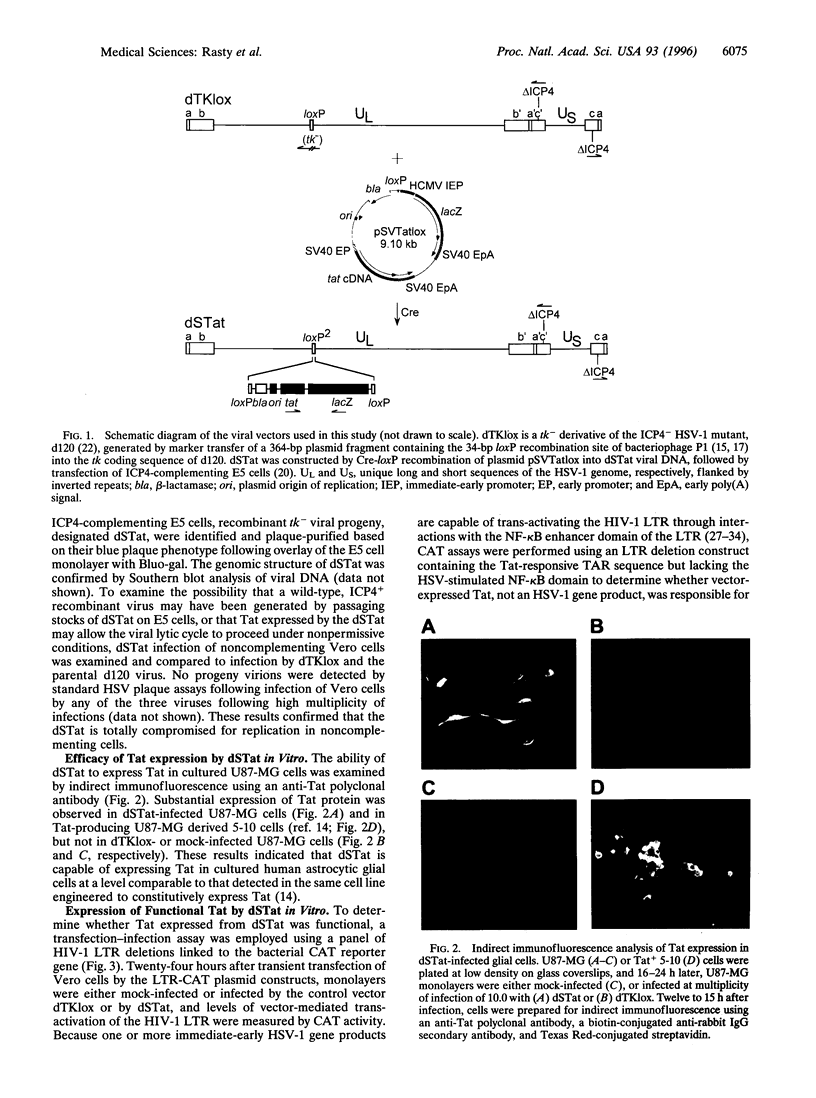
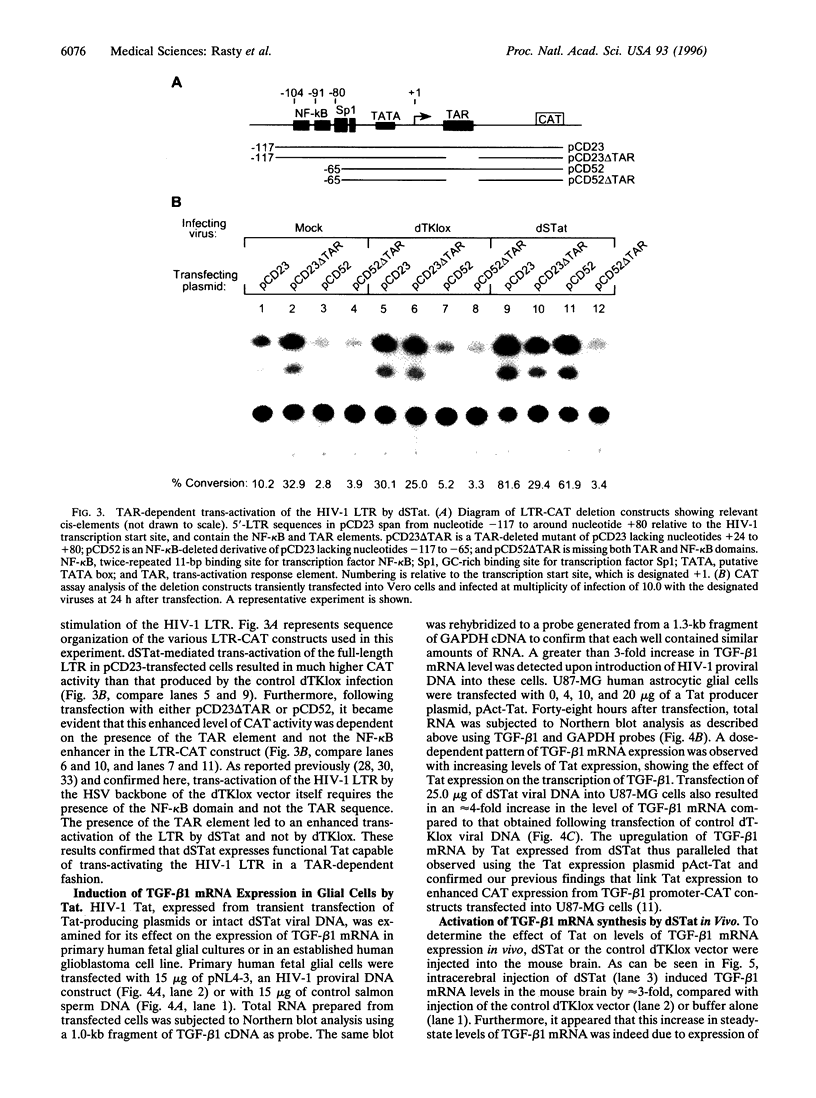
Abstract
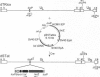
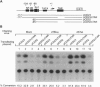
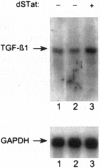
The high incidence of neurological disorders in patients afflicted with acquired immunodeficiency syndrome (AIDS) may result from human immunodeficiency virus type 1 (HIV-1) induction of chemotactic signals and cytokines within the brain by virus-encoded gene products. Transforming growth factor beta1 (TGF-beta1) is an immunomodulator and potent chemotactic molecule present at elevated levels in HIV-1-infected patients, and its expression may thus be induced by viral trans-activating proteins such as Tat. In this report, a replication-defective herpes simplex virus (HSV)-1 tat gene transfer vector, dSTat, was used to transiently express HIV-1 Tat in glial cells in culture and following intracerebral inoculation in mouse brain in order to directly determine whether Tat can increase TGF-beta1 mRNA expression. dSTat infection of Vero cells transiently transfected by a panel of HIV-1 long terminal repeat deletion mutants linked to the bacterial chloramphenicol acetyltransferase reporter gene demonstrated that vector-expressed Tat activated the long terminal repeat in a trans-activation response element-dependent fashion independent of the HSV-mediated induction of the HIV-1 enhancer, or NF-kappaB domain. Northern blot analysis of human astrocytic glial U87-MG cells transfected by dSTat vector DNA resulted in a substantial increase in steady-state levels of TGF-beta1 mRNA. Furthermore, intracerebral inoculation of dSTat followed by Northern blot analysis of whole mouse brain RNA revealed an increase in levels of TGF-beta1 mRNA similar to that observed in cultured glial cells transfected by dSTat DNA. These results provided direct in vivo evidence for the involvement of HIV-1 Tat in activation of TGF-beta1 gene expression in brain. Tat-mediated stimulation of TGF-beta1 expression suggests a novel pathway by which HIV-1 may alter the expression of cytokines in the central nervous system, potentially contributing to the development of AIDS-associated neurological disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Khalili K., Seshamma T., Taylor J. P., Pomerantz R. J. TAR-independent replication of human immunodeficiency virus type 1 in glial cells. J Virol. 1992 Dec;66(12):7522–7528. doi: 10.1128/jvi.66.12.7522-7528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L., Barillari G., Chang H. K., Bohan C. A., Kao V., Morgan R., Gallo R. C., Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992 Dec;66(12):7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse K. A., Cosentino L. M., Weih K. A., Pyle S. W., Robbins P. B., Hochstein H. D., Natarajan V., Farrar W. L. The HIV-1 gp120 envelope protein has the intrinsic capacity to stimulate monokine secretion. J Immunol. 1991 Nov 1;147(9):2892–2901. [PubMed] [Google Scholar]
