of accessible disulfide bonds.
Multiple particle tracking
We used high resolution multiple particle tracking to observe the motions of MPP and measure their diffusion rates in CVM, as previously described.25, 37 Particle solutions at 2% w/v stock concentration were diluted in water 25- to 300-fold (depending on particle size) to ~1010 particles/mL before addition at 3–5% v/v to fresh mucus in 8-well glass chambers (LabTek, Campbell, CA, USA) holding up to 300 WL of mucus or custom-made chambers holding 20–30 WL of mucus. Particles were added to mucus by pipette with gentle stirring to ensure uniform particle distribution throughout the mucus volume (confirmed by microscopy observation). Immediately following addition of particles or pH adjustment, mucus samples were incubated at 37 °C for 2 hr prior to microscopy. Particle trajectories were recorded using a silicon-intensified target camera (VE-1000, Dage-MTI, Michigan, IN, USA) mounted on an inverted epifluorescence microscope (Zeiss, Thornwood, NY, USA) equipped with a 100x oil-immersion objective (N.A., 1.3). Trajectories of n ≥90 particles were analyzed for each experiment. Movies were captured using MetaMorph software (Universal Imaging, Glendale, WI, USA) at a temporal resolution of 66.7 ms for 20 s and tracking resolution of 10 nm.38 Particle transport rates were obtained by transforming the coordinates of particle centroids into time-averaged mean square displacement (MSD), calculated as <MSD(τ)> = [x(t+τ) − x(t)]2 + [y(t+τ) − y(t)]2, where x and y represent the particle coordinates at a given time and τ is the time scale or time lag. Distributions of effective diffusivities were calculated as demonstrated previously,25 and used to estimate mucus pore sizes as described in the following section. This data was also used to identify representative traces of particles with transport rates within one SEM of the mean, which allow visual comparison of the effect of changes in the mucus gel structure at different pH on particle transport rates. Particle transport rates were measured in physiological (pH 4), pH 6–7 and pH 8–9 CVM or physiological and pH 1–2 CVM in sequential order, with a 2 hr incubation at 37 °C following each pH adjustment. For these studies, particles were added to physiological CVM and observed in the same CVM aliquot after each pH adjustment; we have observed similar results performing these studies in parallel with aliquots of the same CVM sample adjusted to different pH before the addition of particles. For mechanistic studies, particle transport rates were measured separately in control or treated CVM at pH 4. At least three independent experiments using individual CVM samples were performed.
Mucus pore size analysis (obstruction-scaling model)
We modeled CVM as a physically-entangled gel and fit an empirically-derived obstruction-scaling model to the effective diffusivities of particles in CVM. The model was initially developed to describe the diffusion of non-interacting solutes in homogeneous hydrogels,39, 40 but it is also applicable to physically entangled and cross-linked hydrogels, such as mucus.22, 41 The diffusivity of a solute (radius rs) in a gel (Dg), with a distribution of pore sizes about a mean (radius R), relative to its diffusivity in water (D0) is given by:
where rf is the radius of the polymer chains (i.e., mucin fibers). By rearranging this equation, we can calculate the average pore size corresponding to a particle’s effective diffusivity. We assumed an rf of 3.5 nm, an estimate based on biochemical analysis and microscopy of mucin fibers,22 and used maximum likelihood estimation to obtain the average pore size from the ensemble effective diffusivities of different sized particles in individual CVM samples at a time scale of 1 s.
Macrorheological characterization
The macrorheological characterization of CVM was performed with a strain-controlled cone and plate rheometer (ARES-100, Rheometrics, Piscataway, NJ) using techniques described previously.32 CVM from on average 5 different donors was pooled to obtain adequate volume for use with a 50 mm cone (~1.3 mL), and stored at 4 °C until use within 8 hr of collection. Pooled samples were incubated at 37 °C for 17 min prior to measurements, and maintained at the same temperature during measurements. Oscillatory deformations of small amplitude (1% strain, within the linear viscoelastic regime for human mucus42) and controlled frequency were applied to extract the frequency-dependent elastic (G') and viscous (G") moduli with minimal shearing damage to the mucus. The rheology of physiological (pH 4), pH 6–7 and pH 8–9 CVM or pH 4 and pH 1–2 CVM was evaluated in sequential order, with a 17 min incubation at 37 °C following each pH adjustment. Three independent experiments were performed.
