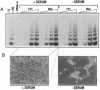
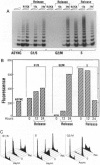
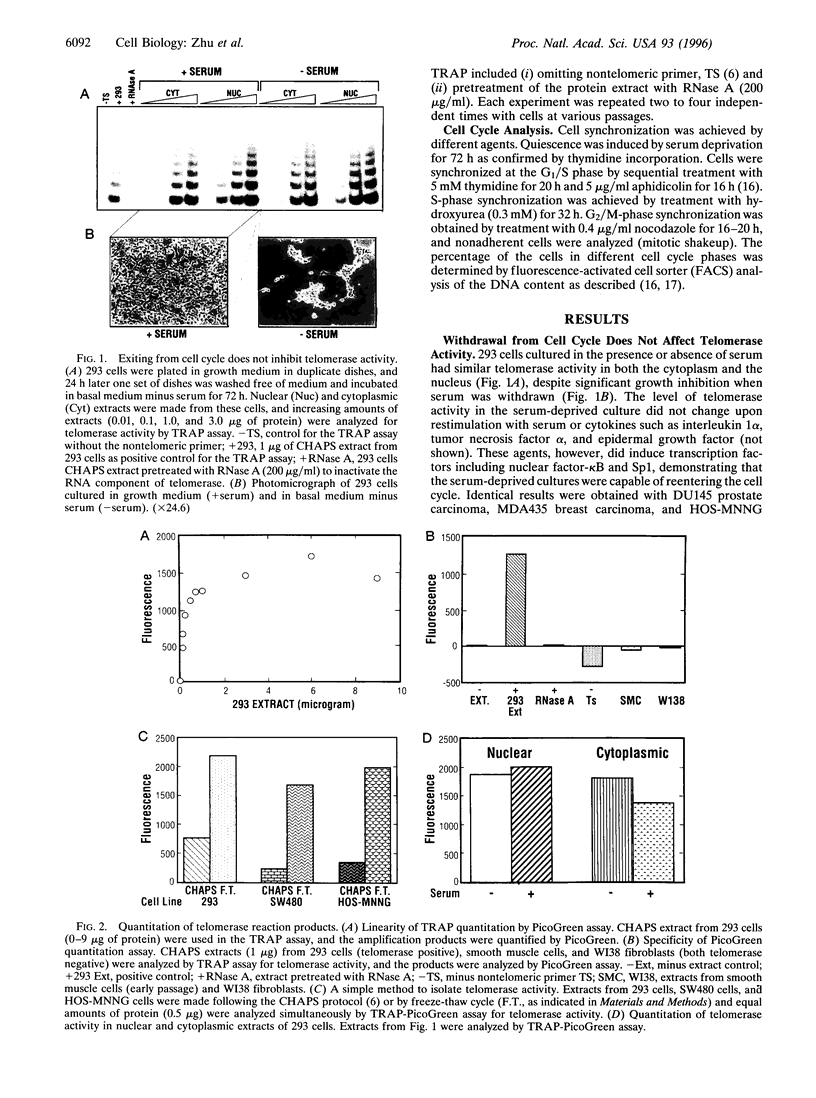
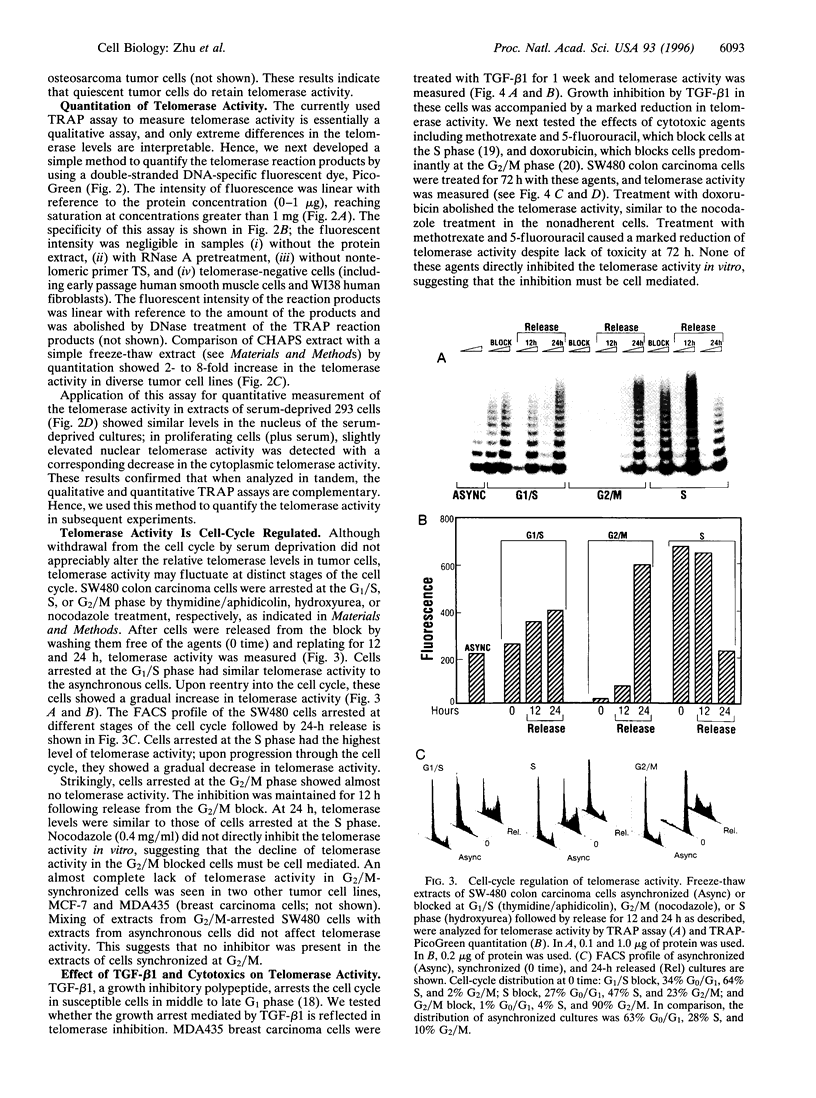
Abstract
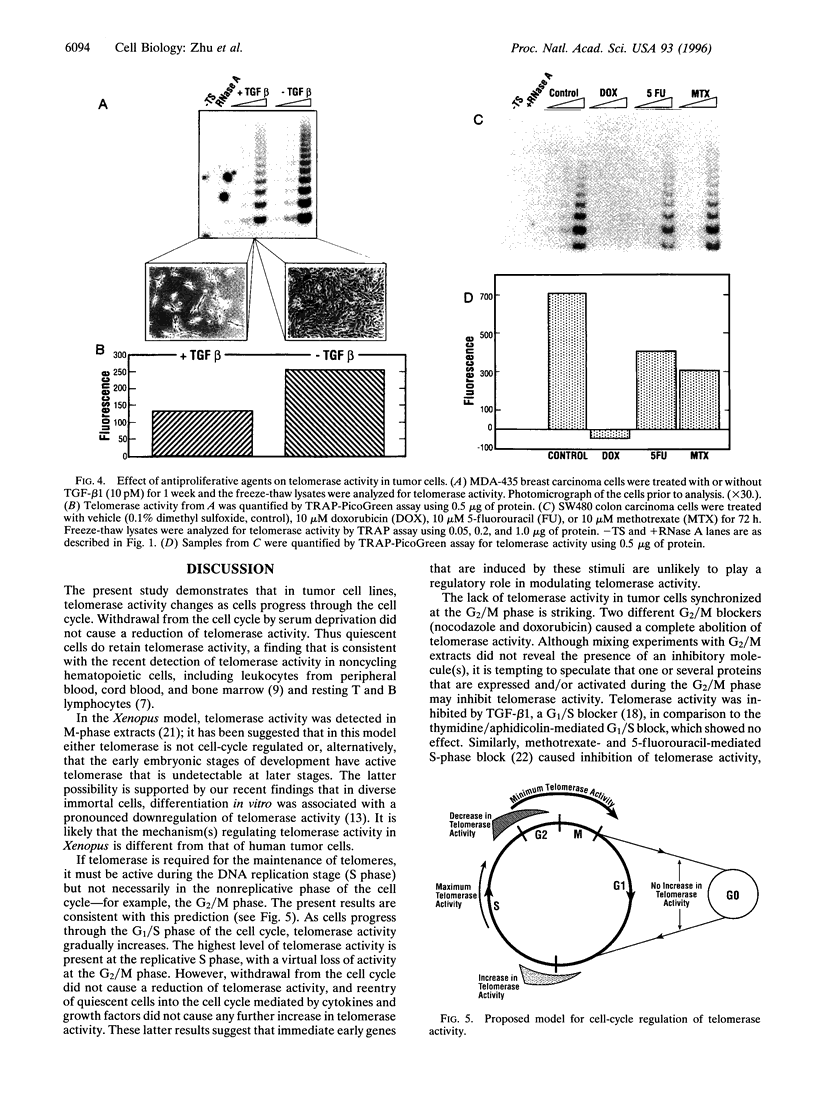
Telomerase is a ribonucleoprotein complex that is thought to add telomeric repeats onto the ends of chromosomes during the replicative phase of the cell cycle. We tested this hypothesis by arresting human tumor cell lines at different stages of the cell cycle. Induction of quiescence by serum deprivation did not affect telomerase activity. Cells arrested at the G1/S phase of the cell cycle showed similar levels of telomerase to asynchronous cultures; progression through the S phase was associated with increased telomerase activity. The highest level of telomerase activity was detected in S-phase cells. In contrast, cells arrested at G2/M phase of the cell cycle were almost devoid of telomerase activity. Diverse cell cycle blockers, including transforming growth factor beta1 and cytotoxic agents, also caused inhibition of telomerase activity. These results establish a direct link between telomerase activity and progression through the cell cycle.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Sedat J. W. Three-dimensional architecture of a polytene nucleus. Nature. 1983 Apr 21;302(5910):676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Broccoli D., Young J. W., de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan T. M., Englezou A., Gupta J., Bacchetti S., Reddel R. R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995 Sep 1;14(17):4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Gupta J., Harley C. B., Leber B., Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995 May 1;85(9):2315–2320. [PubMed] [Google Scholar]
- Fangman W. L., Brewer B. J. A question of time: replication origins of eukaryotic chromosomes. Cell. 1992 Oct 30;71(3):363–366. doi: 10.1016/0092-8674(92)90505-7. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Kastan M. B. Cell cycle control and cancer. Science. 