Abstract
Recently there was yet another clinical trial using antioxidants that failed in patients with critical illness. In this perspective, we suggest that antioxidants likely interfere with the normal immune response, thus contributing to the lack of efficacy in patients with critical illness.
In critical illness, there is evidence that oxidative stress is associated with poorer outcomes and represents a final common pathway that leads to multiorgan failure and death. The imbalance between reactive oxygen species (ROS) production and effective removal by antioxidants and ROS scavengers has been proposed to contribute to many pathological conditions, including critical illnesses such as acute respiratory distress syndrome (ARDS) and sepsis. Thus, oxidative stress has been an attractive therapeutic target in critical illness, and antioxidants have been tested in critically ill patients for decades. Overall the results have been inconsistent without a clear benefit (1). Recently, the largest clinical trial targeting oxidative stress in critically ill patients was completed and reported no clinical efficacy of antioxidant supplementation and a trend toward increased mortality with glutamine administration (2). The reasons for the disappointing results are not clear. Some would argue that the appropriate antioxidants have not been used or that the dose, timing, or delivery or antioxidants were not optimized. In addition, adverse off-target effects may have contributed to their failure. Pertinent to possible off-target effects, it is important to remember that that ROS are critical signaling molecules for cell homeostasis and adaptation to stress (e.g., hypoxia), processes that may be impaired with antioxidants. More recently, it has been recognized that ROS are critical signaling molecules essential for optimal function of innate and adaptive immunity. Both types of immunity are required to fight infection, a frequent contributor to morbidity and mortality in critically ill patients.
Basics of ROS Biology
ROS are intracellular chemical species that are reactive toward lipids, proteins, and DNA (3). ROS include the superoxide anion (O2–), hydrogen peroxide (H2O2), and hydroxyl radicals (OH·). Each ROS has different intrinsic chemical properties, which dictate its reactivity and preferred biological targets. O2– is produced during the one-electron reduction of molecular oxygen (O2). O2– is rapidly converted by superoxide dismutases into H2O2 because O2– can damage the iron–sulfur cluster proteins. H2O2 can modulate signaling by oxidizing thiols within proteins (4). There are enzymes that can reverse this oxidation to return the protein to their native reduced state. This is analogous to phosphorylation/dephosphorylation-dependent signaling prevalent in biology. The levels of H2O2 associated with signaling are likely in the picomolar to nanomolar range; higher levels make proteins inactive by hyperoxidizing thiols (5). There are ample peroxiredoxins and glutathione peroxidases present in the cytosol and mitochondria that convert H2O2 to water to limit levels of H2O2 within the signaling range (6). In the presence of ferrous or cuprous ions, H2O2 can become a hydroxyl radical, which is very reactive and causes oxidation of lipids, proteins, and DNA, resulting in damage to the cell. Thus, iron homeostasis is tightly regulated to prevent the formation of toxic hydroxyl radicals. Redox signaling refers to thiol oxidation–dependent signaling, whereas oxidative stress refers to damage of lipids, proteins, and DNA or disruption of thiol-dependent signaling. Currently the field is hampered by the lack of adequate tools to measure ROS in vivo with appropriate sensitivity and specificity.
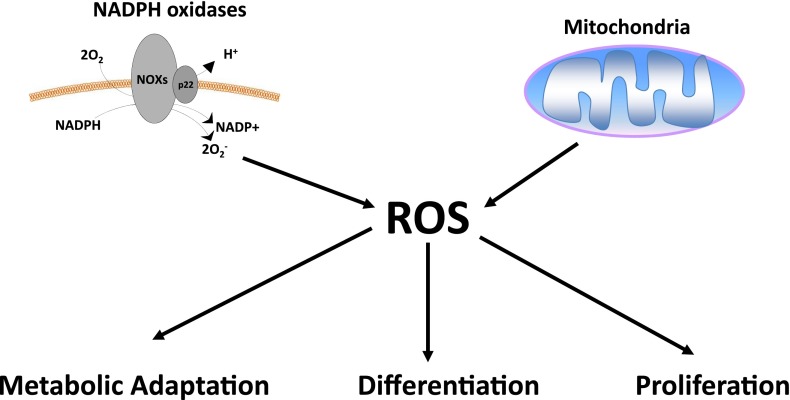
Mitochondria and NADPH oxidases (NOXs) are two main producers of ROS. Mitochondria have eight sites that generate ROS (7). The three best-characterized sites are complex I, II, and III within the mitochondrial respiratory chain, which generate O2– by the one-electron reduction of molecular O2. NOXs proteins use NADPH to generate O2– and are found on plasma membranes as well as endoplasmic reticulum and mitochondrial outer membranes (8). O2– generated by NOXs and mitochondria can be converted to H2O2 by O2– dismutases. There has been accumulating evidence that NOXs and mitochondria-generated H2O2 contribute to multiple processes in cells, including proliferation, differentiation, and metabolic adaptation (Figure 1) (9, 10). A major unanswered question in the field is to define the intracellular protein targets of ROS in different biological processes. An interesting observation highlighting the importance of low levels of ROS-dependent signaling comes from the Caenorhabditis elegans community that have demonstrated elevated ROS levels can extend lifespan (11). It will be of great interest to determine whether small increases in ROS can extend lifespan in mammals.
