Abstract
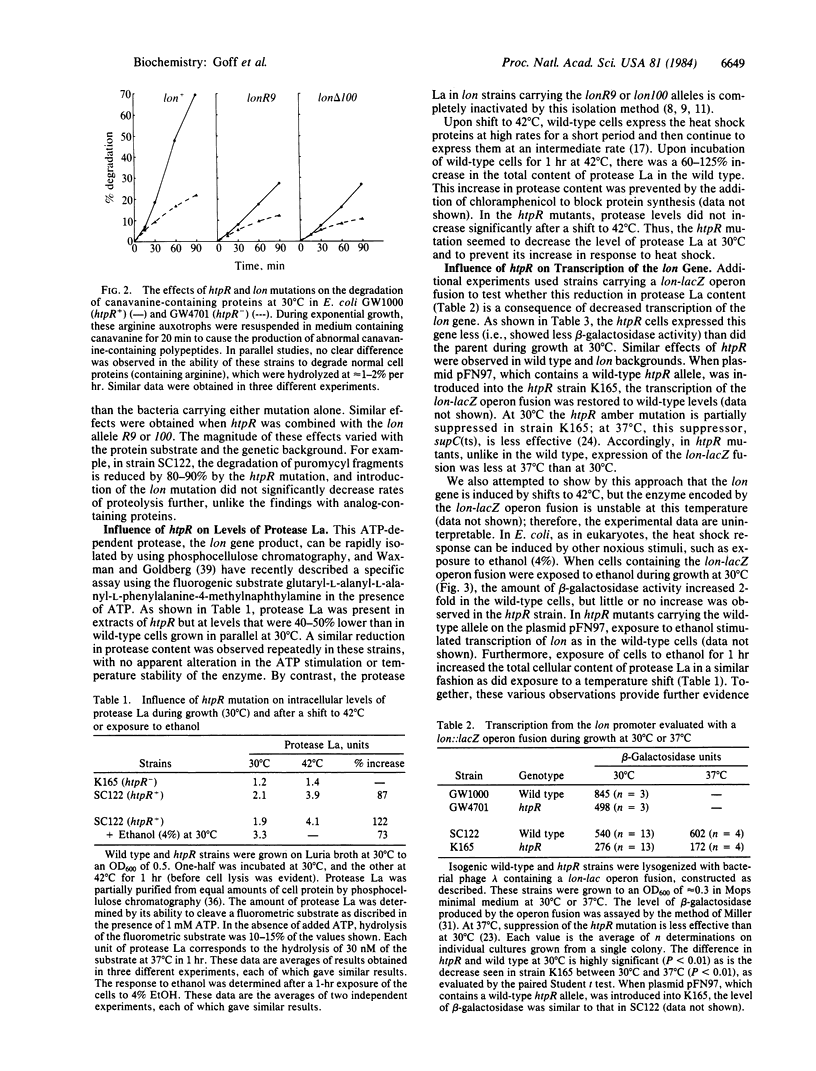
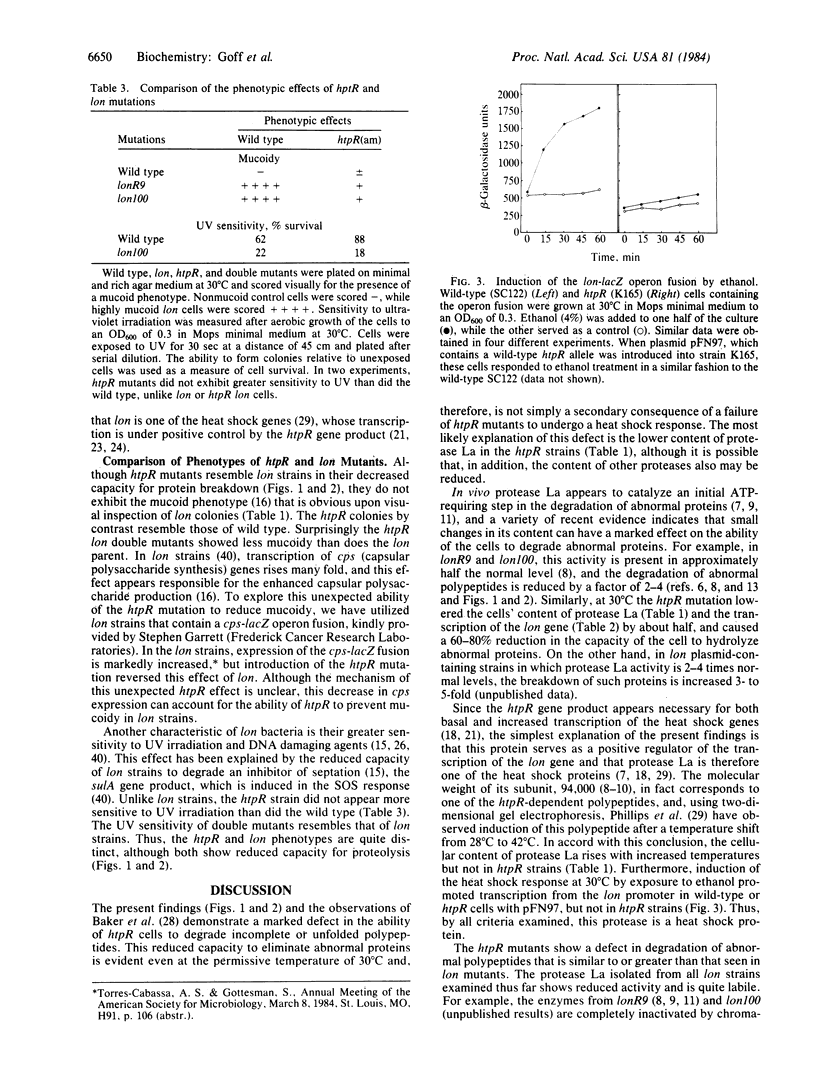
Upon a shift to high temperature, Escherichia coli increase their rate of protein degradation and also the expression of a set of "heat shock" genes. Nonsense mutants of htpR (also called hin), suppressed by a temperature-sensitive suppressor, show lower expression of heat shock genes at 30 degrees C and fail to respond to a shift to 42 degrees C. These mutants were found to have a lower capacity to degrade abnormal or incomplete proteins than that of wild-type cells. This reduction in proteolysis equals or exceeds that in lon mutants, which encode a defective ATP-dependent protease, protease La, and is particularly large in htpR lon double mutants. The activity of protease La was higher in wild-type cells than in htpR mutants grown at 30 degrees C and increased upon shift to 42 degrees C only in the wild type. To determine whether htpR influences transcription of the lon gene, a lon-lacZ operon fusion was utilized. Introduction of the htpR mutation reduced transcription from the lon promoter at 30 degrees C and 37 degrees C. This defect was corrected by a plasmid (pFN97) carrying the wild-type htpR allele. Induction of the heat shock response with ethanol had little or no effect in htpR mutants but stimulated lon transcription 2-3 fold in wild-type cells and htpR cells carrying pFN97. Thus, lon appears to be a heat shock gene, and increased synthesis of protease La under stressful conditions may help to prevent the accumulation of damaged cellular protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Kézdy F. J., Markovitz A. Molecular mechanism for dominance of a mutant allele of an ATP-dependent protease. J Mol Biol. 1982 Dec 5;162(2):503–510. doi: 10.1016/0022-2836(82)90541-1. [DOI] [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Markovitz A. ATP hydrolysis-dependent protease activity of the lon (capR) protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4728–4732. doi: 10.1073/pnas.78.8.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Goldberg A. L. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. H., Waxman L., Goldberg A. L. Studies of the protein encoded by the lon mutation, capR9, in Escherichia coli. A labile form of the ATP-dependent protease La that inhibits the wild type protease. J Biol Chem. 1983 Jan 10;258(1):215–221. [PubMed] [Google Scholar]