Statistical analysis
A one-tailed Student’s t-test assuming unequal variances was used for statistical analysis of unpaired samples. A paired t-test was used to compare different conditions tested in the same samples. Differences were considered significant at p < 0.05. All data are presented as mean ± SEM.
RESULTS AND DISCUSSION
Effect of alkalization on mucus microstructure
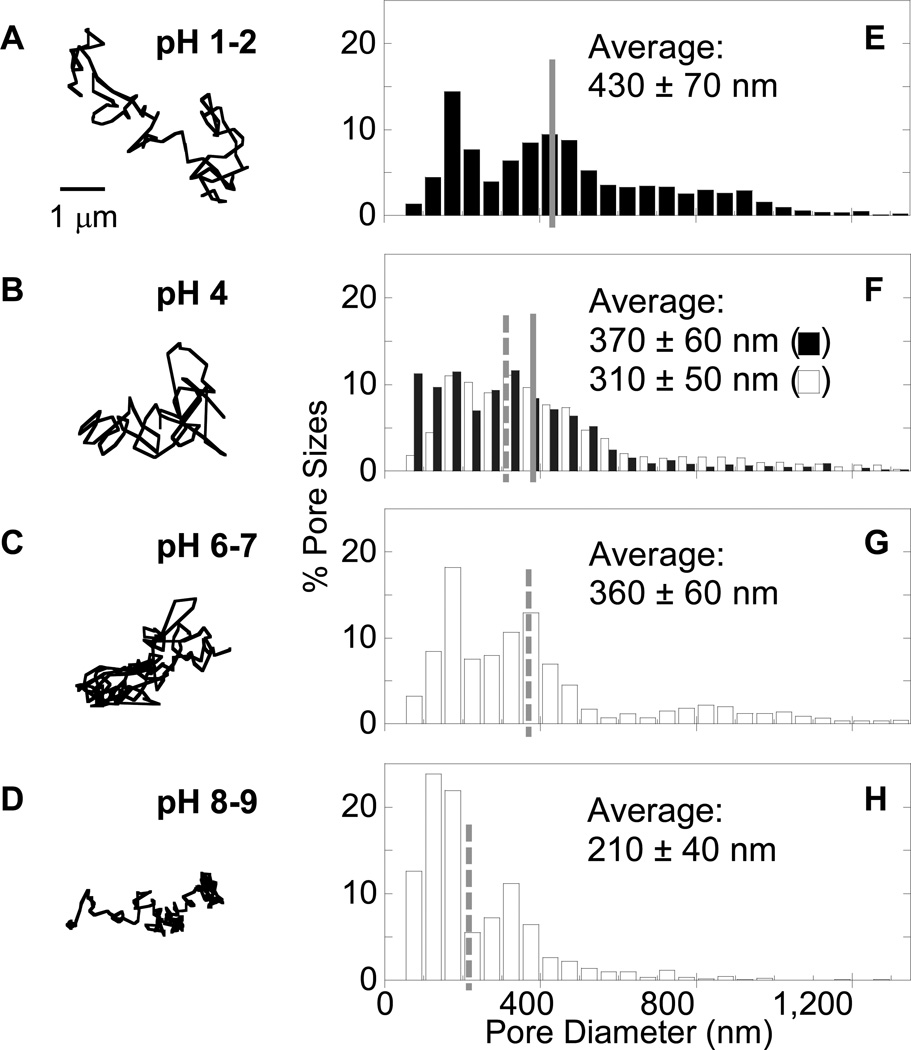
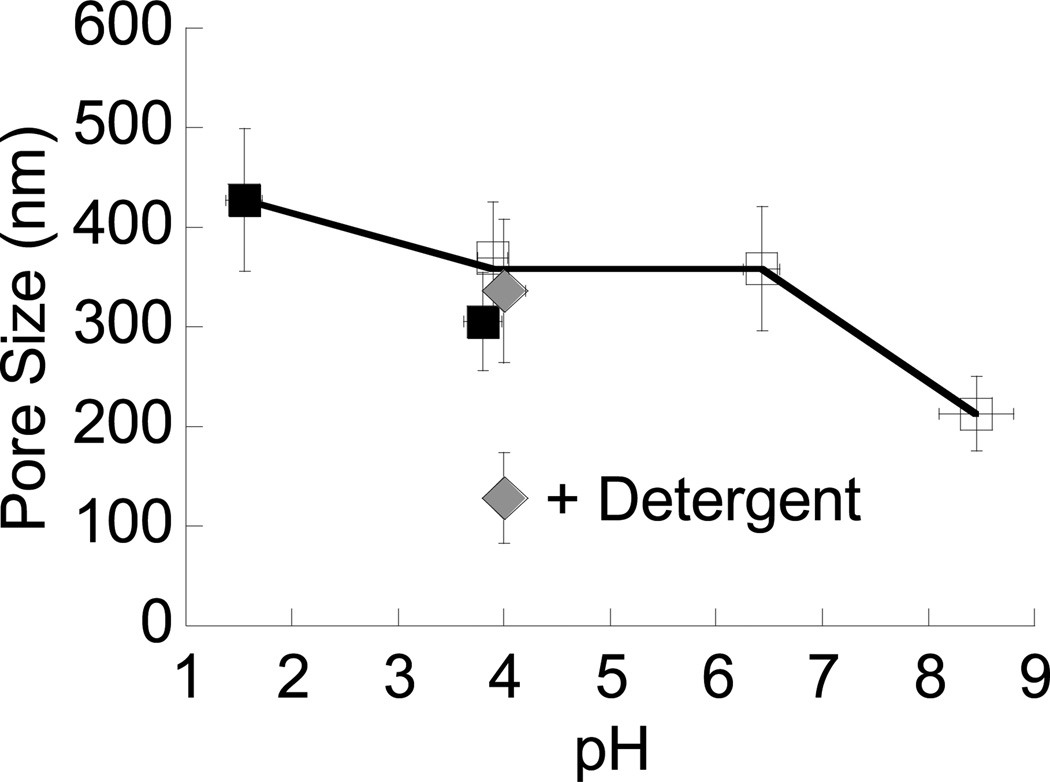
We first quantified the diffusional motions of hundreds of individual MPP 200 nm, 500 nm and 1 Wm in diameter in fresh ex vivo CVM at minimal dilution and physiological pH (Figure 2A–D and Figure S1–S4, see Supporting Information for additional details), and inferred pore sizes from the particle motions (Figure 2E–H and Figure 3). Consistent with our previous findings,28 the average pore size of unmodified CVM (pH ~4) was 370 ± 60 nm. The pH of the same mucus samples (n ≥ 3) was then adjusted to pH 6–7 by addition of small volumes of NaOH (< 3% v/v). Despite the 2–3 unit increase in pH, the average pore size remained essentially unchanged at 360 ± 60 nm. A further increase in mucus pH to 8–9 decreased average pore size to 210 ± 40 nm. Addition of small volumes of saline (3% v/v) rather than NaOH did not detectably alter mucus pore size. The surface charges of the probes were all near neutral in pH 4 and 6–7 solutions (Table S2), suggesting that changes in surface charge were unlikely to have influenced particle motions in mucus over this