- Cupp C., Taylor J. P., Khalili K., Amini S. Evidence for stimulation of the transforming growth factor beta 1 promoter by HIV-1 Tat in cells derived from CNS. Oncogene. 1993 Aug;8(8):2231–2236. [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 1987 Jun 11;15(11):4491–4511. doi: 10.1093/nar/15.11.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Gage P. J., Sauer B., Levine M., Glorioso J. C. A cell-free recombination system for site-specific integration of multigenic shuttle plasmids into the herpes simplex virus type 1 genome. J Virol. 1992 Sep;66(9):5509–5515. doi: 10.1128/jvi.66.9.5509-5515.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J. M., Duh E., Ostrove J. M., Gendelman H. E., Max E. E., Rabson A. B. Activation of the human immunodeficiency virus long terminal repeat by herpes simplex virus type 1 is associated with induction of a nuclear factor that binds to the NF-kappa B/core enhancer sequence. J Virol. 1988 Nov;62(11):4104–4112. doi: 10.1128/jvi.62.11.4104-4112.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M. P., Kim S., Hammer S. M., Ladd E. A., Schaffer P. A., DeLuca N., Albrecht M. A. Activation of human immunodeficiency virus by herpes simplex virus. J Infect Dis. 1992 Sep;166(3):494–499. doi: 10.1093/infdis/166.3.494. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hayman M., Arbuthnott G., Harkiss G., Brace H., Filippi P., Philippon V., Thomson D., Vigne R., Wright A. Neurotoxicity of peptide analogues of the transactivating protein tat from Maedi-Visna virus and human immunodeficiency virus. Neuroscience. 1993 Mar;53(1):1–6. doi: 10.1016/0306-4522(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Helland D. E., Welles J. L., Caputo A., Haseltine W. A. Transcellular transactivation by the human immunodeficiency virus type 1 tat protein. J Virol. 1991 Aug;65(8):4547–4549. doi: 10.1128/jvi.65.8.4547-4549.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekow J., Wachsman W., McCutchan J. A., Cronin M., Carson D. A., Lotz M. Transforming growth factor beta and noncytopathic mechanisms of immunodeficiency in human immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8321–8325. doi: 10.1073/pnas.87.21.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekow J., Wachsman W., McCutchan J. A., Gross W. L., Zachariah M., Carson D. A., Lotz M. Transforming growth factor-beta and suppression of humoral immune responses in HIV infection. J Clin Invest. 