1994 Dec 16;266(5192):1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Hicke B., Rempel R., Maller J., Swank R. A., Hamaguchi J. R., Bradbury E. M., Prescott D. M., Cech T. R. Phosphorylation of the Oxytricha telomere protein: possible cell cycle regulation. Nucleic Acids Res. 1995 Jun 11;23(11):1887–1893. doi: 10.1093/nar/23.11.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirama T., Koeffler H. P. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995 Aug 1;86(3):841–854. [PubMed] [Google Scholar]
- Hiraoka Y., Agard D. A., Sedat J. W. Temporal and spatial coordination of chromosome movement, spindle formation, and nuclear envelope breakdown during prometaphase in Drosophila melanogaster embryos. J Cell Biol. 1990 Dec;111(6 Pt 2):2815–2828. doi: 10.1083/jcb.111.6.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E., Hiyama K., Yokoyama T., Matsuura Y., Piatyszek M. A., Shay J. W. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat Med. 1995 Mar;1(3):249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- Hiyama K., Hiyama E., Ishioka S., Yamakido M., Inai K., Gazdar A. F., Piatyszek M. A., Shay J. W. Telomerase activity in small-cell and non-small-cell lung cancers. J Natl Cancer Inst. 1995 Jun 21;87(12):895–902. doi: 10.1093/jnci/87.12.895. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. Cell. 1991 Sep 20;66(6):1071–1074. doi: 10.1016/0092-8674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993 Apr 23;260(5107):536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- Kumar R., Atlas I. Interferon alpha induces the expression of retinoblastoma gene product in human Burkitt lymphoma Daudi cells: role in growth regulation. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6599–6603. doi: 10.1073/pnas.89.14.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Korutla L., Zhang K. Cell cycle-dependent modulation of alpha-interferon-inducible gene expression and activation of signaling components in Daudi cells. J Biol Chem. 1994 Oct 14;269(41):25437–25441. [PubMed] [Google Scholar]
- Kwok T. T., Mok C. H., Menton-Brennan L. Up-regulation of a mutant form of p53 by doxorubicin in human squamous carcinoma cells. Cancer Res. 1994 Jun 1;54(11):2834–2836. [PubMed] [Google Scholar]
