Summary
Adoptive T-cell transfer (ACT) is a potent and flexible cancer treatment modality that can induce complete, durable regression of certain human malignancies. Long-term follow up of patients receiving tumor-infiltrating lymphocytes (TILs) for metastatic melanoma reveals a substantial subset that experienced complete, lasting tumor regression – and may be cured. Increasing evidence points to mutated gene products as the primary immunological targets of TILs from melanomas. Recent technological advances permit rapid identification of the neoepitopes resulting from these somatic gene mutations and of T cells with reactivity against these targets. Isolation and adoptive transfer of these T cells may improve TIL therapy for melanoma and permit its broader application to non-melanoma tumors. Extension of ACT to other malignancies may also be possible through antigen receptor gene engineering. Tumor regression has been observed following transfer of T cells engineered to express chimeric antigen receptors against CD19 in B-cell malignancies or a T-cell receptor against NY-ESO-1 in synovial cell sarcoma and melanoma. Herein we review recent clinical trials of TILs and antigen receptor gene therapy for advanced cancers. We discuss lessons from this experience and consider how they might be applied to realize the full curative potential of ACT.
Keywords: immunotherapies, gene therapy, T cells, cancer, antigens, tumor immunity
Introduction
The highest goal of cancer therapy is to cure the patient, but decades of failure to reach this goal have contributed to a relatively low bar for therapeutic success in metastatic solid tumors. The primary treatment modality for metastatic cancer continues to be cytotoxic chemotherapy. Curative regimens for advanced stage germ cell tumors and choriocarcinoma were discovered more than 30 years ago. Countless efforts with systemic drug regimens have failed to achieve the same result in any other solid tumor. Newer biologic agents and molecularly targeted drugs have shown activity in cancers refractory to traditional chemotherapeutics, but they too do not appear to be curative and indeed are often minimally effective (1, 2). One emerging cancer treatment platform, however, has demonstrated curative potential and may have the capacity for broad application: adoptive cell transfer therapy (ACT). ACT, infusion of autologous tumor-specific T cells, harnesses the natural ability of T cells to specifically recognize and eliminate widespread target cells and directs it to the treatment of advanced-stage cancers.
The potential to cure metastatic solid tumors by manipulating T-cell responses was established in humans by interleukin-2 (IL-2) treatment for metastatic melanoma and renal cell carcinoma (1, 3–6). Response rates to high-dose bolus IL-2 are a modest 13 to 17%; however 4 to 9% of patients experience complete tumor regression, and these complete responses are generally durable (1, 3–6). In a study at the National Cancer Institute – Surgery Branch, 24 of 33 complete responses to high-dose IL-2 were ongoing with up to 25 years of follow up (1, 4, 5). Building on this success, ACT with tumor-infiltrating lymphocytes (TILs), T cells grown from resected metastatic tumor deposits, has resulted in high response rates and reproducible complete and durable responses in metastatic melanoma. In a pooled analysis of recent protocols, overall response rates and complete response rates for metastatic melanoma were around 50% and 20%, respectively (7). Ninety-five percent of complete responses are ongoing, all with at least five years of follow up (Table 1) (7). Although the term ‘cure’ must be used cautiously, a subset of patients receiving a single infusion of T cells indeed appear be cured by this approach.
Table 1.
Updated results of objective tumor responses by RECIST criteria in clinical trials of TILs for metastatic melanoma (7).