Figure 1.
Reactive oxygen species (ROS) regulate biological responses. ROS generated from nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and mitochondria have been implicated in regulating multiple biological responses including proliferation, differentiation, and metabolic adaptation.
ROS in Lung Injury and Septic Shock (Critical Illness)
Oxidative stress has been implicated in the pathophysiology of acute lung injury and sepsis-induced multiorgan failure, two of the most common causes of morbidity and mortality in critical illness. Investigators have found evidence of oxidative stress, as assessed by various measures, in plasma, alveolar fluid, and exhaled breath condensates of critically ill patients (12–14). In addition, patients with ARDS have decreased antioxidant levels, such as vitamins A, C, and E; glutathione; and selenium (15). Furthermore, some studies have reported that the severity and lack of reversibility of oxidative stress are associated with multiorgan failure and mortality (16).
Trials of Antioxidants in Critical Illness
Based on the reports summarized above and other data, multiple clinical trials targeting oxidative stress have been performed in critically ill patients. Two separate Cochrane reviews have examined the safety and efficacy of N-acetylcysteine (NAC) in sepsis and ARDS (1, 17). These strategies have taken one of two approaches. The first and most common strategy has been to administer exogenous untargeted antioxidants, the most common of which is NAC. The administration of NAC, which can replenish glutathione levels and scavenge ROS, has been tested in multiple lung injury and sepsis trials (18–21). Investigators have generally been successful in increasing glutathione levels in blood, within various cell types, and in bronchoalveolar fluid (19–21). The studies have been unable to demonstrate consistent salutary clinical or physiologic effects and have shown no improvement in mortality (19–21).
A second strategy to combat oxidative stress has been to restore endogenous antioxidants by supplementing vitamins A, C, and E or selenium, alone or in various combinations (22–24). This approach, sometimes referred to as “immune nutrition,” has been studied in various trial designs, including randomized placebo-controlled studies, with inconsistent results. The reasons for failing to demonstrate clinical benefit are unclear but have been speculated to include dose, timing, and route of antioxidant administration and enrollment of insufficient numbers of patients. With this in mind, Heyland and colleagues designed and performed an appropriately powered, multicenter clinical trial in which they tested the efficacy of intravenous and enteral antioxidants (i.e., selenium, zinc, β-carotene, and vitamins E and C) and glutamine (a precursor for glutathione) in mechanically ventilated patients (2). The study had a factorial design and enrolled more than 1,200 patients. For this patient population, the mean APACHE II score was 26, and each patient had to have at least two organ failures. Supplementation was begun within 24 hours of ICU admission and was continued for 28 days or until death or discharge from the ICU. The authors reported that supplementation was associated with an increase in plasma glutamine and selenium levels but found no significant reduction in days of mechanical ventilation or mortality. In patients who received glutamine, there was a trend toward increased mortality at Day 28, which became statistically significant at Day 60. In patients who received antioxidants, there was no difference in mortality at Days 28 or 60. The authors concluded that antioxidant supplementation does not lead to improved outcomes and that glutamine supplementation may be associated with increased mortality.