pH range. Although particle surface charges became more negative at pH 8–9, the rapid diffusion of 200 nm MPP, as well as smaller ~100 nm MPP, indicates particle-mucus interactions were not altered substantially over the entire pH range, and are thus unlikely to account for observed changes in particle mobility and, therefore, pore size.
Figure 2.
Distribution of pore sizes in mucus at various pH ranges, as probed by different sized MPP. (A–D) Representative trajectories for 200 nm MPP exhibiting transport rates within one SEM of the ensemble average at a time scale of 1 s at (A) pH 1–2, (B) pH 4 (physiological), (C) pH 6–7, and (D) pH 8–9. (E–H) Distribution of effective pore sizes in fresh, minimally-diluted healthy human CVM at (E) pH 1–2, (F) pH 4, (G) pH 6–7, and (H) pH 8–9. Open bars indicate mucus samples used for the pH 4, 6–7 and 8–9 comparison, while filled bars indicate those for pH 4 and 1–2. Data represent the ensemble average of at least three independent experiments, with n ≥ 90 particles for each experiment.
Figure 3.
Average pore size in mucus across the pH range of 1 to 9. Data plotted for physiological, unaltered mucus (pH ~4) represent estimates from three distinct data sets: mucus samples used to compare pH 4, 6–7 and 8–9 (open squares), those used to compare pH 4 and 1–2 (filled squares), and those from a previous study28 (filled diamond). The average pore size of CVM treated with a nonionic detergent is indicated by “+ Detergent”. Error bars are presented as S.E.M.