| Lymphoconditioning regimen | N | PR (%) (duration in months) |
CR (%) (duration in months) |
OR (%) |
|---|---|---|---|---|
| No TBI | 43 | 16 (37%) (84, 36, 29, 28, 14, 12, 11, 7, 7, 7, 7, 4, 4, 2, 2, 2) |
5 (12%) (108+, 106+, 105+, 93+, 88+) |
21 (49%) |
| 200 TBI | 25 | 8 (32%) (14, 9, 6, 6, 5, 4, 3, 3) |
5 (20%) (95+, 91+, 87+, 84+, 81+) |
13 (52%) |
| 1200 TBI | 25 | 8 (32%) (21, 13, 7, 6, 6, 5, 3, 2) |
10 (40%) (75+, 73+, 71+, 71+, 66+, 65+, 65+, 65+, 64+, 19) |
18 (72%) |
TBI = Total body irradiation, PR = Partial response, CR = Complete responses, OR = Overall responses (PR+CR)
The complete and durable responses to TIL therapy have established the principle that infusion of tumor-specific T cells can eradicate advanced cancer in humans. However, application of TIL therapy has been limited to melanoma. Ongoing efforts are focused not only on improving TIL therapy but also on extending TIL and other cellular therapies to diverse malignancies (Table 2). One promising strategy to expand the range of ACT is to administer T cells that have been genetically engineered to express tumor-specific antigen receptors. These receptors can be chimeric antigen receptors (CARs), which are antibody single-chain variable fragments joined with T-cell receptor (TCR) and T-cell costimulatory receptor signaling domains, which recognize cell-surface antigens in a non-major histocompatibility (MHC)-restricted manner; or they can be traditional αβ TCRs, which recognize epitopes of intracellular antigens presented by MHC molecules. Clinical responses following administration of genetically engineered cells have been observed in B-cell malignancies, melanoma, and synovial sarcoma, and trials in other types of cancer are ongoing.
Table 2.
Program for the application of adoptive T-cell therapy to a wide variety of human cancers
| Target | Receptor type | Cancers | Protocol status |
|---|---|---|---|
| Antigen receptor gene therapy | |||
| NY-ESO-1 | TCR | Epithelial malignancies and sarcoma | Accruing |
| CD19 | CAR | Lymphomas | Accruing |
| VEGFR2 | CAR | All cancers | Accruing |
| EGFRvIII | CAR | Glioblastoma | Accruing |
| Mesothelin | CAR | Pancreatic, ovarian, mesothelioma | Accruing |
| 2G-1 | TCR | Kidney | Closed |
| MART-1 | TCR | Melanoma | Closed |
| gp100 | TCR | Melanoma | Closed |
| CEA | TCR | Colorectal | Closed |
| MAGE-A3 (MHC I) | TCR | Epithelial malignancies | Closed |
| SSX-2 | TCR | Epithelial malignancies | In development |
| CSP4 (HMWAg) | CAR | Melanoma, breast, pancreatic | In development |
| HPV-16 E6 | TCR | Oropharyngeal, cervical, other anogenital | In development |
| MAGE-A3 (MHC II) | TCR | Epithelial malignancies | In development |
| TIL therapy | |||
| Cutaneous melanoma | |||
| Ocular melanoma | |||
| Gastrointestinal cancers | |||
| Human papillomavirus-positive cancers (uterine cervix, oropharynx, anus, etc.) | |||
In this review, we examine recent and ongoing clinical trials of TIL therapy for melanoma and other malignancies, and we look at the how the lessons from these trials are shaping the next generation of cellular therapies. We discuss emerging data testing genetically engineered T cells for the treatment of a variety of advanced-stage malignancies, discussing recent advances as well as ongoing challenges encountered with this approach. Finally, we look at how lessons from antigen receptor gene therapy clinical trials and our increasing understanding of T-cell biology can come together for continued progress in the field.
Adoptive T-cell therapy using tumor-infiltrating lymphocytes
Complete and durable response to TIL therapy
TIL therapy can induce long-lasting, complete regression of metastatic melanoma. A series of recent clinical trials in which TILs were administered following three different lymphoconditioning regimens resulted in objective clinical responses in 52/93 patients (56%) and complete responses in 20/93 patients (22%) (7). Of the complete responses, 19/20 (95%) were durable, i.e. ongoing after at 64 to 109 months of follow up (7) (Table 1). Other centers, too, have reported high response rates and long-lasting tumor regression following a single infusion of TILs for melanoma. Radvanyi et al. (8)r eported the MD Anderson Cancer Center experience with overall responses in 13/31 patients (42%). Two patients had complete responses, both ongoing at more than a year after treatment. Itzhaki et al. (9) described a clinical trial conducted in Israel with tumor responses in 15/31 patients (48%), four of which were complete, and all of which were ongoing at one to four years of follow up. Using low-dose IL-2 as an adjuvant after cell infusion, a group in Denmark reported complete responses in 2/6 patients, both ongoing (10). Thus, TIL therapy can induce complete and durable responses in metastatic melanoma, a finding that has been reproduced by at least four treatment centers in three countries. The challenge now is to improve TIL treatments for melanoma and to extend this promising platform to other types of cancer.