Why Antioxidants May Not Have Worked
The failure of antioxidants in critical illness mirrors the experience in diseases such as diabetes, cancer, inflammatory disorders, and neurodegeneration (25). To reconcile the failure of antioxidants with their known role in the pathophysiology of many diseases, it is important to remember the role ROS play as signaling molecules for normal cellular function and adaptation to cellular stress. One such adaptation is in response to infection, which is both a cause and result of critical illness. We speculate that antioxidants might disrupt the normal signaling processes that control the usual response to severe infection. In support of this idea, there is growing evidence that NOX and mitochondrial-generated ROS are involved in optimal activation of lymphocytes and monocytes, which are important for an ideal response to infectious agents (26, 27). The activation of multiple Toll-like receptors, which are required for bacterial clearance, and the RIG-I–like receptors, which are essential for viral clearance, requires mitochondrial ROS production (28). The inflammasome recognizes a large range of pathogen-associated molecular patterns and damage-associated molecular patterns to induce proteolytic processing of the proinflammatory cytokines IL-1β and IL-18, which are crucial for host defense to pathogens. Recent studies indicate that mitochondrial ROS are essential for inflammasome activation (29). The activation and proliferation of T cells is also dependent on mitochondrial ROS (30). In many of these studies, the widely used antioxidant NAC has been shown to reduce the activation of signaling process involved in adaptive and innate immunity. Thus, an emerging idea is that ROS promote a normal immune response to infection and that antioxidants might interfere with these normal functions (Figure 2).
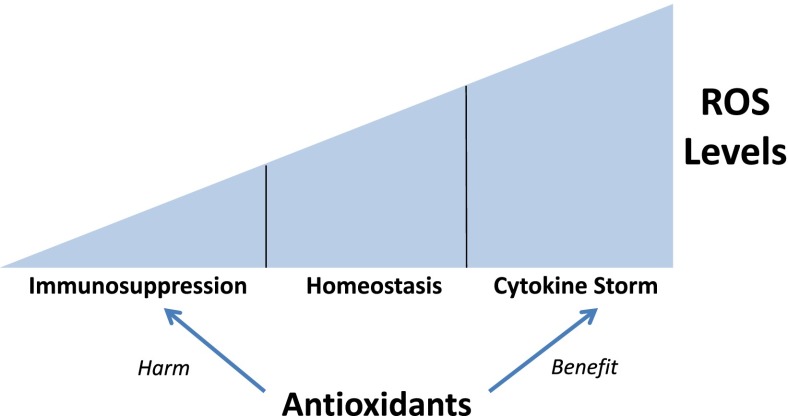
Figure 2.
Reactive oxygen species (ROS) levels dictate immune responses. The levels of ROS contribute to the physiological responses of inflammatory cells. High levels of intracellular ROS levels are associated with exaggerated inflammatory responses associated with cytokine storm, and relatively low ROS levels are associated with a hypoinflammatory response leading to immunosuppression. ROS levels in the intermediate range are associated with normal immune cell function. Therefore, the efficacy of antioxidants depends on ROS production within inflammatory cells.
The evolving understanding of ROS biology is reminiscent of the rethinking of the role of inflammation in critical illness. For many years, the prevailing thought was that the pathophysiology of sepsis related to uncontrolled inflammation (i.e., cytokine storm), leading to cardiovascular collapse, organ failure, and death. This has led over the past two decades to testing of multiple agents (e.g., tumor necrosis factor, IL-1 blockers, and corticosteroids) targeting inflammation in large clinical trials. No agent targeting cytokines or inflammation has shown benefit, and some have caused increased mortality. Some investigators have found that inflammation response profiles in septic patients are more complex than originally thought (31). Patients who survive the initial phase of sepsis, which may be marked by a cytokine storm, may develop a period of relative immunosuppression, during which they are at increased risk of nosocomial infection or viral reactivation. Alternatively, older patients or those with significant comorbidities may be in an immunosuppressed state at presentation, which may or may not persist during the course of their critical illness (31). Given the important role of ROS in activating lymphocytes and monocytes, we speculate that variability in ROS production in septic patients may contribute to the variability in inflammatory responses. If true, this complexity may have implications for the use of antioxidants in critical illness. We hypothesize that the use of antioxidants may be beneficial during periods of exaggerated inflammatory responses but may be detrimental during periods of relative immunosuppression. Thus, the efficacy of antioxidants may depend on an individual’s inflammatory response profile with timing and duration of antioxidant administration critical to demonstrating a salutary effect.