Effect of acidification on mucus microstructure
We next characterized the effect of acidification on the microstructure of CVM. In the stomach, pH decreases sharply from the epithelial surface (pH 7) to the gastric lumen (pH 1–2). Studies using purified porcine gastric mucin solutions have shown that mucins aggregate as pH is lowered, which was thought critical to producing a more dense gel that protects the stomach from acid.11 To determine whether this phenomenon occurs in fresh human mucus, we acidified CVM samples to pH 1–2 by addition of HCl and characterized pore sizes in acidified mucus vs. the same physiological mucus samples. Surprisingly, acidifying CVM did not cause significant mucin aggregation, as the average pore size increased by less than 40% from 310 ± 50 nm at pH 4 to 430 ± 70 nm at pH 1–2. Again, addition of 3% v/v saline rather than HCl did not detectably alter mucus pore size, and particle surface charges remained near neutral at pH 1–2. For all pH values, the distribution of pore sizes spanned a wide range, from as small as ~50 nm to more than 1 Wm. This distribution was narrower and shifted toward smaller pores in CVM at pH 8–9, and broader and shifted toward larger pores at pH 1–2. Nevertheless, the difference in average pore size between the pH extremes was only about 2-fold, an unexpectedly small change given the large 6–8 pH unit span. It should be noted that some inherent variation in average pore size may be expected across donor samples, but the average pore sizes of 310–370 nm observed here in unmodified CVM did not differ significantly from the average of 340 nm determined in a previous study based on samples from 8 donors.28
Characterization of bulk rheology
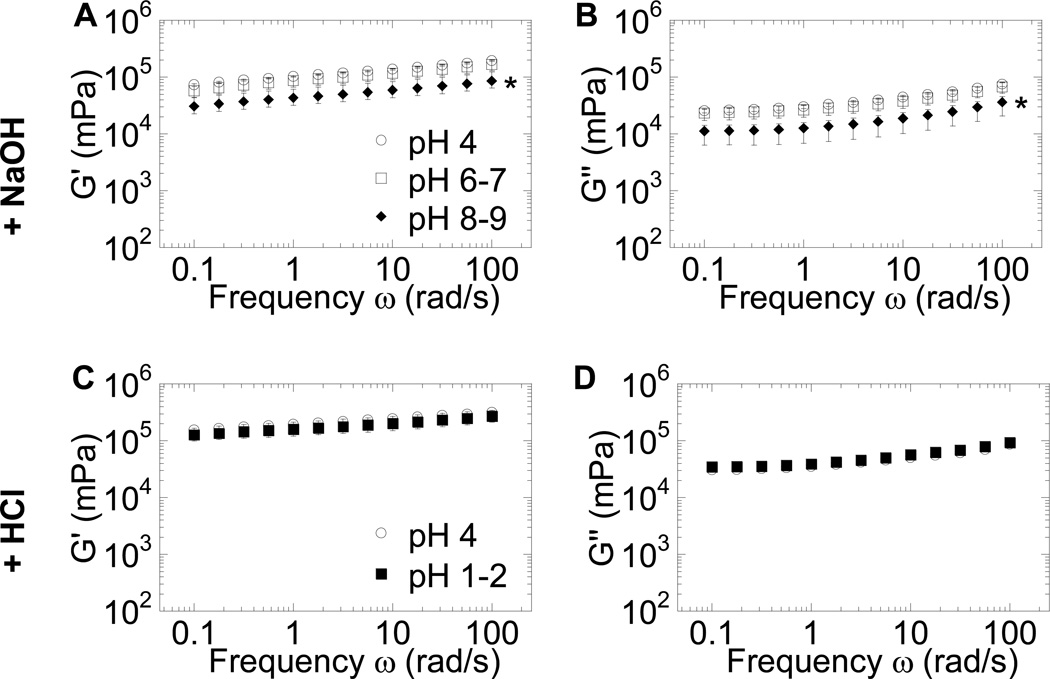
Given that large changes in pH had only small effects on mucus pore size, we hypothesized that, unlike gels of purified mucins, the bulk rheology of fresh human mucus would be largely unaltered by major changes in pH. To test this hypothesis, we measured the bulk rheology of fresh CVM samples, titrated as above to different pH values, using a sensitive strain-controlled cone and plate rheometer under small-amplitude oscillatory shear. We found that the bulk viscoelasticity of CVM changed minimally, with at most a ~2-fold decrease in the viscous and elastic moduli for mucus from pH 4 to pH 8–9 (Figure 4). The discrepancy between our finding and previous publications may be due to biochemical and structural differences between physiological human mucus and reconstituted mucin gels. The isolation and purification of mucins eliminates salts, lipids, and other mucus components13 that may confer pH tolerance in native mucus, and may also directly alter the physicochemical properties of the mucins themselves, including their MW and folding.17 These changes likely alter mucin-mucin interactions to the extent that it is not possible to recover the native mucus gel structure upon reconstitution,17 causing the gel to become susceptible to changes in pH.