Improving and extending TIL therapy
Depleting negative regulatory cells
Strategies for improving TIL therapy have been suggested by mouse models, studies of human tissues, and testing in clinical trials. One focus of these studies has been immunosuppressive cells in the tumor microenvironment, which might be ablated or reprogrammed to improved ACT. These populations include the phenotypically heterogeneous myeloid-derived suppressor cells (MDSCs), which can acquire potent immunosuppressive traits in murine tumor models (11–13). Interestingly, myeloid cell subsets from human melanomas occur at the same frequency and possess the same phenotypes as those from peripheral blood; however, myeloid cells from peripheral blood but not tumor suppress T-cell proliferation, suggesting a role for circulating rather than tumor-resident myeloid cells in inhibiting T-cell responses (14). Another type of suppressive cell, CD4+CD25+FoxP3+ regulatory T (Treg) cells, has also been studied extensively in murine models and in patients with cancer. In mice, small numbers of Treg cells can abrogate effective CD8+ T-cell-mediated adoptive immunotherapy (15). Treg cells may be important in human cancer, as suggested by the selective accumulation of Treg cells in metastatic melanoma tumor deposits (16). Furthermore, Treg cells appear to have biological relevance in TIL therapy, as evinced by a negative correlation between levels of CD4+ FoxP3+ cells and clinical response in reconstituting patients treated with TILs (17). Notably, addition of total body irradiation (TBI) to a preparative regimen of cyclophosphamide and fludarabine is associated with decreased Treg reconstitution, suggesting a possible benefit to increased intensity lymphoconditioning (17). Another approach to reduce Treg cells is to administer CD8+ T cells only. A randomized selection trial compared standard ‘bulk’ TILs (a mixture of CD4+ and CD8+ T cells) to CD8-enriched TILs; 12/34 patients (35%) responded to standard TILs and 7/35 patients (20%) responded to CD8-enriched TILz, a difference that was not statistically significant. Interpretation of this result is confounded by removal of CD4+ T-helper (Th) cells as well as Treg cells from the cell product in the CD8-enriched group. Furthermore, recent studies indicate that the CD4+ CD25+ cells present in infused TILs are not Tregs (17). In the clinical trial of standard versus CD8-enriched TILs, response rates in both groups were lower than historical comparisons, possibly because TILs were generated from whole-tumor digests rather than multiple individually cultured tumor fragments. Outgrowth of TIsL from fragments rather than digests is theoretically advantageous, because the individual cultures can be tested for autologous tumor recognition and the most reactive cultures can be selected for administration (18). Taken together, these data suggest that Treg cells may be important in TIL therapy but that Treg cells from the reconstituting host rather than from the infused cell product may suppress anti-tumor responses.
Increased intensity lymphoconditioning
Increased intensity lymphoconditioning appears to impart a more lasting depletion of Treg cells, but it may have other benefits as well. In mice, lymphoconditioning not only reduces Treg cells but also increases levels of the homeostatic cytokines IL-7 and IL-15 (19, 20). This elevation of IL-7 and IL-15 was also observed following administration of three different lymphoconditioning regimens to patients receiving TIL therapy and was greater in patients receiving chemotherapy combined with 12 Gy TBI than in those receiving chemotherapy without TBI (21). In mice, escalation from non-myleoablative to myeloablative (with autologous hematopoietic stem cell rescue) conditioning improves the efficacy of transferred anti-tumor CD8+ T cells, through a HSC-mediated mechanism (19). Furthermore, in mice, increased doses of pre-treatment TBI correlate with enhanced tumor regression (22). Studies in humans receiving TIL therapy for metastatic melanoma also suggest a benefit to increased intensity lymphoconditioning. In three sequential non-randomized trials, overall response rates for patients pre-treated with chemotherapy, chemotherapy plus 2 Gy TBI, or chemotherapy plus 12 Gy TBI were 49%, 52%, and 72%, respectively (7). Complete response rates for the same regimens were 12%, 20%, and 40%, respectively. Thus, more intensive preparative regimens may enhance tumor responses, but patient numbers were small in these non-randomized trials. A randomized trial to test this hypothesis is presently accruing at the Surgery Branch, National Cancer Institute (NCI).