1991 Mar;87(3):1010–1016. doi: 10.1172/JCI115059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A. Human immunodeficiency virus-infected macrophages, gp120, and N-methyl-D-aspartate receptor-mediated neurotoxicity. Ann Neurol. 1993 Feb;33(2):227–228. doi: 10.1002/ana.410330218. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Sucher N. J., Kaiser P. K., Dreyer E. B. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991 Jul;7(1):111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- Magnuson D. S., Knudsen B. E., Geiger J. D., Brownstone R. M., Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995 Mar;37(3):373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- Marcuzzi A., Weinberger J., Weinberger O. K. Transcellular activation of the human immunodeficiency virus type 1 long terminal repeat in cocultured lymphocytes. J Virol. 1992 Jul;66(7):4228–4232. doi: 10.1128/jvi.66.7.4228-4232.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. E., Koyanagi Y., Chen I. S. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989 Oct;63(10):4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. E., Koyanagi Y., Zack J., Thomas L., Martin F., Chen I. S. Induction of interleukin-1 and tumor necrosis factor alpha in brain cultures by human immunodeficiency virus type 1. J Virol. 1992 Apr;66(4):2217–2225. doi: 10.1128/jvi.66.4.2217-2225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossmann M. C., Kossmann T., Wahl S. M. Cytokines and neuropathology. Trends Pharmacol Sci. 1992 Jul;13(7):286–291. doi: 10.1016/0165-6147(92)90087-m. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G. J., Rice S. A., Knipe D. M., Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988 Mar 11;239(4845):1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Leonard J., Weck K. E., Rabson A. B., Gendelman H. E. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3726–3732. doi: 10.1128/jvi.61.12.3726-3732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Sabatier J. M., Vives E., Mabrouk K., Benjouad A., Rochat H., Duval A., Hue B., Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991 Feb;65(2):961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Whealy M., Robbins A., Enquist L. Site-specific insertion of DNA into a pseudorabies virus vector. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9108–9112. doi: 10.1073/pnas.84.24.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Southgate C. D., Green M. R. The HIV-1 Tat protein activates transcription from an upstream DNA-binding site: implications for Tat function. Genes Dev. 1991 Dec;5(12B):2496–2507. doi: 10.1101/gad.5.12b.2496. [DOI] [PubMed] [Google Scholar]
