Abstract
Objectives
To compare outcomes and cost-effectiveness of various initial imaging strategies in the management of stable chest pain in a long-term prospective randomised trial.
Setting
Regional cardiothoracic referral centre in the east of England.
Participants
898 patients (69% man) entered the study with 869 alive at 2 years of follow-up. Patients were included if they presented for assessment of stable chest pain with a positive exercise test and no prior history of ischaemic heart disease. Exclusion criteria were recent infarction, unstable symptoms or any contraindication to stress MRI.
Primary outcome measures
The primary outcomes of this follow-up study were survival up to a minimum of 2 years post-treatment, quality-adjusted survival and cost-utility of each strategy.
Results
898 patients were randomised. Compared with angiography, mortality was marginally higher in the groups randomised to cardiac MR (HR 2.6, 95% CI 1.1 to 6.2), but similar in the single photon emission CT-methoxyisobutylisonitrile (SPECT-MIBI; HR 1.0, 95% CI 0.4 to 2.9) and ECHO groups (HR 1.6, 95% CI 0.6 to 4.0). Although SPECT-MIBI was marginally superior to other non-invasive tests there were no other significant differences between the groups in mortality, quality-adjusted survival or costs.
Conclusions
Non-invasive cardiac imaging can be used safely as the initial diagnostic test to diagnose coronary artery disease without adverse effects on patient outcomes or increased costs, relative to angiography. These results should be interpreted in the context of recent advances in imaging technology.
Trial registration
ISRCTN 47108462, UKCRN 3696.
Strengths and limitations of this study.
This is the only large randomised prospective trial of a strategy of non-invasive gate-keeper cardiac imaging versus upfront angiography in the literature.
This is one of only very few comparative effectiveness studies in cardiac imaging to include data on stress perfusion cardiac magnetic resonance imaging.
The cost-utility data are derived from National Health Service tariffs and our results are not necessarily directly transferrable to other healthcare systems.
Introduction
Coronary artery disease (CAD) is common and its management is costly.1 Revascularisation using bypass surgery (coronary artery bypass graft, CABG) or percutaneous coronary intervention (PCI) is effective for patients with severe disease2 but a significant minority of patients do not gain symptomatic relief.3 Data from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial did not show prognostic benefits from revascularisation in any patient subgroup.4 The yield of coronary angiography (CA) is variable with one recent large study of nearly 400 000 patients demonstrating a normalcy rate approaching 40%.5 Therefore non-invasive testing to identify those with a low likelihood of obstructive CAD may be safer, cheaper and more appropriate than upfront angiography. This approach is codified in multiple American Heart Association/American College of Cardiology (AHA/ACC) guidelines on the investigation of stable or suspected angina pectoris in which initial non-invasive imaging is rated as highly appropriate.6–9
The ‘Cost-Effectiveness of non-invasive Cardiac Testing’ (CECaT) trial was an unblinded non-inferiority trial designed to assess three functional tests—stress echocardiography, single photon emission CT (SPECT) and stress cardiac MR (CMR)—as a gate-keeper to CA in patients referred for CA with suspected CAD. Early clinical and cost-effectiveness estimates have been published and showed that the CMR group had slightly lower mean exercise treadmill time according to the Bruce protocol but otherwise the tests could be considered equally effective.10 This report provides the main cost-effectiveness and mortality outcomes up to 6 years after randomisation.
Methods
Study design
The design of the study has been described elsewhere10 and is reviewed briefly here. All patients referred to Papworth Hospital, UK for non-urgent angiography were eligible for the study. Inclusion criteria were: established or suspected chronic stable angina and a positive exercise tolerance test result with subsequent referral for angiography. Exclusion criteria were: recent MI (<3 months), revascularisation (<6 months); urgent need for revascularisation; contraindication to adenosine or CMR; inability to exercise.
Trial design was of four parallel arms with a 1:1:1:1 randomisation ratio. Randomisation was computer generated and stratified according to Pryor risk assessment.11 Within each Pryor risk group, randomisation was in blocks of length 6 or 8. Sequentially numbered group designation was held in the research and development (R&D) office and was not available to trial personnel. Patients were randomised to their initial diagnostic test—stress SPECT, stress CMR, stress echocardiography or angiography—by R&D within 4 weeks of recruitment and only after they had given consent and been registered.
Non-invasive imaging results were returned with a recommendation to proceed with angiography only when reported as ‘positive’ for inducible ischaemia. Protocol adherence to this recommendation was not mandated by trial design and patients proceeded to angiography if considered clinically indicated. Treatment with PCI or CABG (performed within 6 months of angiography) or medical therapy was according to standard practice.
Coronary angiography
Standard diagnostic angiography was performed from the right femoral artery approach.12 A minimum of five views of the left and three views of the right coronary system were taken.13 All examinations were reported by an experienced staff cardiologist and segmental location of disease (if any) was recorded.
Stress echocardiography
β-Blocker medications were stopped 2 days beforehand. Dobutamine was infused at rates of 10–40 µg/kg/min, increased at 3 min intervals. If necessary, 300–600 μg of atropine were added at peak stress to achieve 90% of target heart rate. Images were acquired in standard planes in the final minute of each 3 min stage. Intravenous microspheres were used to delineate the endocardial surface. All examinations were reported by one of two staff cardiologists experienced in stress echocardiography. Studies were positive for ischaemia if stress-induced deterioration in contractility was observed.
SPECT
Rest/stress 99mTc sestamibi (99mTc MIBI) SPECT was performed (2 day protocol). A 6 min adenosine infusion (140 g/kg/min) was employed. 400 MBq 99m-Tc MIBI was administered at 3 min after infusion of adenosine was started. Gated SPECT imaging occurred 60 min after injection. Tomographic images were assessed for fixed and reversible defects by a single observer (as per established criteria).14
CMR
Stress CMR was performed at a standard similar to that which was subsequently recommended by the Society of CMR.15 A 1.5 T mobile CMR system and four-channel phased array surface coil were used (Signa CV/i, GE Medical Systems). Stress/rest dynamic first-pass perfusion imaging was performed. A hybrid fast gradient ECHO/echoplanar sequence was employed.16 Adenosine was infused at 140 µg/kg/min for 4 min. After 3 min, a bolus of gadolinium-diethylenetriaminepenta acetic acid (0.1 mmol/kg) was delivered at 5 mL/s, followed by a 25 mL saline flush. First-pass imaging of the heart occurred over 80 heart beats. A volumetric notched-saturation prepulse preserved a constant saturation-recovery time during slice acquisition.17 Six to eight short axis slices were obtained every two heart beats. Rest imaging was repeated after a minimum interval of 15 min. Cine steady state free precession images and late gadolinium enhancement images were also acquired as described in the original CECaT protocol.10 Studies were reported as positive if there was an inducible perfusion defect visible for at least five frames either: (1) in a region of normal wall thickness or (2) in a region of myocardial thinning in the absence of a history of prior myocardial infarction (MI).