Harnessing innate immunity
One of the manifold mechanisms by which lymphoconditioning enhances ACT may be through activation of innate immune cells. Studies in mice indicate that TBI induces microbial translocation from the gut triggering, through lipopolysaccharide ligation of Toll-like receptor 4, dendritic cell activation, and dendritic cell-mediated stimulation of adoptively transferred anti-tumor CD8+ T cells (23). Similarly, in a murine model, bone marrow-derived tumor stromal cells, including CD11b+ F4/80hi macrophages, CD11b+ MHCIIhi CD11chi dendritic cells, and CD11b+ Gr-1hi myeloid derived suppressor cells can be stimulated through local delivery of IL-12 by CD8+ T cells genetically engineered to secrete a single-chain IL-12 molecule (24). These activated antigen-presenting cells (APCs) can stimulate the anti-tumor IL-12-secreting CD8+ T cells, thereby improving their anti-tumor activity. The mechanism for this increased efficacy appears to involve APC upregulation of Fas and reverse signaling through Fas ligand expressed by CD8+ T cells (25). In humans, systemic administration of IL-12 can cause severe toxicity, so strategies for direct delivery of the cytokine in the tumor environment are crucial (24, 26). One strategy is to transduce tumor-specific T cells with a vector encoding nuclear factor of activated T cells (NFAT)-inducible single chain IL-12. This vector design links IL-12 transcription to TCR signaling (27). A phase II clinical trial testing this strategy with small numbers of adoptively transferred, IL-12-transduced TILs (without IL-2 administration) is presently active in the Surgery Branch, NCI.
Another approach to harnessing the ability of APCs to enhance anti-tumor T-cell responses is to combine TIL therapy with small molecule anti-cancer agents that induce tumor apoptosis. The rationale for this strategy is that tumor antigens from dying malignant cells might be taken up by APCs in the tumor and tumor-draining lymph nodes, and processed and presented to adoptively transferred TILs, thereby increasing the activation and anti-tumor activity of these cells. A conspicuous candidate for combination therapy is vemurafenib, a small molecule with high activity in melanomas harboring BRAF V600E mutations (28–30). Vemurafenib has been reported to increase expression of melanocyte differentiation antigens by melanomas thereby increasing T-cell recognition of these antigens (31). In metastatic melanoma, it has high response rates (about 50%) but modest progression-free survival benefit (median of 3.7 months compared with dacarbazine), and complete responses are rare (1–6%) (28, 29). The combination of vemurafenib with TILs might increase the frequency of complete tumor responses and lead to more durable disease regression.
Predictive biomarkers
Another tactic for improving TIL therapy is to identify biological correlates of responses, either TILs or tumor characteristics, that might be used not only to select the most efficacious cells for administration but also to identify the patients most likely to respond to treatment (and spare those least likely to respond the risks of cell administration). However, identification of predictive biomarkers has been challenging and confounded by difficulty identifying both the true tumor regression antigens and the T cells against these antigens. Initial indications of which traits distinguish effective from ineffective cells have come from mouse models of ACT. In these models there is a, perhaps counterintuitive, inverse relationship between T-cell acquisition of effector function in vitro and ability to mediate tumor regression in vivo (32). The loss of anti-tumor efficacy that is coupled to differentiation into a more effector-like cell is linked to diminished proliferative potential, and measures of proliferative potential have been applied to human cells in an effort to identify correlates of clinical response to TILs. The capacity of T cells to replicate is governed, in part, by the length of their telomeres. Telomeres are specialized DNA-protein structures at the ends of chromosomes that undergo progressive shortening with cell division. Telomere length of TILs correlates positively with clinical response to therapy (21, 33). Another marker of T-cell differentiation state, CD27, has also been examined in studies of TIL therapy. CD27 is expressed by naive and memory T cells but downregulated in late stage effector T cells (34–37). Higher numbers of infused CD8+CD27+ T cells are positively associated with clinical response to treatment with TILs (38). These observations point to proliferative potential as a determinant of effective ACT in humans; however, there is considerable overlap in both telomere length and CD27 expression among responding and non-responding patients, which precludes use of these markers to select or exclude patients from treatment.
