Abstract
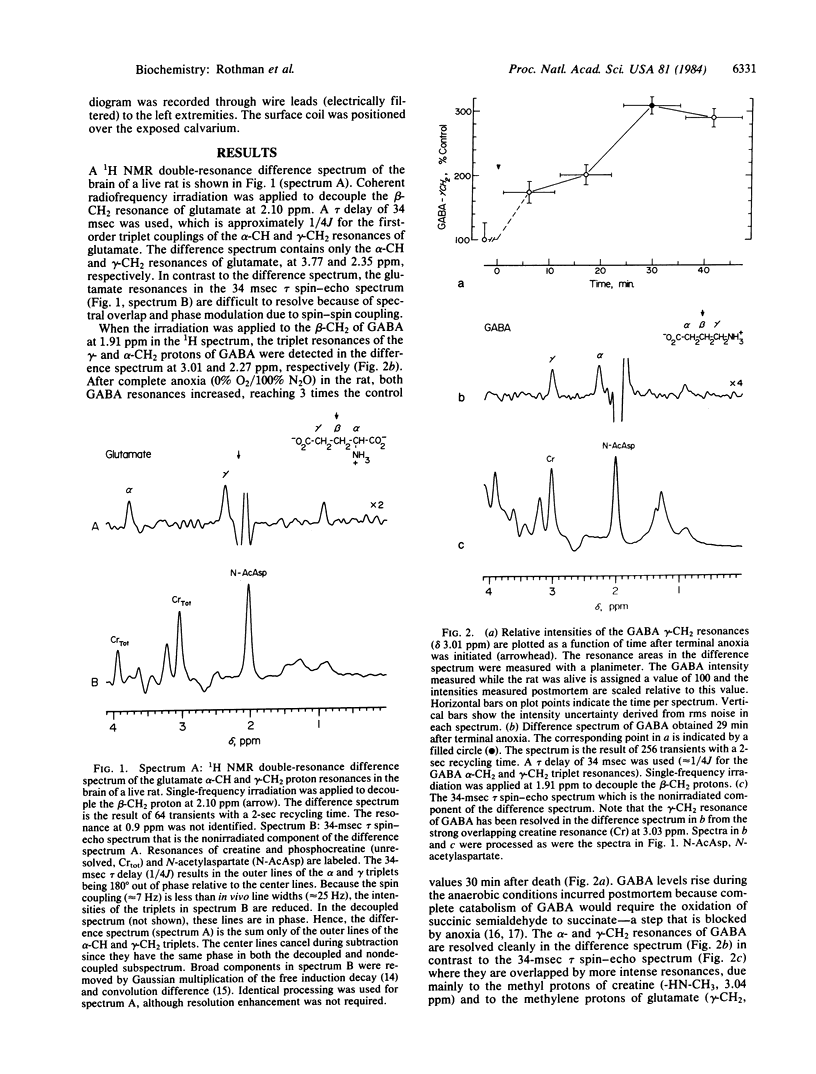
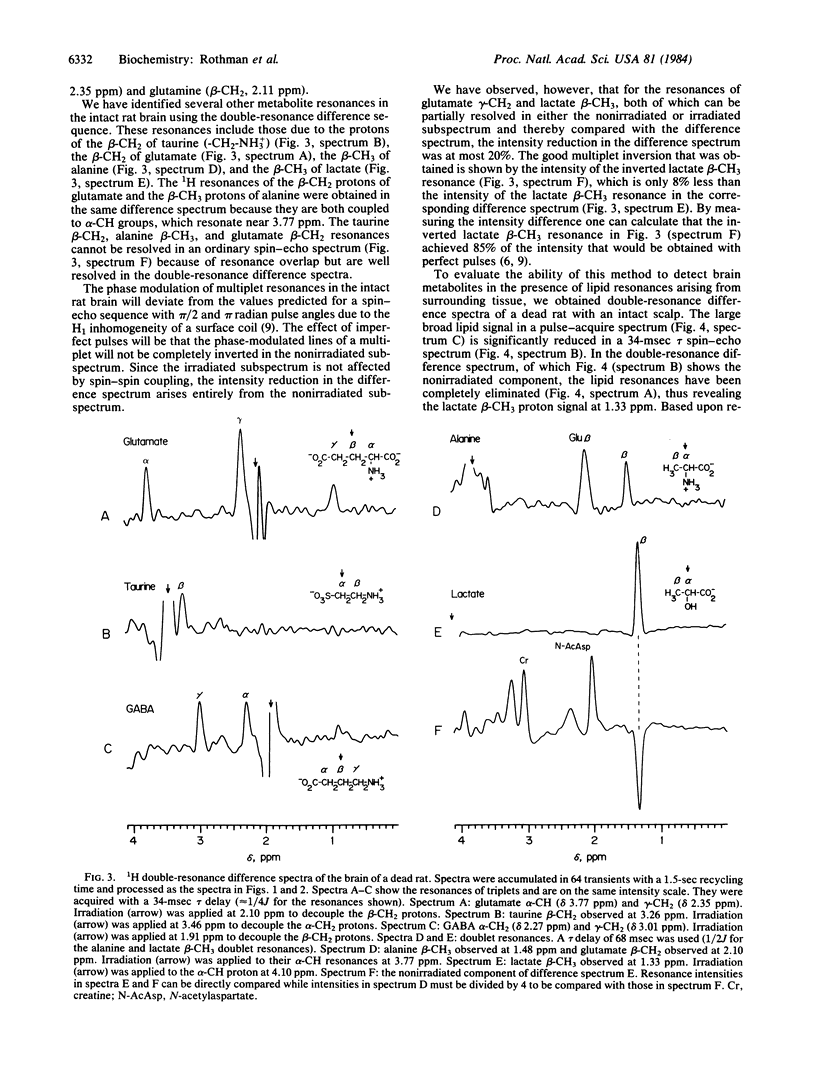
We have used 1H homonuclear double-resonance difference spectroscopy at 360.13 MHz to resolve specific metabolite resonances in the brains of intact rats. Metabolite resonances resolved include previously obscured proton resonances of alanine, gamma-aminobutyric acid (GABA), glutamate, and taurine. The gamma-aminobutyric acid alpha- and gamma-CH2 proton resonances were observed in the living rat in the difference spectrum obtained upon irradiation of the beta-CH2 proton resonance at 1.91 ppm. A 3-fold increase in the intensity of the alpha- and gamma-CH2 resonances of gamma-aminobutyric acid was observed 30 min after death. The alpha-CH and gamma-CH2 resonances of glutamate were also resolved in vivo by selective irradiation of the beta-CH2 protons to which they are spin-coupled. In addition, this technique was used to observe the beta-CH3 protons of lactate through the intact scalp of a rat. Large lipid signals arising from scalp tissue were eliminated in the difference spectrum, revealing the lactate beta-CH3 resonance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger J. R., Sillerud L. O., Behar K. L., Gillies R. J., Shulman R. G., Gordon R. E., Shae D., Hanley P. E. In vivo carbon-13 nuclear magnetic resonance studies of mammals. Science. 1981 Nov 6;214(4521):660–662. doi: 10.1126/science.7292005. [DOI] [PubMed] [Google Scholar]
- Behar K. L., Rothman D. L., Shulman R. G., Petroff O. A., Prichard J. W. Detection of cerebral lactate in vivo during hypoxemia by 1H NMR at relatively low field strengths (1.9 T). Proc Natl Acad Sci U S A. 1984 Apr;81(8):2517–2519. doi: 10.1073/pnas.81.8.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar K. L., den Hollander J. A., Stromski M. E., Ogino T., Shulman R. G., Petroff O. A., Prichard J. W. High-resolution 1H nuclear magnetic resonance study of cerebral hypoxia in vivo. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4945–4948. doi: 10.1073/pnas.80.16.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle K. M., Boyd J., Campbell I. D., Porteous R., Soffe N. Observation of carbon labelling in cell metabolites using proton spin echo NMR. Biochem Biophys Res Commun. 1982 Dec 15;109(3):864–871. doi: 10.1016/0006-291x(82)92020-4. [DOI] [PubMed] [Google Scholar]