Figure 4.
Macrorheological characterization of fresh human CVM at different pH ranges under dynamic oscillatory shear. (A) Elastic modulus (G′) and (B) viscous modulus (G″) for pH 4 (physiological) mucus compared to the same mucus samples adjusted to pH 6–7 and pH 8–9. (C) G′ and (D) G″ for pH 4 mucus compared to the same mucus samples adjusted to pH 1–2. Data represent the average of three independent experiments, where each experiment characterized CVM pooled from at least three donors. * indicates a statistically significant difference at p < 0.05 compared to pH 4 values, across all frequencies probed. Error bars are presented as S.E.M.
Role of biochemical components in the pH stability of mucus
To gain further insight into the molecular mechanisms that contribute to pH tolerance in CVM, we considered possible biochemical interactions that may occur between mucins and other mucus components. While not yet fully understood, recent evidence suggests mucin macromolecules likely associate with each other to form fiber-like bundles that comprise the primary structural features of physiological mucus gels.28 Specific biochemical features that may mediate mucin bundling as well as give rise to the pH tolerance of these gels include: (i) disulfide crosslinks between mucin fibers; (ii) densely glycosylated tandem repeats, which are highly hydrophilic and can undergo electrostatic repulsion, hydrogen bonding and salt bridging interactions; and (iii) nonglycosylated protein domains that can aggregate via hydrophobic interactions,12, 43 which may bundle mucin fibers together to create large pores.28 Disulfide bonds remain stable at pH values below the pKa of cysteine thiols (~8.5–9.5),44 and hence likely contribute to the structural stability of the mucin network between pH 1–2 and 8–9. As CVM is alkalinized from pH 4, electrostatic interactions between mucin fibers are also unlikely to change significantly, since the negative charge of mucins is dominated by the already highly negatively charged glycosylated segments. However, bases have detergent-like effects – as reflected by the use of basic cleaning solutions (e.g., household ammonia) to dissolve oily/hydrophobic substances – and disruption of the lipid-coated or naked protein domains of mucins could in turn partially disrupt mucin bundling via hydrophobic interactions,28 leading to the slight decrease in pore size observed at pH 8–9. In contrast, as mucus pH is decreased to 1–2, negatively charged sialic acid sugar groups (pKa ~2.645) become neutralized, reducing solubility and electrostatic repulsion and perhaps increasing hydrophobic aggregation (bundling).46, 47 In addition, decreasing the number of negatively charged sialic acids may increase pore size by diminishing the ability of Ca2+ ions to crosslink the glycosylated tandem repeat domains of mucins.48 It is also possible that the increase in hydrogen bonding (due to greater availability of hydroxyl groups on sialic acid carboxyls) is sufficient to promote mucin-mucin bundling. However, the presence of physical entanglements and disulfide crosslinks in the mesh network of native mucus gels, as opposed to purified mucin solutions, as well as the hydrophilicity of the mucins, may prevent them from completely collapsing together, limiting the extent to which mucins can bundle and increase pore sizes. It is interesting to note that gastric mucins may be further fortified against microstructural changes at low pH, since they possess a higher proportion of ‘acid-resistant’ sulfate groups (pKa < 1) than sialic acid groups.1 These interactions are summarized in Table S3.