Tumor regression antigens in melanoma
Study of T-cell traits that predict response to therapy is complicated by the challenge of identifying from heterogeneous, polyclonal TIL cultures the T cells that have specificity for tumor antigens and determining which of these cells are responsible for tumor regression. Recent observations have shed light on the tumor antigens recognized by TILs and the likely specificities of the T cells mediating tumor destruction. Early studies identified melanoma TILs and other T cells with reactivity against the shared tumor/self-melanocyte differentiation antigens (MDAs) (e.g. gp100, melanoma antigen recognized by T cells 1 (MART1), tyrosinase, tyrosinase-related protein 1 (TYRP1) and TYRP2 (39–42). MDAs are expressed by normal melanocytes in tissues such as skin and retina as well as by melanomas. TIL therapy rarely induces autoimmune toxicities against these normal tissues (e.g. vitiligo or vision loss) even in patients who experience profound tumor regression, and the frequency of MDA-specific T cells in bulk TIL populations is generally low (21, 43). Conversely, TCR gene therapies that specifically and potently target MDAs consistently induce autoimmune adverse events and rarely mediate tumor regression (as discussed below) (44). Thus, T cells recognizing MDAs appear not to account for the major anti-tumor effects of TILs.
Increasing evidence points instead to mutated gene products as the targets of melanoma TILs and the primary cancer regression antigens in melanoma. Melanomas are characterized by high numbers of mutational events (45–54). These mutations result in neoepitopes that can be recognized by TILs, and T cells with specificity for these neoepitopes can constitute the dominant tumor-reactive populations in TILs (46–48, 50, 51, 55, 56). T-cell affinity for mutated epitopes is not limited by thymic negative selection as it is for self-antigens such as MDAs; TIL clones against mutated gene products routinely display high target antigen avidity and robust recognition of autologous tumor lines (55, 56). Although TILs can be raised from many cancers, only those from melanomas consistently possess specific reactivity against the tumors from which they were generated, and melanoma is the only cancer for which TIL have shown clinical activity (57, 58). It seems likely, therefore, that the immunogenicity of melanoma is linked to the high frequency of mutational events in this cancer and that T cells specific for mutated gene products are responsible for tumor regression in patients receiving TIL therapy.
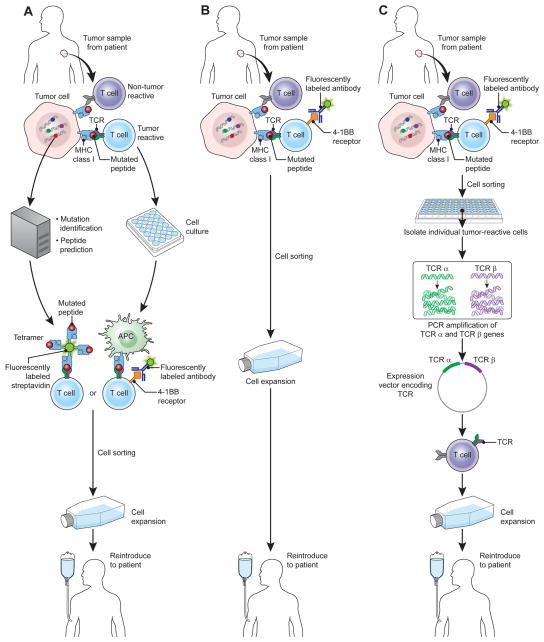
Targeting of the mutational epitopes of melanoma with antigen receptor gene therapy is difficult because the mutations, while frequent, are rarely shared between patients. An exception is the Braf mutation shared by about half of melanomas, although it has thus far not been possible to generate T cells targeting this mutation. It may, however, be possible to target other mutated antigens by selecting T cells against them from TILs or peripheral blood (59). Isolation of T cells against mutated epitopes might be accomplished on a patient-to-patient basis (Fig. 1). With increasingly reliable and inexpensive DNA sequencing technology, the expressed genes of a tumor might be sequenced to identify mutations. Mutated epitopes could be predicted using protein processing and MHC-binding prediction algorithms. Predicted peptides could then be used to identify T cells from TILs or possibly peripheral blood, a technique has been employed successfully to identify the targets of tumor-reactive TILs from bulk populations (56). Isolation of the T cells might be accomplished with cell sorting based on binding to labeled MHC-peptide complexes or by stimulation with peptides followed by selection based on T-cell activation markers [i.e. 4-1BB, CD40L, programmed death-1 (PD-1)] (60–63). Alternatively, tumor-reactive T cells might be isolated directly from tumor digests using markers of T-cell activation, a strategy that would shorten the timeline for producing TIL cell products and that might enable extension of this approach to other types of cancers that have rare but avid tumor-specific populations in their TILs. Finally, as single cell polymerase chain reaction (PCR) technology and gene transfer systems evolve, it may be possible to isolate individual tumor-reactive cells, sequence their TCRs, construct DNA vectors for gene transfer, and generate TCR-genetically engineered T cells on a patient-to-patient basis.