- Brown F. F., Campbell I. D., Kuchel P. W., Rabenstein D. C. Human erythrocyte metabolism studies by 1H spin echo NMR. FEBS Lett. 1977 Oct 1;82(1):12–16. doi: 10.1016/0014-5793(77)80875-2. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M., Jeminet G., Williams R. J. Pulsed NMR methods for the observation and assignment of exchangeable hydrogens: application to bacitracin. FEBS Lett. 1974 Dec 1;49(1):115–119. doi: 10.1016/0014-5793(74)80645-9. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M., Williams R. J., Wright P. E. Pulse methods for the simplification of protein NMR spectra. FEBS Lett. 1975 Sep 1;57(1):96–99. doi: 10.1016/0014-5793(75)80160-8. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Dawson M. J., Wilkie D. R., Gordon R. E., Shaw D. Clinical use of nuclear magnetic resonance in the investigation of myopathy. Lancet. 1982 Mar 27;1(8274):725–731. doi: 10.1016/s0140-6736(82)92635-6. [DOI] [PubMed] [Google Scholar]
- LOVELL R. A., ELLIOTT S. J., ELLIOTT K. A. THE GAMMA-AMINOBUTYRIC ACID AND FACTOR I CONTENT OF BRAIN. J Neurochem. 1963 Jul;10:479–488. doi: 10.1111/j.1471-4159.1963.tb09850.x. [DOI] [PubMed] [Google Scholar]
- Ogino T., Arata Y., Fujiwara S. Proton correlation nuclear magnetic resonance study of metabolic regulations and pyruvate transport in anaerobic Escherichia coli cells. Biochemistry. 1980 Aug 5;19(16):3684–3691. doi: 10.1021/bi00557a008. [DOI] [PubMed] [Google Scholar]
- Shank R. P., Aprison M. H. Post mortem changes in the content and specific radioactivity of several amino acids in four areas of the rat brain. J Neurobiol. 1971;2(2):145–151. doi: 10.1002/neu.480020207. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Brown T. R., Ugurbil K., Ogawa S., Cohen S. M., den Hollander J. A. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979 Jul 13;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Seo Y., Nishikawa H. High-resolution proton magnetic resonance spectra of muscle. Biochim Biophys Acta. 1981 Dec 4;678(2):283–291. doi: 10.1016/0304-4165(81)90218-x. [DOI] [PubMed] [Google Scholar]