Based on the possible interactions described above, we treated ex vivo CVM samples at their physiological pH of ~4 with various agents, measured particle probe speeds, and calculated the consequent pore sizes (Figure 5). These agents include (i) nonoxynol-9 (N9), a nonionic detergent that disrupts hydrophobic interactions between mucins;28 (ii) ethylene glycol tetraacetic acid (EGTA), a calcium chelator; and (iii) tris(2-carboxyethyl)phosphine (TCEP), a disulfide reducing agent. None of these reagents altered probe size or surface charge (Table S2). N9: Similar to our previous finding,28 N9 decreased the average mucus pore size at pH 4 significantly compared to the same untreated samples (p < 0.05), likely by debundling mucin fibers to create a finer mesh. Given the detergent-like effect of bases, our finding that the decrease in pore size caused by N9 was similar to that induced at pH 8–9 may support the hypothesis that strong bases may reduce mucus pore size via a similar mechanism as detergents. EGTA: Consistent with published literature,48 the removal of Ca2+ ions by EGTA at pH 4 led to a significant increase in average pore size (p < 0.05). EGTA did not increase pore size to the same extent as that observed with acidification to pH 1–2, perhaps due to a reduction in the chelation efficiency of EGTA at lower pH or to strongly bound Ca2+ ions that are difficult to chelate. Nevertheless, these results are consistent with the notion that salt bridges mediated by Ca2+ ions may help control pore sizes at pH 4. TCEP: Finally, to determine how disulfide bonds contribute to the pH stability of mucus, we treated CVM with TCEP, which can reduce disulfide bonds over a broad range of pH (at least 1.5 to 8.549), unlike other reducing agents (e.g., DTT). TCEP treatment also caused a significant increase in pore size (p < 0.05), suggesting disulfide bonds, along with physical entanglements, may restrict the extent of pore size enlargement possible as mucus pH decreases. It is possible that TCEP reduced only some of the otherwise accessible mucin disulfide bonds (see Materials and Methods for an estimate of the number of accessible disulfide bonds), since a large fraction of disulfide bonds in physiological mucus gels is expected to be buried within the hydrophobic regions of mucin bundles.1, 50, 51
Figure 5.
Effect of treatment with various reagents on mucus pore size. The average pore size of CVM at pH 4 treated (“+”) with N9 (a nonionic detergent), EGTA (a calcium chelator) or TCEP (a disulfide reducing agent) is compared to that measured for the same untreated CVM sample (“−”) at pH 4. Dashed lines indicate average pore sizes for untreated CVM adjusted to pH 1–2 or 8–9. Pore sizes were estimated from the transport rates of 500 nm probe particles; data represent the ensemble average of at least three independent experiments. * indicates a statistically significant difference at p < 0.05 compared to the same untreated CVM samples at pH 4. Error bars are presented as S.E.M.
CONCLUSIONS
Using muco-inert nanoparticles as non-perturbing probes, we have shown that the microstructure of fresh, ex vivo human CVM is largely stable across a wide range of physiological pH values. Our studies suggest that this pH stability likely arises from complex biochemical interactions between physically entangled and crosslinked mucins as well as other mucus components, such as lipids, ions and proteins. Although our studies focused on CVM, we expect that the microstructure and bulk rheology of other human mucus secretions may also be relatively pH insensitive. The major gel-forming mucins (MUC2, MUC5AC and MUC5B) expressed at most mucosal surfaces52, 53 are biochemically homologous, sharing the critical structural features described above, although they may exhibit diverse glycan structures.52, 54 Moreover, the composition (water, ion, lipid contents) and bulk phase rheology of diverse human mucus gels (including respiratory, gastrointestinal and cervicovaginal) are generally similar.3, 4 CVM is currently the only readily available source of significant quantities of fresh, unaltered and healthy human mucus gel, making it a convenient and valuable ex vivo model; the unchanged microstructure of CVM from pH 4 to 6–7 also suggests CVM may be a valid substitute for studying other human mucus secretions, many of which are near neutral pH. These studies may not only help further elucidate the structural basis for the protective functions of physiological mucus gels, but may also provide inspiration for the design of new synthetic biopolymers with pH-resistant properties.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Integrated Imaging Center at Johns Hopkins University. We also thank Mr. Tao Yu for experimental assistance.