Outcomes
The primary outcome in the original CECaT trial was exercise treadmill time at 18 months postrandomisation using the modified Bruce protocol, in which exercise intensity was increased every 3 min. There was a range of secondary outcomes including diagnostic accuracy, clinical events, health-related quality and cost-effectiveness of life up to 18 months after randomisation.10
The primary outcomes of this follow-up study were survival up to a minimum of 2 years post-treatment, quality-adjusted survival and cost-utility of each strategy. Survival status at the end of follow-up was determined from the Office for National Statistics database, UK (http://www.ons.gov.uk/).
Quality of life was measured using the EuroQoL EQ-5D questionnaire18 which was completed at baseline, 6 and 18 months postrandomisation, and at 6, 18 and 24 months post-treatment. The social tariff for the EQ-5D was applied in order to calculate utility values.19 Because post-treatment measurements were at variable times postrandomisation (randomisation date is time zero for a randomised trial) daily utilities were estimated using linear interpolation.
Sample size calculations
The sample size of 898 patients was based on exercise performance and was calculated according to the methodology published in the initial report of the CECaT study.10
Statistical and economic analysis
For this study, survival was summarised using Kaplan-Meier estimates and the groups were compared using Cox proportional hazards regression. This assumes that the instantaneous risk of death (hazard) for a reference value of a covariate will vary through time, but that the hazards for other values of the covariate will be a constant multiple of this baseline hazard, and this multiple will not vary through time. This assumption was tested using Schoenfeld residuals and there was little evidence against it. The diagnostic test was entered into the Cox regression as a four-level fixed covariate, with angiography as the reference category. In sensitivity analysis CABG and PCI were included in the regression analyses as time-varying covariates, taking a value 0 up to the time of intervention and 1 thereafter, to ensure that any differences between the groups was not due to differences in treatment. Inclusion of treatment (CABG/PCI) did not affect comparison of diagnostic strategies and results of these analyses are not included here.
Patient-specific hospital resource use was collected for 2 years post-treatment with revascularisation or medical management. Costs were based on National Health Service reference costs 2005/2006 prices. An annual discount rate of 3.5% was applied to all costs and quality-adjusted life-years (QALYs) after 12 months postrandomisation. Resources costed were: imaging tests; revascularisation; inpatient/outpatient episodes; adverse events; cardiac-related medications. Patient-reported admissions for MI were verified with the admitting hospital and adjudicated.
Quality-adjusted survival and cost-estimates were censored at the last follow-up at 2 years after treatment, resulting in varying duration of follow-up from the time of randomisation to the different diagnostic strategies, so that mean values over a range of time horizons were estimated using inverse weighting methods.20 This method allows for differing follow-up times between patients by splitting follow-up time into intervals, and up-weighting the observed quality-adjusted survival and costs in an interval in proportion to the inverse of the Kaplan-Meier estimate of the proportion observed during the interval. In the base case we used a time horizon of 3 years since it was the longest period over which results were stable, with acceptable precision. CIs for costs and QALYs were estimated using bootstrapping with 5000 samples.21
Sensitivity analysis
Sensitivity of cost-utility results for different time horizons was assessed by re-estimating results up to 12, 18, 24, 36 and 48 months after randomisation. In addition, cardiologists were divided into those who did and did not perform PCI as part of their routine clinical practice, and the results were recalculated for each subgroup. With the exception of this post hoc data interrogation, all other results presented derive from the intention-to-treat analysis.
The study had Institutional Review Board (IRB) approval and full written informed consent was obtained from all participants. All authors had full access to the data and take responsibility for the manuscript as written.
Results
Recruitment and compliance
Between September 2001 and September 2004, 3201 patients were assessed, 1981 were excluded and 322 refused entry to the trial. Refusals were more likely to come from women (46% compared with 31% enrolled into the study, p<0.001) and were significantly older (mean age 64.6, (SD 10.1) compared with study group mean age 61.8, (9.4), p<0.001).
In total 898 patients were randomised. Groups were well matched at baseline (table 1). In each group 69% of patients were high risk for CAD (Pryor score >0.8). The trial was closed to recruitment in September 2004 after enrolling the prespecified number of participants.
Table 1.
Baseline characteristics*
| Demographics | Angiography (n=222) | SPECT (n=224) | Cardiac MRI (n=226) | Stress ECHO (n=226) |
|---|---|---|---|---|
| Mean (SD) age (years) | 60.7 (9.1) | 62.1 (9.5) | 62.2 (9.0) | 61.9 (9.9) |
| Males (%) | 149 (67%) | 157 (70%) | 153 (68%) | 160 (71%) |
| History/risk factors | ||||
| Previous MI (%) | 63 (28%) | 52 (23%) | 69 (31%) | 59 (26%) |
| Previous CVA (%) | 10 (5%) | 13 (6%) | 8 (4%) | 12 (5%) |
| Diabetes (%) | ||||
| IDDM | 12 (5%) | 8 (4%) | 11 (5%) | 5 (2%) |
| NIDDM | 16 (7%) | 18 (8%) | 21 (9%) | 22 (10%) |
| Family history CAD | 60 (27%) | 55 (25%) | 63 (28%) | 59 (26%) |
| Smoking history (%) | ||||
| <25 pack-years | 149 (67%) | 162 (72%) | 148 (65%) | 155 (69%) |
| ≥25 pack-years | 73 (33%) | 62 (28%) | 78 (35%) | 71 (31%) |
| Treated hyperlipidaemia (%) | 164 (74%) | 171 (76%) | 179 (79%) | 179 (79%) |
| Treated hypertension (%) | 117 (53%) | 132 (59%) | 115 (51%) | 129 (57%) |
| Exercise tolerance using the modified Bruce protocol | ||||
| Mean (SD) total exercise time (min) | 11.29 (4.56) | 10.46 (4.41) | 10.43 (4.43) | 10.89 (4.36) |
| ECG changes on exercise test | ||||
| 1–2 mm ST depression with symptoms | 53 (24%) | 43 (19%) | 54 (24%) | 57 (25%) |
| ≥2 mm ST depression without symptoms | 16 (7%) | 24 (11%) | 20 (9%) | 24 (11%) |
| ST elevation†/no change | 153 (69%) | 157 (70%) | 152 (67%) | 145 (64%) |
| CCS class | ||||
| 0–I | 60 (27%) | 54 (24%) | 78 (35%) | 58 (26%) |
| II | 138 (62%) | 144 (64%) | 122 (54%) | 132 (58%) |
| III–IV | 24 (11%) | 26 (12%) | 26 (11%) | 36 (16%) |
*There were no significant differences between the groups in any variable.
†ST elevation was observed in three angiography, two CMR and one ECHO patients.
CAD, coronary artery disease; CCS, Canadian Cardiovascular Society CVA; cerebrovascular accident; IDDM, Insulin-dependent diabetes mellitus; MI, myocardial infarction; NIDDM, non-insulin-dependent diabetes mellitus; SPECT, single photon emission CT.