- Mantell L. L., Greider C. W. Telomerase activity in germline and embryonic cells of Xenopus. EMBO J. 1994 Jul 1;13(13):3211–3217. doi: 10.1002/j.1460-2075.1994.tb06620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B. Is telomerase a universal cancer target? J Natl Cancer Inst. 1995 Jun 21;87(12):859–861. doi: 10.1093/jnci/87.12.859. [DOI] [PubMed] [Google Scholar]
- Petru E., Sevin B. U., Haas J., Ramos R., Perras J. A correlation of cell cycle perturbations with chemosensitivity in human ovarian cancer cells exposed to cytotoxic drugs in vitro. Gynecol Oncol. 1995 Jul;58(1):48–57. doi: 10.1006/gyno.1995.1182. [DOI] [PubMed] [Google Scholar]
- Sandell L. L., Zakian V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993 Nov 19;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Sharma H. W., Maltese J. Y., Zhu X., Kaiser H. E., Narayanan R. Telomeres, telomerase and cancer: is the magic bullet real? Anticancer Res. 1996 Jan-Feb;16(1):511–515. [PubMed] [Google Scholar]
- Sharma H. W., Sokoloski J. A., Perez J. R., Maltese J. Y., Sartorelli A. C., Stein C. A., Nichols G., Khaled Z., Telang N. T., Narayanan R. Differentiation of immortal cells inhibits telomerase activity. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12343–12346. doi: 10.1073/pnas.92.26.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloski J. A., Blair O. C., Carbone R., Sartorelli A. C. Induction of the differentiation of synchronized HL-60 leukemia cells by tiazofurin. Exp Cell Res. 1989 May;182(1):234–241. doi: 10.1016/0014-4827(89)90294-2. [DOI] [PubMed] [Google Scholar]
- Tahara H., Nakanishi T., Kitamoto M., Nakashio R., Shay J. W., Tahara E., Kajiyama G., Ide T. Telomerase activity in human liver tissues: comparison between chronic liver disease and hepatocellular carcinomas. Cancer Res. 1995 Jul 1;55(13):2734–2736. [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- de Lange T. Activation of telomerase in a human tumor. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2882–2885. doi: 10.1073/pnas.91.8.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. Human telomeres are attached to the nuclear matrix. EMBO J. 1992 Feb;11(2):717–724. doi: 10.1002/j.1460-2075.1992.tb05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]