Figure 1.
Extension of TILs to non-melanoma cancers
For some epithelial malignancies, tumor infiltration by CD8+ T cells has been linked to improved clinical outcomes suggesting a potential therapeutic role for T cells (64, 65). TILe can be grown from varied cancers, but, with the exception of melanoma, they rarely demonstrate specific cytolysis of the tumors from which they were generated. In one study, TILs from 43/115 melanomas displayed preferential killing of autologous tumor targets over other cell lines and tumor digests (57). In the same study, TILs from only 2/12 kidney cancers, 0/10 breast cancers, and 0/10 colon cancers displayed specificity for autologous tumor. However, using more sensitive techniques based on upregulation of costimulatory molecules by reactive T cells, rare populations of TILs with tumor specificity have been identified in metastatic gastrointestinal tract adenocarcinomas (personal correspondence, Dr. Simon Turcotte). Study of these cells might provide insight into new tumor antigens, and it may be possible to isolate these low-frequency T-cell populations for therapeutic use through cell sorting techniques.
Testing of TIL therapy has been limited to few types of cancer other than melanoma (57). Other cancers might be rational targets for TIL therapy because of their expression of potentially immunogenic mutational epitopes or even foreign proteins. Lung carcinomas, specifically those in smokers, demonstrate a high frequency of somatic mutations (66). Furthermore, the sensitivity of these malignancies to treatment with T-cell checkpoint blockade using anti- PD-1 monoclonal antibodies suggests that patients with these cancers harbor anti-tumor T cells that could potentially be isolated and used in adoptive immunotherapy (67). Head and neck cancer also is tobacco related, and it too displays frequent somatic mutations (68, 69). T-cell infiltration of these tumors is associated with improved clinical outcomes, suggesting that T cells may have a therapeutic role (70, 71). Head and neck cancers are also attractive targets for TIL therapy due to the association of oropharyngeal tumors with human papillomavirus (HPV) infection. In HPV-associated tumors, malignant transformation is driven by the actions of the high-risk HPV E6 and E7 oncoproteins, viral antigens that are constitutively expressed by malignant cells and contribute to their growth and survival, and therefore are attractive therapeutic targets (72). Although TIL responses against E6 and E7 have not been carefully studied in oropharyngeal cancers, they are common in cervical cancers (which are necessarily associated with high-risk HPV), indicating that these tumor antigens can be the targets of naturally occurring T cells (73, 74). As with head and neck cancers, cervical cancer prognosis is linked to tumor infiltration by T cells intimating a role for T cells in anti-tumor immunity against HPV-positive cancers (75, 76). In addition to oropharygneal and cervical cancers, malignancies of the anus, vulva, vagina, and penis are associated with HPV and therefore might be reasonable candidates for TIL therapy. Study of TILs for HPV+ cancers has the advantage that the tumor antigens, E6 and E7, are shared between patients facilitating identification of tumor-specific T cells from TILs and hence studies aiming to relate the characteristics of TILs to clinical outcomes (i.e predictive biomarkers). Furthermore, it may be possible to isolate HPV-specific T cells from TILs (or even peripheral blood) and adoptively transfer these cells for cancer treatment. A clinical protocol testing TIL therapy for metastatic HPV-positive cancers from all sites is presently accruing in the Surgery Branch, NCI.