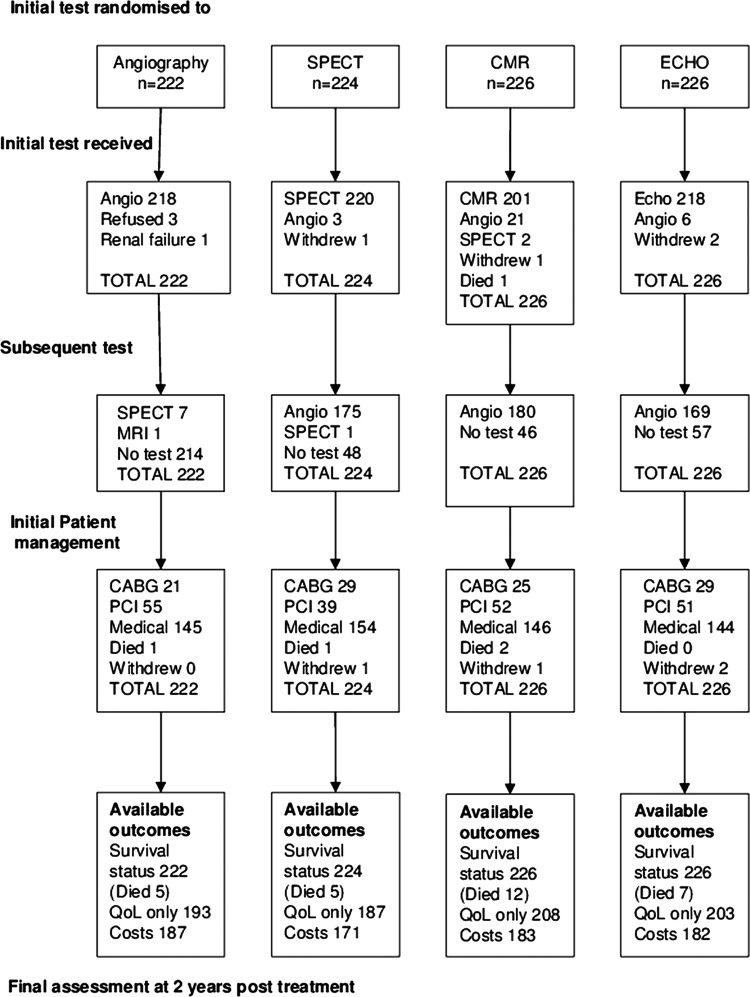
One hundred and seventy-five (78%) SPECT patients, 180 (80%) CMR patients and 169 (75%) stress ECHO patients were referred on for angiography (figure 1). Between 20% and 25% of patients undergoing non-invasive tests did not require further investigation. Twenty-one per cent of patients who had negative tests were referred for angiography and the proportion was similar in each group (SPECT n=45, CMR n=50, ECHO n=48, p=0.858). Of these 14 (31%) SPECT, 26 (52%) CMR and 23 (48%) ECHO resulted in a positive angiogram (p=0.130). Four patients died and four withdrew from the trial early on. Of the remaining patients, revascularisation was required in 34% (301/890—see figure 1 for numbers in each arm). There was no significant difference between the groups in initial patient management (figure 1; p=0.527). Beyond the initial management strategy 42 subsequent revascularisation procedures were required in the angiography arm compared with 30 in the SPECT, 44 in the CMR and 41 in the ECHO arms. The median times between randomisation and initial revascularisation were 122 days in the angiography group, 192 days for SPECT, 184 days for CMR and 177.5 days for ECHO, resulting in a delay due to functional testing of approximately 2 months.
Figure 1.
CONSORT diagram describing recruitment and randomisation.
Survival
During the study there were 7 (3%), 7 (3%), 18 (8%) and 11 (5%) deaths in the angiography, SPECT, CMR and ECHO groups respectively. Kaplan-Meier survival curves for the four groups are plotted in figure 2. Survival over the whole trial period in the SPECT (HR 1.0, 95% CI 0.4 to 2.9) and stress ECHO (HR 1.6, 95% CI 0.6 to 4.0) groups were not significantly different from angiography but the CMR group had higher mortality, with HR 2.6 (95% CI 1.1 to 6.2), p=0.032. The significant effect of CMR on survival remained when CABG or PCI were included in the models. However, mortality was low in all groups and the absolute mean difference in survival was less than 1 month over 3 years (table 2). Mean survival estimates over 3 years with 95% Cls are shown in table 2.
Figure 2.
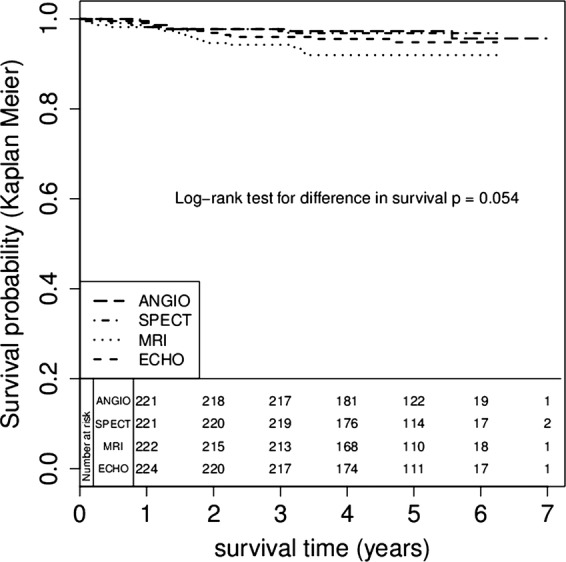
Kaplan-Meier survival estimates according to initial modality of diagnosis.
Table 2.
Cost-effectiveness summaries to 3 years postrandomisation
| Angiography (n=222) | SPECT (n=224) | Cardiac MRI (n=226) | Stress ECHO (n=226) | |
|---|---|---|---|---|
| HR for death | Baseline | 0.99 | 2.60 | 1.42 |
| (95% CI) | (0.35 to 2.85) | (1.09 to 6.22) | (0.54 to 3.74) | |
| Mean survival (years) | 2.96 | 2.95 | 2.90 | 2.94 |
| (95% CI) | (2.92 to 2.99) | (2.90 to 2.99) | (2.83 to 2.95) | (2.89 to 2.97) |
| Mean difference vs CA | − | −0.01 | −0.06 | −0.02 |
| (95% CI) | − | (−0.07 to 0.05) | (−0.14 to 0.01) | (−0.08 to 0.04) |
| Mean QALYs | 2.24 | 2.27 | 2.18 | 2.27 |
| (95% CI) | (2.16 to 2.31) | (2.20 to 2.33) | (2.11 to 2.25) | (2.19 to 2.33) |
| Mean difference vs CA | − | 0.03 | −0.05 | 0.03 |
| (95% CI) | − | (−5.21 to 1.38) | (−5.21 to 1.38) | (−3.04 to 4.21) |
| Mean discounted costs (£) | 5243 | 4644 | 4947 | 5530 |
| (95% CI) | (4282 to 6461) | (4194 to 5126) | (4480 to 5431) | (4857 to 6262) |
| Mean difference vs CA | − | −599 | −296 | 287 |
| (95% CI) | (−1901 to 503) | (−1603 to 824) | (−1109 to 1537) | |
| Probability cost-effective at £20K per QALY | − | 0.82 | 0.29 | 0.55 |
| Probability cost-effective at £30K per QALY | − | 0.79 | 0.25 | 0.59 |
CA, coronary angiography; SPECT, single photon emission CT; QALYs, quality-adjusted life-years.