Funding Sources
This work was supported by the National Institutes of Health Grants 5U01AI066726, R21 AI094519, R33 AI079740-01, 1R01CA140746, and R01HD062844, and by National Science Foundation graduate research fellowships (Y.-Y.W. and L.E.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- CVM
cervicovaginal mucus
- MPP
mucus-penetrating particle
- PEG
polyethylene glycol
- N9
nonoxynol-9
- EGTA
ethylene glycol tetraacetic acid
- TCEP
tris(2-carboxyethyl)phosphine
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
SUPPORTING INFORMATION AVAILABLE
Supplemental text – probe particle mobility in human CVM as a function of pH; Table S1 – physiological pH ranges in the human body; Table S2 – characterization of particle probes; Figures S1-S4 – additional particle traces and transport rates in CVM; Table S3 – mechanisms that may govern pH response of mucus microstructure. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Cone RA. Adv. Drug Deliv. Rev. 2009;61:75. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Powell DW. Ion and water transport in the intestine. In: Andreoli, Hoffman, Fanestil, Schultz, editors. Physiology of Membrane Disorders. 2nd ed. New York, NY: Plenum, Inc; 1986. p. 559. [Google Scholar]
- 3.Lai SK, Wang YY, Hanes J. Adv. Drug Deliv. Rev. 2009;61:158. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai SK, Wang YY, Wirtz D, Hanes J. Adv. Drug Deliv. Rev. 2009 doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wine JJ, Joo NS. Proc. Am. Thorac. Soc. 2004;1:47. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 6.Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton DJ. Am. J. Respir. Crit. Care Med. 2008;178:1033. doi: 10.1164/rccm.200803-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olmsted SS, Meyn LA, Rohan LC, Hillier SL. Sex. Transm. Dis. 2003;30:257. doi: 10.1097/00007435-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Science. 2004;304:1301. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 9.MacKintosh FC, Kas J, Janmey PA. Phys. Rev. Lett. 1995;75:4425. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 10.Palmer A, Xu J, Kuo SC, Wirtz D. Biophys. J. 1999;76:1063. doi: 10.1016/S0006-3495(99)77271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhaskar KR, Gong DH, Bansil R, Pajevic S, Hamilton JA, Turner BS, LaMont JT. Am. J. Physiol. 1991;261:G827. doi: 10.1152/ajpgi.1991.261.5.G827. [DOI] [PubMed] [Google Scholar]
- 12.Celli JP, Turner BS, Afdhal NH, Ewoldt RH, McKinley GH, Bansil R, Erramilli S. Biomacromolecules. 2007;8:1580. doi: 10.1021/bm0609691. [DOI] [PubMed] [Google Scholar]
- 13.Cao X, Bansil R, Bhaskar KR, Turner BS, LaMont JT, Niu N, Afdhal NH. Biophys. J. 1999;76:1250. doi: 10.1016/S0006-3495(99)77288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhaskar KR, Garik P, Turner BS, Bradley JD, Bansil R, Stanley HE, LaMont JT. Nature. 1992;360:458. doi: 10.1038/360458a0. [DOI] [PubMed] [Google Scholar]
- 15.Lieleg O, Vladescu I, Ribbeck K. Biophys. J. 2010;98:1782. doi: 10.1016/j.bpj.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S, Bansil R. Proc. Natl Acad. Sci. U. S. A. 2009;106:14321. doi: 10.1073/pnas.0903438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocevar-Nared J, Kristl J, Smid-Korbar J. Biomaterials. 1997;18:677. doi: 10.1016/s0142-9612(96)00180-9. [DOI] [PubMed] [Google Scholar]
- 18.Crater JS, Carrier RL. Macromol Biosci. 2010;10:1473. doi: 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- 19.Boskey ER, Cone RA, Whaley KJ, Moench TR. Hum. Reprod. 2001;16:1809. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 20.Gipson IK, Ho SB, Spurr-Michaud SJ, Tisdale AS, Zhan Q, Torlakovic E, Pudney J, Anderson DJ, Toribara NW, Hill JA., 3rd Biol. Reprod. 1997;56:999. doi: 10.1095/biolreprod56.4.999. [DOI] [PubMed] [Google Scholar]
- 21.Ceric F, Silva D, Vigil P. J. Electron. Microsc. (Tokyo) 2005;54:479. doi: 10.1093/jmicro/dfh106. [DOI] [PubMed] [Google Scholar]
- 22.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Biophys. J. 2001;81:1930. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunelli R, Papi M, Arcovito G, Bompiani A, Castagnola M, Parasassi T, Sampaolese B, Vincenzoni F, De Spirito M. FASEB J. 2007;21:3872. doi: 10.1096/fj.07-8189com. [DOI] [PubMed] [Google Scholar]