Another family of virally associated cancers that might be targeted with TILs or other cellular therapies are induced by Epstein-Barr virus (EBV). EBV-related cancers include most Burkitt’s lymphoma, undifferentiated nasopharyngeal carcinoma (NPC), and lymphoproliferative disorders (LPD), and some Hodgkin’s disease (HD), non-Hodgkin’s lymphoma, gastrointestinal lymphoma, and gastric carcinoma (77–79). EBV expresses latency genes in transformed cells, and these viral proteins can be targeted by T cells (80). The potential for this strategy to succeed has been demonstrated most clearly in EBV-related LPD in transplant recipients. Heslop et al. (81) reported the long-term outcomes from 114 patients at three different centers administered EBV-specific T cells for the prevention or treatment of EBV-positive LPD following hematopoietic stem cell transplant (HSCT). The infused EBV-specific T cells were generated by in vitro stimulation of donor lymphocytes with EBV-lymphoblastoid cells and culture with interleukin-2. With median follow up of 10 years, LPD developed in 0/101 patients treated prophylactically, and remission occurred in 11/13 patients treated for active disease. A similar strategy has been employed successfully in a multicenter study mostly of solid organ transplant recipients with LPD (82). Partially MHC-matched donor lymphocytes were used, because donor blood was not available. Complete remission occurred in 14/33 patients; an additional 3 patients showed partial responses (overall response rate of 52% at 6 months). EBV-specific T cells have also demonstrated, in small studies, modest activity against NPC. In one study, partial responses occurred in 2/10 patients with stage IV NPC (83). In another study of stage III and stage IV NPC, 7/15 patients experienced objective responses, five of which were complete (although in two active disease was unconfirmed) (84). Finally, a clinical trial using the same approach in HD showed tumor responses in 3/11 patients, two complete and one partial (85). Thus, evidence from clinical trials for three different EBV-related cancers suggests that adoptive of EBV-specific T cells can mediate tumor regression in humans. These results might be improved upon by more advanced methods of isolating EBV-specific cells from peripheral blood or by adoptive transfer of TILs or genetically engineering of T cells with high affinity TCRs against EBV antigens.
Adoptive T-cell therapy using T cells transduced to express anti-tumor receptors
The potency of TIL therapy can be coupled with the flexibility to specifically target diverse tumor antigens through antigen receptor gene engineering with CARs or TCRs. CARs are constructed of antibody single-chain variable fragments joined with TCR and T-cell costimulatory receptor domains, and they can confer to T cells non-major histocompatibility (MHC)-restricted recognition of cell surface antigens. However, the unique strength of T-cell therapy may be in its ability to target intracellular antigens. TCR genetically engineered T cells can recognize intracellular tumor antigens through MHC-dependent presentation of epitopes generated by antigen processing. The primary limitation of TCR-based antigen recognition is MHC restriction; target cells must have intact antigen processing and presentation and the treatment population must share a MHC allele [e.g. human leukocyte antigen (HLA)-A2] (86). Nonetheless, TCR gene engineering permits targeting of a host of antigens that cannot be accessed by other means, and this added reach may be crucial to the increased application of cellular therapies (87).