All patients had complete adverse event data up to 18 months postrandomisation during which there were 178 non-fatal adverse events in 114 patients, mostly hospital admissions for chest pain (table 3). Beyond this time only adverse events that resulted in admissions were recorded as they were relevant for the economic analysis. No patient suffered any adverse event at the time of the initial randomised imaging test.
Table 3.
Summary adverse events during initial 18 months of follow-up*
| Adverse event | Angiography (n=222) | SPECT (n=224) | Cardiac MRI (n=226) | Stress ECHO (n=226) |
|---|---|---|---|---|
| Total adverse events | 38 | 34 | 44 | 62 |
| Chest pain (not myocardial infarction) | 21 | 20 | 28 | 35 |
| Angina | 7 | 5 | 4 | 3 |
| Myocardial infarction | 2 | 0 | 3 | 6 |
*Note that beyond this time only events that required hospital admission were recorded.
SPECT, single photon emission CT.
Cost-utility
Table 4 shows some of the highest incurred follow-up costs for the four groups and shows that patient management varied substantially between individuals. Although angiography was the most expensive of the four initial diagnostic tests, the strategy of initial angiography had lower mean overall cost than stress ECHO, and was similar to CMR up to 3 years (table 2). Extra costs for patients in the three non-invasive groups were largely due to patients undergoing follow on angiography. There were no significant differences in overall costs between the groups.
Table 4.
Summary of the frequency of use of the main resource use elements during follow-up of up to 3 years (excluding initial diagnostic test)
| Resource use (unit cost) | Angiography (n=222) | SPECT (n=224) | Cardiac MRI (n=226) | Stress ECHO (n=226) |
|---|---|---|---|---|
| CABG (£7195) | 28 | 33 | 28 | 34 |
| PCI (£3660) | 57 | 46 | 60 | 62 |
| Other hospital admission (£467/day) | 36 | 29 | 28 | 53 |
| Angiography (£625) | 12 | 183 | 175 | 181 |
| SPECT (£405) | 16 | 3 | 3 | 6 |
| Cardiac MRI (£565) | 5 | 5 | 12 | 5 |
| Echocardiography (£435) | 30 | 17 | 24 | 18 |
| PET scan (£842) | 0 | 3 | 0 | 1 |
| Preadmission clinic (£85) | 22 | 21 | 27 | 24 |
| Follow-up clinic (£85) | 19 | 22 | 31 | 21 |
| Outpatient visits (£143) | 247 | 270 | 300 | 284 |
*Cardiac drugs were also included but are not shown here due to many different combinations of drugs and doses prescribed.
CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; PET, positron emission tomography; SPECT, single photon emission CT.
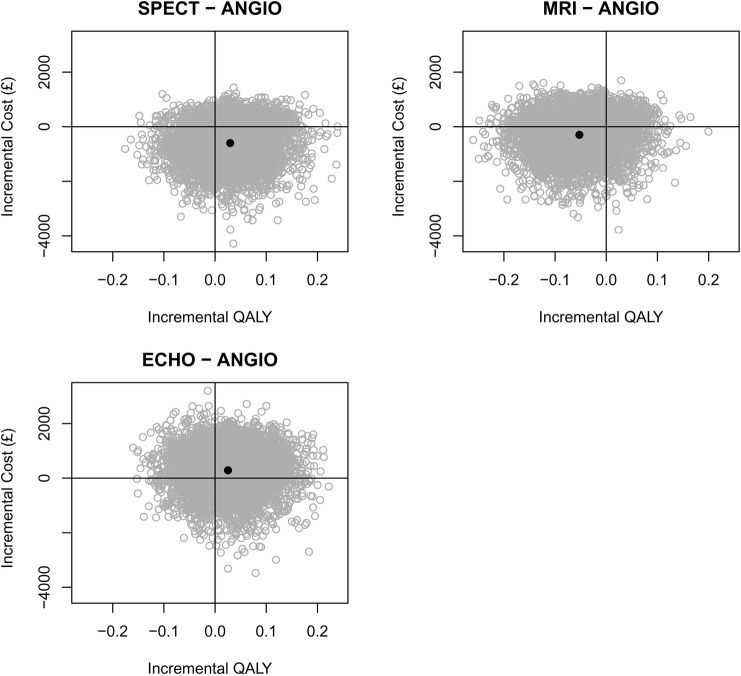
During the study there were no significant differences in EQ-5D between the groups. Figure 3 shows daily mean EQ-5D utility over time based on interpolation between measurements for survivors who completed the EQ-5D. Using inverse-weighting we calculated QALYs over different time horizons and these are presented up to 3 years in table 2. Mean QALYs over 3 years in the angiography group was 2.24, which was not significantly different from the other groups. Figure 4 shows the joint distribution of the difference in mean cost against the difference in mean QALY for each diagnostic strategy group and angiography alone, and shows the uncertainty in these estimates. Figure 5 shows the cost-effectiveness acceptability curve and demonstrated that SPECT has a high chance (>70%) of being cost-effective whatever the willingness-to-pay threshold is but for CMR and ECHO there is much less certainty about this decision. The mean differences between groups were close to zero in all three cases so that the cost-per-QALY estimates are unstable and a cost-minimisation approach may be more appropriate. This would favour SPECT, which was both cheaper and more effective on average than angiography, and had the lowest overall cost.
Figure 3.
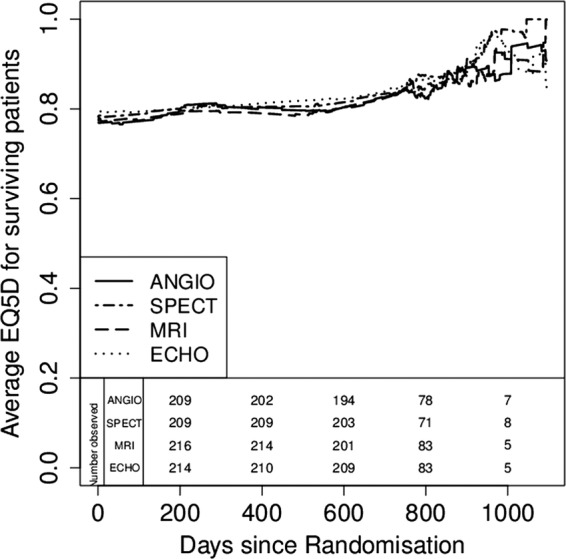
Quality of life assessed by EQ5D over time.
Figure 4.
Bootstrapped estimates of the joint distribution of mean cost-difference against mean quality-adjusted life-year difference up to 3-years postrandomisation.
Figure 5.