- Taylor J. P., Cupp C., Diaz A., Chowdhury M., Khalili K., Jimenez S. A., Amini S. Activation of expression of genes coding for extracellular matrix proteins in Tat-producing glioblastoma cells. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9617–9621. doi: 10.1073/pnas.89.20.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. P., Kundu M., Khalili K. TAR-independent activation of HIV-1 requires the activation domain but not the RNA-binding domain of Tat. Virology. 1993 Aug;195(2):780–785. doi: 10.1006/viro.1993.1430. [DOI] [PubMed] [Google Scholar]
- Taylor J. P., Pomerantz R., Bagasra O., Chowdhury M., Rappaport J., Khalili K., Amini S. TAR-independent transactivation by Tat in cells derived from the CNS: a novel mechanism of HIV-1 gene regulation. EMBO J. 1992 Sep;11(9):3395–3403. doi: 10.1002/j.1460-2075.1992.tb05418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas S. M., Masliah E., Rockenstein E. M., Rall G. F., Abraham C. R., Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994 Jan 13;367(6459):188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Viscidi R. P., Mayur K., Lederman H. M., Frankel A. D. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science. 1989 Dec 22;246(4937):1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- Vlach J., Pitha P. M. Differential contribution of herpes simplex virus type 1 gene products and cellular factors to the activation of human immunodeficiency virus type 1 provirus. J Virol. 1993 Jul;67(7):4427–4431. doi: 10.1128/jvi.67.7.4427-4431.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlach J., Pitha P. M. Herpes simplex virus type 1-mediated induction of human immunodeficiency virus type 1 provirus correlates with binding of nuclear proteins to the NF-kappa B enhancer and leader sequence. J Virol. 1992 Jun;66(6):3616–3623. doi: 10.1128/jvi.66.6.3616-3623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Corcoran M. L., Pyle S. W., Arthur L. O., Harel-Bellan A., Farrar W. L. Human immunodeficiency virus glycoprotein (gp120) induction of monocyte arachidonic acid metabolites and interleukin 1. Proc Natl Acad Sci U S A. 1989 Jan;86(2):621–625. doi: 10.1073/pnas.86.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., McCartney-Francis N., Morganti-Kossmann M. C., Kossmann T., Ellingsworth L., Mai U. E., Mergenhagen S. E., Orenstein J. M. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991 Apr 1;173(4):981–991. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M., Ho D. D., Schooley R. T., Hirsch M. S., Richardson E. P., Jr Subacute encephalomyelitis of AIDS and its relation to HTLV-III infection. Neurology. 1987 Apr;37(4):562–569. doi: 10.1212/wnl.37.4.562. [DOI] [PubMed] [Google Scholar]