- 24.Round AN, Rigby NM, Garcia de la Torre A, Macierzanka A, Mills EN, Mackie AR. Biomacromolecules. 2012;13:3253. doi: 10.1021/bm301024x. [DOI] [PubMed] [Google Scholar]
- 25.Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Proc. Natl Acad. Sci. U. S. A. 2007;104:1482. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai SK, Suk JS, Pace A, Wang YY, Yang M, Mert O, Chen J, Kim J, Hanes J. Biomaterials. 2011;32:6285. doi: 10.1016/j.biomaterials.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suk JS, Lai SK, Wang YY, Ensign LM, Zeitlin PL, Boyle MP, Hanes J. Biomaterials. 2009;30:2591. doi: 10.1016/j.biomaterials.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai SK, Wang YY, Hida K, Cone R, Hanes J. Proc. Natl Acad. Sci. U. S. A. 2010;107:598. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Angew. Chem. Int. Ed. Engl. 2008;47:9726. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boskey ER, Moench TR, Hees PS, Cone RA. Sex. Transm. Dis. 2003;30:107. doi: 10.1097/00007435-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. J. Virol. 2009;83:11196. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai SK, Wang YY, Cone R, Wirtz D, Hanes J. PLoS ONE. 2009;4:e4294. doi: 10.1371/journal.pone.0004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen DH, Katz DF. Contraception. 1999;59:91. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 34.Wagner G, Levin RJ. J. Reprod. Fertil. 1980;60:17. doi: 10.1530/jrf.0.0600017. [DOI] [PubMed] [Google Scholar]
- 35.Carlstedt I, Lindgren H, Sheehan JK, Ulmsten U, Wingerup L. Biochem. J. 1983;211:13. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabachnik NF, Blackburn P, Cerami A. J. Biol. Chem. 1981;256:7161. [PubMed] [Google Scholar]
- 37.Suh J, Dawson M, Hanes J. Adv. Drug Deliv. Rev. 2005;57:63. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Apgar J, Tseng Y, Fedorov E, Herwig MB, Almo SC, Wirtz D. Biophys. J. 2000;79:1095. doi: 10.1016/S0006-3495(00)76363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amsden B. Macromolecules. 1998;31:8382. [Google Scholar]
- 40.Amsden B. Macromolecules. 1999;32:874. [Google Scholar]
- 41.Shen H, Hu Y, Saltzman WM. Biophys. J. 2006;91:639. doi: 10.1529/biophysj.105.077404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson M, Wirtz D, Hanes J. J. Biol. Chem. 2003;278:50393. doi: 10.1074/jbc.M309026200. [DOI] [PubMed] [Google Scholar]
- 43.Bromberg LE, Barr DP. Biomacromolecules. 2000;1:325. doi: 10.1021/bm005532m. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert HF. Methods Enzymol. 1995;251:8. doi: 10.1016/0076-6879(95)51107-5. [DOI] [PubMed] [Google Scholar]
- 45.Bettelheim FA. Ann. N. Y. Acad. Sci. 1963;106:247. doi: 10.1111/j.1749-6632.1963.tb16642.x. [DOI] [PubMed] [Google Scholar]
- 46.Bansil R, Turner BS. Curr. Opin. Colloid Interface Sci. 2006;11:164. [Google Scholar]
- 47.Hong Z, Chasan B, Bansil R, Turner BS, Bhaskar KR, Afdhal NH. Biomacromolecules. 2005;6:3458. doi: 10.1021/bm0505843. [DOI] [PubMed] [Google Scholar]
- 48.Raynal BD, Hardingham TE, Sheehan JK, Thornton DJ. J. Biol. Chem. 2003;278:28703. doi: 10.1074/jbc.M304632200. [DOI] [PubMed] [Google Scholar]
- 49.Han JC, Han GY. Anal. Biochem. 1994;220:5. doi: 10.1006/abio.1994.1290. [DOI] [PubMed] [Google Scholar]
- 50.Cone RA, Mucus . In: Mucosal Immunlogy. 3rd ed. Lamm ME, Strober W, McGhee JR, Mayer L, Mestecky J, Bienenstock J, editors. San Diego: Academic Press; 1999. p. 43. [Google Scholar]
- 51.Sheehan JK, Thornton DJ, Somerville M, Carlstedt I. Am Rev Respir Dis. 1991;144:S4. doi: 10.1164/ajrccm/144.3_pt_2.S4. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Vilar J, Hill RL. J. Biol. Chem. 1999;274:31751. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 53.Wickstrom C, Davies JR, Eriksen GV, Veerman EC, Carlstedt I. Biochem. J. 1998;334(Pt 3):685. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roussel P, Lamblin G, Lhermitte M, Houdret N, Lafitte JJ, Perini JM, Klein A, Scharfman A. Biochimie. 1988;70:1471. doi: 10.1016/0300-9084(88)90284-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.