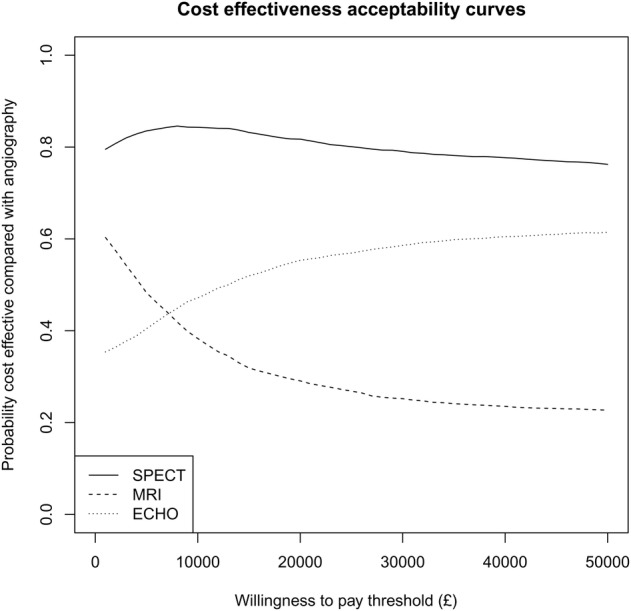
Estimated probability of being cost-effective compared with angiography alone against the amount (£) a health provider is willing to pay for one additional quality-adjusted life-year.
Sensitivity analysis
The comparisons between the diagnostic strategy groups did not change substantially when we varied the time horizon; the main effect of this was that the variation surrounding estimates increased as the time horizon lengthened due to the heavy censoring (results not shown). Tables 5 and 6 show results for interventional and non-invasive cardiologists respectively. Patients who were managed by interventional cardiologists incurred higher costs due to the greater number of tests and revascularisation procedures performed, with minimal incremental benefit in QALY.
Table 5.
Cost-effectiveness summaries for patients managed by interventional cardiologists to 3 years postrandomisation
| Angiography (n=73) | SPECT (n=96) | Cardiac MRI (n=93) | Stress ECHO (n=98) | |
|---|---|---|---|---|
| Mean survival (years) | 2.96 | 2.97 | 2.89 | 2.96 |
| (95% CI) | (2.91 to 2.98) | (2.93 to 3.00) | (2.80 to 2.97) | (2.90 to 2.98) |
| Mean difference vs CA | − | 0.01 | −0.07 | 0 |
| (95% CI) | − | (−0.05 to 0.07) | (−0.17 to 0.02) | (−0.07 to 0.05) |
| Mean QALYs | 2.25 | 2.29 | 2.21 | 2.2 |
| (95% CI) | (2.14 to 2.36) | (2.19 to 2.38) | (2.10 to 2.32) | (2.10 to 2.29) |
| Mean difference vs CA | − | 0.03 | −0.04 | −0.06 |
| (95% CI) | − | (−5.31 to 2.30) | (−4.96 to 2.41) | (−3.06 to 6.42) |
| Mean discounted costs (£) | 5754 | 5205 | 5307 | 6329 |
| (95% CI) | (4651 to 6941) | (4475 to 5979) | (4610 to 6032) | (5120 to 7713) |
| Mean difference vs CA | − | −549 | −447 | 574 |
| (95% CI) | (−1973 to 799) | (−1841 to 897) | (−1097 to 2262) | |
| Probability cost-effective at £20K per QALY | 0.72 | 0.42 | 0.20 | |
| Probability cost-effective at £30K per QALY | 0.70 | 0.39 | 0.21 |
CA, coronary angiography; SPECT, single photon emission CT; QALYs, quality-adjusted life-years.
Table 6.
Cost-effectiveness summaries for patients managed by non-interventional cardiologists to 3 years postrandomisation
| Angiography (n=149) | SPECT (n=128) | Cardiac MRI (n=133) | Stress ECHO (n=128) | |
|---|---|---|---|---|
| Mean survival (years) | 2.95 | 2.93 | 2.9 | 2.92 |
| (95% CI) | (2.89 to 2.99) | (2.85 to 2.98) | (2.81 to 2.97) | (2.85 to 2.98) |
| Mean difference vs CA | − | −0.02 | −0.05 | −0.03 |
| (95% CI) | − | (−0.11 to 0.06) | (−0.15 to 0.04) | (−0.11 to 0.05) |
| Mean QALYs | 2.23 | 2.25 | 2.17 | 2.31 |
| (95% CI) | (2.14 to 2.31) | (2.16 to 2.33) | (2.07 to 2.26) | (2.22 to 2.40) |
| Mean difference vs CA | − | 0.02 | −0.06 | 0.09 |
| (95% CI) | − | (−6.92 to 1.90) | (−5.50 to 3.45) | (−5.45 to 3.71) |
| Mean discounted costs (£) | 4936 | 4216 | 4723 | 4780 |
| (95% CI) | (3681 to 6665) | (3635 to 4799) | (4068 to 5381) | (4136 to 5467) |
| Mean difference vs CA | − | −719 | −212 | −156 |
| (95% CI) | (−2527 to 695) | (−2007 to 1258) | (−1990 to 1353) | |
| Probability cost-effective at £20K per QALY | 0.75 | 0.29 | 0.85 | |
| Probability cost-effective at £30K per QALY | 0.72 | 0.27 | 0.86 |
CA, coronary angiography; SPECT, single photon emission CT; QALYs, quality-adjusted life-years.
Discussion
CECaT is the first completed prospective randomised trial to look at the clinical and cost-effectiveness of non-invasive imaging in the diagnosis and management of angina. To the best of the authors’ knowledge there has been no comparable outcomes trial published on this scale evaluating three independent non-invasive ‘gatekeeper’ modalities versus initial angiography in stable chest pain. The trial is also unusual in the length of prospective follow-up extending to 6 years for mortality outcomes.
We have demonstrated that use of any one of stress CMR, stress ECHO or stress SPECT as the initial test for a stable chest pain patient leads to non-inferior outcomes in quality of life and cost-utility compared with patients randomised to upfront invasive CA. Typically, non-invasive tests perform well in low-risk populations because of a negative predictive value which is usually better than the positive predictive value. However, the patient risk profile was relatively high in our study, and despite this there was no significant difference between an initial functional or anatomic approach.
There are several reasons why initial angiography may not have led to clear benefit in our study. First, although angiography has stood at the heart of the diagnostic chest pain pathway, the traditional binary cut-off values of 50% or 70% stenosis by visual estimation were shown in the FAME study to bear little relation to the true physiological significance of luminal narrowing.22
Second, data from various countries suggest that not only is CA often inappropriate when formally rated by expert observers23 24 but that disparate national or regional rates of angiography do not translate into clear mortality benefits between countries25–28 and on occasion may even demonstrate an inverse relationship.29 Contemporary US data from approximately 500 000 PCI procedures collected prospectively in the National Cardiovascular Data Registry demonstrated that, of 145 000 elective PCI cases, roughly 12% were judged inappropriate. Furthermore, the rate of inappropriate elective PCI between hospital sites ranged from 0% to 55% suggesting significant variability in practice.30 The data suggest that a better way of selecting patients for invasive investigation is needed.