In summary, the biology of ROS has undergone an evolution in the past two decades from toxins that cause cellular damage to essential molecules regulating essential cellular signaling pathways. This conceptual shift should make us consider whether administering antioxidants could have beneficial and/or detrimental effects within the same patient at different time points. As a therapeutic intervention, individualizing dosing will likely be a crucial element in optimizing the potential of an antioxidant strategy.
Footnotes
This work was supported by National Institutes of Health grants 5P01HL071643 (N.S.C.) and by a VA Administration Merit Review (M.J.).
Originally Published in Press as DOI: 10.1164/rccm.201307-1380CP on October 11, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012;9:CD006616. doi: 10.1002/14651858.CD006616.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG. Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–1497. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 3.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee S. Cell signaling: H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 5.Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. 2012;5:pe10. doi: 10.1126/scisignal.2002943. [DOI] [PubMed] [Google Scholar]
- 6.Murphy MP. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 7.Brand M. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambeth JD. Nox enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 9.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso de Vega JM, Diaz J, Serrano E, Carbonell LF. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit Care Med. 2002;30:1782–1786. doi: 10.1097/00003246-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin SR, Simon RH, Grum CM, Ketai LH, Boxer LA, Devall LJ. Oxidant activity in expired breath of patients with adult respiratory distress syndrome. Lancet. 1986;1:11–14. doi: 10.1016/s0140-6736(86)91895-7. [DOI] [PubMed] [Google Scholar]
- 14.Sznajder JI, Fraiman A, Hall JB, Sanders W, Schmidt G, Crawford G, Nahum A, Factor P, Wood LD. Increased hydrogen peroxide in the expired breath of patients with acute hypoxemic respiratory failure. Chest. 1989;96:606–612. doi: 10.1378/chest.96.3.606. [DOI] [PubMed] [Google Scholar]
- 15.Bowler RP, Velsor LW, Duda B, Chan ED, Abraham E, Ware LB, Matthay MA, Day BJ. Pulmonary edema fluid antioxidants are depressed in acute lung injury. Crit Care Med. 2003;31:2309–2315. doi: 10.1097/01.CCM.0000085090.06078.8C. [DOI] [PubMed] [Google Scholar]
- 16.Cowley HC, Bacon PJ, Goode HF, Webster NR, Jones JG, Menon DK. Plasma antioxidant potential in severe sepsis: a comparison of survivors and nonsurvivors. Crit Care Med. 1996;24:1179–1183. doi: 10.1097/00003246-199607000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2004:CD004477. doi: 10.1002/14651858.CD004477.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rank N, Michel C, Haertel C, Lenhart A, Welte M, Meier-Hellmann A, Spies C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med. 2000;28:3799–3807. doi: 10.1097/00003246-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants n-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 20.Ortolani O, Conti A, De Gaudio AR, Masoni M, Novelli G. Protective effects of n-acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock. 2000;13:14–18. doi: 10.1097/00024382-200013010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Laurent T, Markert M, Feihl F, Schaller MD, Perret C. Oxidant-antioxidant balance in granulocytes during ARDS: effect of n-acetylcysteine. Chest. 1996;109:163–166. doi: 10.1378/chest.109.1.163. [DOI] [PubMed] [Google Scholar]
- 22.Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Galley HF, Howdle PD, Walker BE, Webster NR. The effects of intravenous antioxidants in patients with septic shock. Free Radic Biol Med. 1997;23:768–774. doi: 10.1016/s0891-5849(97)00059-2. [DOI] [PubMed] [Google Scholar]
- 24.Angstwurm MW, Schottdorf J, Schopohl J, Gaertner R. Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med. 1999;27:1807–1813. doi: 10.1097/00003246-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 26.Segal BH, Grimm MJ, Khan AN, Han W, Blackwell TS. Regulation of innate immunity by NADPH oxidase. Free Radic Biol Med. 2012;53:72–80. doi: 10.1016/j.freeradbiomed.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tschopp J, Schroder K. Nlrp3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 30.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. Mitochondria are required for antigen-specific t cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]