- Cooper S., Ruettinger T. A temperature sensitive nonsense mutation affecting the synthesis of a major protein of Escherichia coli K12. Mol Gen Genet. 1975 Aug 5;139(2):167–176. doi: 10.1007/BF00264696. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Hohn B., Hohn T. Activity of empty, headlike particles for packaging of DNA of bacteriophage lambda in vitro. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2372–2376. doi: 10.1073/pnas.71.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui I., Maltman K., Little R., Hastrup S., Johnsen M., Fiil N., Dennis P. Insertions of transposon Tn5 into ribosomal protein PNA polymerase operons. J Bacteriol. 1982 Dec;152(3):1022–1032. doi: 10.1128/jb.152.3.1022-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Goldberg A. L. Intermediate steps in the degradation of a specific abnormal protein in Escherichia coli. J Biol Chem. 1977 Dec 10;252(23):8350–8357. [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Lin S., Zabin I. Beta-galactosidase. Rates of synthesis and degradation of incomplete chains. J Biol Chem. 1972 Apr 10;247(7):2205–2211. [PubMed] [Google Scholar]
- Mizusawa S., Gottesman S. Protein degradation in Escherichia coli: the lon gene controls the stability of sulA protein. Proc Natl Acad Sci U S A. 1983 Jan;80(2):358–362. doi: 10.1073/pnas.80.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Lau E. T. Molecular cloning and expression of a gene that controls the high-temperature regulon of Escherichia coli. J Bacteriol. 1983 Feb;153(2):597–603. doi: 10.1128/jb.153.2.597-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Phillips T. A., VanBogelen R. A., Neidhardt F. C. lon gene product of Escherichia coli is a heat-shock protein. J Bacteriol. 1984 Jul;159(1):283–287. doi: 10.1128/jb.159.1.283-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman L., Goldberg A. L. Protease La from Escherichia coli hydrolyzes ATP and proteins in a linked fashion. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4883–4887. doi: 10.1073/pnas.79.16.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Temperature-induced synthesis of specific proteins in Escherichia coli: evidence for transcriptional control. J Bacteriol. 1980 Jun;142(3):843–851. doi: 10.1128/jb.142.3.843-851.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnbauer B. A., Markovitz A. Cloning of gene lon (capR) of Escherichia coli K-12 and identification of polypeptides specified by the cloned deoxyribonucleic acid fragment. J Bacteriol. 1980 Aug;143(2):852–863. doi: 10.1128/jb.143.2.852-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