Ischaemia-driven revascularisation has been shown to be of benefit in a number of trials, most recently in the FAME study in which an invasive method of measuring the flow reserve of the microcirculation (Fractional Flow Reserve, FFR) was used to allocate patients to intervention or observation versus a clinical decision on intervention based on angiography alone.31 At 2 years of follow-up there were clear survival and MACE benefits to the FFR-based approach.
The ACRE study reported that, up to 6 years after diagnosis, medical management was a more cost-effective strategy for angina compared with PCI.32 The lack of evidence for survival from revascularisation was also seen in the COURAGE trial.4 Critics have suggested this may be because randomisation to PCI versus optimal medical therapy was made after CA had been performed, potentially leading to a recruitment bias of patients with less severe disease. In the CECaT trial this bias was avoided by randomisation to a management strategy defined by the non-invasive test result for each of the three functional arms of the trial. In a relatively high-risk population, we demonstrated no clinically significant survival or economic detriment from using non-invasive imaging as a gate-keeper to catheterisation. Similarly, quality of life was not significantly different across all four groups and these differences extended to a warranty period of at least 3 years.
We did observe a marginal decrease in survival in the CMR arm. The reasons for the difference are unclear but do not relate to patient characteristics or management with CABG or PCI. Although statistically significant, the mean survival difference from the other groups was only 4 weeks over 3 years and is unlikely to be clinically meaningful. Since recent work has established a strong correspondence between FFR measurements and stress CMR perfusion indices it would be surprising if CMR were genuinely inferior to other modalities for risk stratification.33 Indeed several recent publications have highlighted the incremental prognostic data (above that obtained from clinical variables) derived from several thousand perfusion CMR studies.34 35
Given the recent publication of the CEMARC trial36 in which a clear diagnostic superiority was demonstrated for stress CMR over SPECT, it is interesting that our data nonetheless show an equivalence in functional health status between those randomised to SPECT versus CMR in the CECaT trial. The implication may be that although CMR detects the presence of any ischaemia with a greater sensitivity it is the overall burden of ischaemia that alters a patient's prognosis. As such, it has not yet been demonstrated that the higher diagnostic accuracy of CMR translates into better long-term patient outcomes—a fact acknowledged by Greenwood37 subsequent to CEMARC's publication. In this context the CECaT nuclear results are congruent with numerous past publications and reconfirm the reassuring warranty period of a normal SPECT study.
Cost-effectiveness
There was no significant difference in cost-effectiveness between the angiography-as-default group and the non-invasive test groups up to 3 years, perhaps relating to the higher-than-anticipated rate of referral for angiography after negative functional tests. Protocol deviation of this kind is not infrequent in trials of non-invasive technology. In the recent PARR-2 study of patients undergoing fluorodeoxyglucose positron emission tomography for the assessment of viability, roughly 25% of the study population did not adhere to the protocol.38 The willingness of a cardiologist to defer referral for CA in the face of a normal non-invasive study may in part reflect individual prejudices and job description (interventional vs non-invasive) as demonstrated in a recent survey of cardiology attitudes39 and was also reflected in our own data.
In CECaT, 20–25% of patients receiving a non-invasive test did not go on to have angiography. A proportion of the additional cost in the non-invasive arms related to angiography and PCI in the patients with a negative test, although only very few subsequently required CABG—a robust marker of significant disease—during follow-up. This readiness to employ PCI in a group in whom the indication/benefit is debatable was also seen in the ACRE trial32 and reflects understandable clinical response to uncertainty but also the easy access to PCI in healthcare systems without barriers to self-referral.40 Similarly, studies from the USA have demonstrated a greater willingness to use CA when available ‘on site’ as is increasingly seen even in small-to-medium sized hospitals.41–43
Cost-effectiveness of each non-invasive technique
Nuclear myocardial perfusion imaging
The END study used propensity matching to compare a large cohort of patients referred for either gate-keeper myocardial perfusion imaging or upfront angiography—this non-randomised study demonstrated a significant cost-reduction in the nuclear arm.44 In contrast to this and other work45–47 we were unable to show a significant difference in cost-effectiveness in our own study. To some extent this reflected the participating physician bias towards angiography during the period of trial recruitment (2001–2006) with many patients referred for angiography despite normal perfusion studies. This continues in the contemporary era with Chan et al30 demonstrating that almost 80% of Delphi-adjudicated inappropriate elective PCIs were performed following either low-risk ischaemia imaging in mildly symptomatic patients or intermediate risk imaging in entirely asymptomatic patients.
In the CECaT study, when PCI was performed despite a negative initial non-invasive test, this occurred because subsequent angiography indicated ‘significant’ stenosis. This was a clinical judgment based on ‘eyeball assessment’ of lesion severity as has been reported in clinical trials with angiographic end points.48 However, the severity and functional significance of many stenoses may be over-called, even by quantitative assessment, when compared with the physiological assessment of fractional flow reserve across the lesion.49 50 Further improvement in cost-effectiveness could likely have been achieved in the nuclear arm of the trial. Our post-trial practice is to perform the stress SPECT first—and if this is negative—cancel the rest test, likely augmenting the relative cost-effectiveness of SPECT.51
CMR
There are relatively few data available regarding the cost-effectiveness of CMR. One recent mathematical modelling study estimated that CMR likely has greater cost-effectiveness/utility compared with SPECT despite greater base case cost of the former.52 The economic superiority of CMR was also recently described by the CEMARC group, although interestingly the base case costs employed for CMR and SPECT in their analysis differed by only a few pounds. Their own sensitivity analysis in fact demonstrated that if the costs of the two tests varied by more than 100 pounds (which was the case in our study) then in fact—as we found—SPECT became the dominant strategy in a low-to-intermediate risk population.53
Stress ECHO
Stress echocardiography may be a more cost-effective strategy than angiography for men aged 50–60 with CAD prevalence of 50%.47 54 There is also some evidence that stress ECHO is more cost-effective than SPECT as an initial test,55 56 especially in women with suspected CAD.57 A similar benefit was not seen in our study probably because of the high disease prevalence in our population. The lack of superiority of either stress echocardiography or a combined strategy of exercise testing and stress ECHO compared with upfront catheterisation was also evident in a recent Polish study of 600 patients with a similar age, gender and disease prevalence to our own study population.58
Taken overall, our data clearly demonstrate a limited future role for cost-effective non-invasive imaging if referring physicians are not willing to accept a negative result as ground truth. This might be interpreted as reflecting a need for greater physician education since we showed a clear difference in onward referral rates for angiography after a negative test between interventional and non-invasive cardiologists.
Clinical effectiveness
We demonstrated that SPECT can obviate the need for CA for a significant number of patients without any clinical detriment. In the stress ECHO group clinical outcomes were also comparable to the angiography subgroup at 18 months. The CMR group had statistically marginally poorer survival and this follows our earlier finding that CMR patients had significantly worse exercise tolerance at 18 months after randomisation.10 This is difficult to explain on the basis of Pryor risk score or other baseline clinical variables. However, the mean difference in survival between the CMR arm and the other groups was only 4 weeks and thus of marginal clinical significance. More importantly, the CMR group were not otherwise disadvantaged—compared with the angiographic control group—with respect to major adverse events, other resource use, or quality of life.
Limitations
This study was carried out in a single specialist cardiothoracic centre with a significant proportion of high risk, predominantly white European, male patients. Those eligible who refused the trial were older and were more likely to be women.
Survival data from the national registry did not include cause of death so that deaths due to cardiovascular causes could not be reported separately.
The trial completed recruitment in 2004 and we used the technology that was available to us at the onset of the trial. At that time, we were not able to use attenuation correction for SPECT imaging; however this was also not used in the most recent CEMARC trial.36 Similarly, we performed our CMR examinations in a mobile facility on a 1.5 T scanner with only modest coil technology and limited temporal and anatomic coverage that would compare unfavourably with the 3 T whole heart high-resolution perfusion studies available today.
The trial aimed to reflect the strategy of using non-invasive imaging as a gateway to angiography in contemporary clinical practice. The test results were considered in conjunction with other information available at the time. Thus it was not the aim to formally assess diagnostic accuracy of the non-invasive tests in this context. This trial-based study was limited to 3 year cost-effectiveness follow-up—longer-term economic models would provide lifetime estimates of the cost-effectiveness of the non-invasive strategies and could include advances in imaging technology.
Conclusions
We have demonstrated in the CECaT trial that stress ECHO, stress CMR and stress SPECT may each be used to defer invasive CA without clinical detriment or significant excess costs in an outpatient population with stable chest pain.
Supplementary Material
Footnotes
Collaborators: Johanna Armstrong, Martin Buxton, Noreen Caine, Richard Coulden, Andrew Crean, Matthew Dyer, Margaret Gillham, Hester Goddard, Kim Goldsmith, Vikki Hughes, Evelyn Lee, Roger Patel, Peter Schofield, Linda Sharples, Emer Sonnex, David Stone, Carmen Treacy.
Contributors: HT performed literature review, data analysis and interpretation; NW was involved in image analysis, drafting the manuscript and critical revision; VH was involved in recruiting the patients, data management, administering health questionnaires, data analysis and drafting the manuscript; MD and MB were responsible for data analysis, health economic assessment, drafting the manuscript and critical revision; LDS was responsible for study design, trial management, statistical analysis, drafting the manuscript and critical revision; CJ performed statistical and health economic analysis, drafting the manuscript and critical revision; AMC was involved in study design, patient recruitment, image interpretation, trial management, drafting the manuscript and critical revision and is the overall guarantor of manuscript integrity. All authors have read the manuscript in its submitted form and have provided final approval for publication.
Funding: The original CECaT study was funded by a grant from the UK National Health Service R&D Health Technology Assessment Program (project no. 99/26/04).
Competing interests: HT, CJ and LDS were supported by the Medical Research Council (Programme number U015232027).
Ethics approval: Papworth Hospital Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Contributor Information
Collaborators: Johanna Armstrong, Martin Buxton, Noreen Caine, Richard Coulden, Andrew Crean, Matthew Dyer, Margaret Gillham, Hester Goddard, Kim Goldsmith, Vikki Hughes, Evelyn Lee, Roger Patel, Peter Schofield, Linda Sharples, Emer Sonnex, David Stone, and Carmen Treacy
References
- 1.NICE Myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. National Institute for Clinical Excellence, 2003; Technology Appraisal Guidance 73 [Google Scholar]
- 2.Boden WE. Surgery, angioplasty, or medical therapy for symptomatic multivessel coronary artery disease: is there an indisputable ‘winning strategy’ from evidence-based clinical trials? J Am Coll Cardiol 2004;43:1752–4 [DOI] [PubMed] [Google Scholar]
- 3.Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery. Quality of life in patients randomly assigned to treatment groups. Circulation 1983;68:951–60 [DOI] [PubMed] [Google Scholar]
- 4.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16 [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 2002;40:1531–40 [DOI] [PubMed] [Google Scholar]
- 7.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. J Am Coll Cardiol 2008;51:1127–47 [DOI] [PubMed] [Google Scholar]
- 8.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol 2009;53:2201–29 [DOI] [PubMed] [Google Scholar]
- 9.Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2462–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharples L, Hughes V, Crean A, et al. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial. Health Technol Assess 2007;11:iii–iv, ix–115 [DOI] [PubMed] [Google Scholar]
- 11.Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med 1993;118:81–90 [DOI] [PubMed] [Google Scholar]
- 12.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol 1953;39:368–76 [DOI] [PubMed] [Google Scholar]
- 13.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 1999;33:1756–824 [DOI] [PubMed] [Google Scholar]
- 14.Wackers FJ. Exercise myocardial perfusion imaging. J Nucl Med 1994;35:726–9 [PubMed] [Google Scholar]
- 15.Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. J Cardiovasc Magn Reson 2004;6:727–65 [DOI] [PubMed] [Google Scholar]
- 16.Reeder SB, Atalar E, Faranesh AZ, et al. Multi-echo segmented k-space imaging: an optimized hybrid sequence for ultrafast cardiac imaging. Magn Reson Med 1999;41:375–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canet EP, Janier MF, Revel D. Magnetic resonance perfusion imaging in ischemic heart disease. J Magn Reson Imaging 1999;10:423–33 [DOI] [PubMed] [Google Scholar]
- 18.Kind P. The EuroQol instrument: an index of health-related quality of life. In: Spiker B. ed. Quality of life and pharmacoeconomics in clinical trials. Philadelphia, PA: Lippincott-Raven, 1996 [Google Scholar]
- 19.Dolan P, Gudex C, Kind P. A social tariff for EuroQol: results from a UK general population survery. Centre for Health Economics, University of York, 1995 [Google Scholar]
- 20.Willan AR, Lin DY, Cook RJ, et al. Using inverse-weighting in cost-effectiveness analysis with censored data. Stat Methods Med Res 2002;11:539–51 [DOI] [PubMed] [Google Scholar]
- 21.Efron BTR. An introduction to the bootstrap. San Francisco: Chapman and Hall, 1993 [Google Scholar]
- 22.Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816–21 [DOI] [PubMed] [Google Scholar]
- 23.Mozes B, Shabtai E. The appropriateness of performing coronary angiography in two major teaching hospitals in Israel. Int J Qual Health Care 1994;6:245–9 [DOI] [PubMed] [Google Scholar]
- 24.Gray D, Hampton JR. Methods of establishing criteria for purchasing coronary angiography in the investigation of chest pain. J Public Health Med 1994;16:399–405 [DOI] [PubMed] [Google Scholar]
- 25.Guadagnoli E, Hauptman PJ, Ayanian JZ, et al. Variation in the use of cardiac procedures after acute myocardial infarction. N Engl J Med 1995;333:573–8 [DOI] [PubMed] [Google Scholar]
- 26.Mark DB, Naylor CD, Hlatky MA, et al. Use of medical resources and quality of life after acute myocardial infarction in Canada and the United States. N Engl J Med 1994;331:1130–5 [DOI] [PubMed] [Google Scholar]
- 27.Rouleau JL, Moye LA, Pfeffer MA, et al. A comparison of management patterns after acute myocardial infarction in Canada and the United States. The SAVE investigators. N Engl J Med 1993;328:779–84 [DOI] [PubMed] [Google Scholar]
- 28.Tu JV, Pashos CL, Naylor CD, et al. Use of cardiac procedures and outcomes in elderly patients with myocardial infarction in the United States and Canada. N Engl J Med 1997;336:1500–5 [DOI] [PubMed] [Google Scholar]
- 29.Selby JV, Fireman BH, Lundstrom RJ, et al. Variation among hospitals in coronary-angiography practices and outcomes after myocardial infarction in a large health maintenance organization. N Engl J Med 1996;335:1888–96 [DOI] [PubMed] [Google Scholar]
- 30.Chan PS, Patel MR, Klein LW, et al. Appropriateness of percutaneous coronary intervention. JAMA 2011;306:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol 2010;56:177–84 [DOI] [PubMed] [Google Scholar]
- 32.Griffin SC, Barber JA, Manca A, et al. Cost effectiveness of clinically appropriate decisions on alternative treatments for angina pectoris: prospective observational study. BMJ 2007;334:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins S, McGeoch R, Lyne J, et al. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation 2009;120:2207–13 [DOI] [PubMed] [Google Scholar]
- 34.Krittayaphong R, Chaithiraphan V, Maneesai A, et al. Prognostic value of combined magnetic resonance myocardial perfusion imaging and late gadolinium enhancement. Iint J Cardiovasc Imaging 2011;27:705–14 [DOI] [PubMed] [Google Scholar]
- 35.Bingham SE, Hachamovitch R. Incremental prognostic significance of combined cardiac magnetic resonance imaging, adenosine stress perfusion, delayed enhancement, and left ventricular function over preimaging information for the prediction of adverse events. Circulation 2011;123:1509–18 [DOI] [PubMed] [Google Scholar]
- 36.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwood JP. CMR versus SPECT for diagnosis of coronary heart disease—authors’ reply. Lancet 2012;379:2147–8 [DOI] [PubMed] [Google Scholar]
- 38.Beanlands RS, Nichol G, Huszti E, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol 2007;50:2002–12 [DOI] [PubMed] [Google Scholar]
- 39.Maron DJ, Stone GW, Berman DS, et al. Is cardiac catheterization necessary before initial management of patients with stable ischemic heart disease? Results from a Web-based survey of cardiologists. Am Heart J 2011;162:1034–43 e13 [DOI] [PubMed] [Google Scholar]
- 40.Nallamothu BK, Rogers MA, Chernew ME, et al. Opening of specialty cardiac hospitals and use of coronary revascularization in medicare beneficiaries. JAMA 2007;297:962–8 [DOI] [PubMed] [Google Scholar]
- 41.Feit F, Mueller HS, Braunwald E, et al. Thrombolysis in Myocardial Infarction (TIMI) phase II trial: outcome comparison of a ‘conservative strategy’ in community versus tertiary hospitals. The TIMI Research Group. J Am Coll Cardiol 1990;16:1529–34 [DOI] [PubMed] [Google Scholar]
- 42.Blustein J. High-technology cardiac procedures. The impact of service availability on service use in New York State. JAMA 1993;270:344–9 [DOI] [PubMed] [Google Scholar]
- 43.Every NR, Larson EB, Litwin PE, et al. The association between on-site cardiac catheterization facilities and the use of coronary angiography after acute myocardial infarction. Myocardial Infarction Triage and Intervention Project Investigators. N Engl J Med 1993;329:546–51 [DOI] [PubMed] [Google Scholar]
- 44.Shaw LJ, Hachamovitch R, Berman DS, et al. The economic consequences of available diagnostic and prognostic strategies for the evaluation of stable angina patients: an observational assessment of the value of precatheterization ischemia. Economics of Noninvasive Diagnosis Multicenter Study Group. J Am Coll Cardiol 1999;33:661–9 [DOI] [PubMed] [Google Scholar]
- 45.Mowatt G, Vale L, Brazzelli M, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Health Technol Assess 2004;8:iii–iv, 1–207 [DOI] [PubMed] [Google Scholar]
- 46.Underwood SR, Godman B, Salyani S, et al. Economics of myocardial perfusion imaging in Europe—the EMPIRE Study. Eur Heart J 1999;20:157–66 [DOI] [PubMed] [Google Scholar]
- 47.Underwood SR, Shaw LJ, Anagnostopoulos C, et al. Myocardial perfusion scintigraphy and cost effectiveness of diagnosis and management of coronary heart disease. Heart 2004;90(Suppl 5):v34–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uchida T, Popma J, Stone GW, et al. The clinical impact of routine angiographic follow-up in randomized trials of drug-eluting stents: a critical assessment of ‘oculostenotic’ reintervention in patients with intermediate lesions. JACC Cardiovasc Interv 2010;3:403–11 [DOI] [PubMed] [Google Scholar]
- 49.Christou MA, Siontis GC, Katritsis DG, et al. Meta-analysis of fractional flow reserve versus quantitative coronary angiography and noninvasive imaging for evaluation of myocardial ischemia. Am J Cardiol 2007;99:450–6 [DOI] [PubMed] [Google Scholar]
- 50.Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging 2009;2:1009–23 [DOI] [PubMed] [Google Scholar]
- 51.Chang SM, Nabi F, Xu J, et al. Normal stress-only versus standard stress/rest myocardial perfusion imaging: similar patient mortality with reduced radiation exposure. J Am Coll Cardiol 2010;55:221–30 [DOI] [PubMed] [Google Scholar]
- 52.Boldt J, Leber AW, Bonaventura K, et al. Cost-effectiveness of cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary artery disease in Germany. J Cardiovasc Magn Reson 2013;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker S, Girardin F, McKenna C, et al. Cost-effectiveness of cardiovascular magnetic resonance in the diagnosis of coronary heart disease: an economic evaluation using data from the CE-MARC study. Heart 2013;99:873–81 [DOI] [PubMed] [Google Scholar]
- 54.Kuntz KM, Fleischmann KE, Hunink MG, et al. Cost-effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med 1999;130:709–18 [DOI] [PubMed] [Google Scholar]
- 55.Hayashino Y, Nagata-Kobayashi S, Morimoto T, et al. Cost-effectiveness of screening for coronary artery disease in asymptomatic patients with Type 2 diabetes and additional atherogenic risk factors. J Gen Intern Med 2004;19:1181–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan SS, Oppe M, Zoet-Nugteren SK, et al. A microcosting study of diagnostic tests for the detection of coronary artery disease in the Netherlands. Eur J Radiol 2009;72:98–103 [DOI] [PubMed] [Google Scholar]
- 57.Kim C, Kwok YS, Saha S, et al. Diagnosis of suspected coronary artery disease in women: a cost-effectiveness analysis. Am Heart J 1999;137:1019–27 [DOI] [PubMed] [Google Scholar]
- 58.Lewandowski M, Szwed H, Kowalik I. Searching for the optimal strategy for the diagnosis of stable coronary artery disease. Cost-effectiveness of the new algorithm. Cardiol J 2007;14:544–51 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.