CARs in clinical trials
CARs against wide-ranging tumor antigens have been tested in recent clinical trials, and the results of these studies have greatly expanded our understanding of the biology of these treatments and the important qualities of therapeutic targets for cellular therapies. Most of these trials have targeted overexpressed antigens, non-mutated self-proteins expressed more highly in malignant than normal tissues. Cell therapy against these antigens seeks to exploit a theoretical therapeutic window in which, due greater target expression by tumor, tumor regression might occur in the absence of intolerable autoimmune toxicities. One attractive antigen to target with this approach is carbonic anhydrase IX (CAIX). CAIX is strongly expressed in renal cell carcinoma (88–90). However, it is also present in normal tissues including liver, small intestine, and gastric mucosa (88–90). A study in which patients with metastatic renal cell carcinoma were given cycles of five daily, escalating doses of T cells transduced to express an anti-CAIX CAR was performed (91). Cells were administered without lymphoconditioning but with adjuvant subcutaneous interleukin-2. After four to five infusions, liver enzyme elevations occurred in all of the first three patients. This liver toxicity required cessation of treatment or reduction in cell dose for all patients, and one patient required corticosteroids. A liver biopsy from the first treated patient showed cholangitis with T-cell infiltration around the bile ducts, and CAIX expression by bile duct epithelial cells suggesting a T-cell mediated cholangitis likely caused by the transferred cells. An additional cohort of five patients was treated in a 3 × 3 phase I dose-escalation study design starting at 1 × 108 CAR T cells per dose. Two of these patients developed grade 3 liver enzyme abnormalities that required cessation of therapy and administration of corticosteroids (92). Biopsies from these patients again showed T-cell infiltration around bile ducts and CAIX expression by bile duct epithelial cells. None of the eight patients treated with CAR-transduced cells experienced tumor regression. A third cohort of patients was pretreated with anti-CAIX antibodies that block CAR-CAIX interactions. These patients did not experience hepatic adverse events (or tumor regression) providing additional evidence for direct, CAIX-specific, T-cell-mediated liver injury in the first two cohorts. Thus, targeting of CAIX with a CAR-expressing T cells resulted in CAR-mediated autoimmunity against normal tissues expressing the targeted antigen but did not cause tumor regression.
CAR T cells have also been tested in a clinical trial for cancers with amplification of the ERBB2 gene. ERBB2 encodes the receptor tyrosine-protein kinase erbB-2 (ERBB2) protein, an epidermal growth factor receptor family member overexpressed in common malignancies including subsets of breast, colon, ovarian, gastric, and kidney cancers, and melanoma. Trastuzumab, a monoclonal antibody against the extracellular domain of ERBB2 has clinical activity (alone or in combination with other agents) in metastatic breast cancers that overexpress the protein. However, cardiac and gastrointestinal toxicities occur with the drug, and ERBB2 is expressed by normal tissues including heart, lung, gastrointestinal tract, and kidney. A CAR with the single-chain Fv fragment from the trastuzumab antibody was created, and T cells transduced to express this receptor were used to treat a single patient with metastatic colon cancer (93). Following cell infusion, the patient rapidly developed respiratory distress and hemodynamic instability requiring intensive care unit admission, mechanical ventilation, and vasopressors, as well as corticosteroids to suppress the transferred cells. Elevated levels of interferon-γ (IFNγ), granulocyte macrophage-colony stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), IL-6, and IL-10 were detected in her serum four hours after cell administration. The patient died five days after treatment, apparently from the sequelae of cytokine release syndrome. On postmortem examination, the highest levels of CAR-transduced T cells were detected in her lungs and abdominal and mediastinal lymph nodes (higher than in tumor deposits). Given the presence of ERBB2 and anti-ERBB2 CAR cells in the lungs and the elevated cytokine levels shortly after cell infusion, it is most likely that the CAR-transduced T cells were activated by target antigen in the lungs triggering cytokine release syndrome. Important lessons can be taken from this single patient experience. First, some antigens that are safely targeted with antibodies may not be suitable for targeting with the more potent platform of adoptive T-cell transfer. Second, targeting of self-antigens with highly active CARs should be approached with a dose-escalation trial design. And finally, considered in combination with the results of CAIX-directed CAR-based treatment, targeting of antigens expressed by vital normal tissues must be approached with great caution.
The greatest experience and most promising results from gene therapy with CARs have been with CD19-based targeting of B-cell malignancies. CD19 is a B-lineage surface antigen that is expressed by certain lymphoid cancers as well as normal mature B cells, B-cell precursors, and plasma cells. Targeting of the B-cell surface antigen CD20 has been a successful strategy for antibody-based treatment of non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. B-cell depletion resulting from these treatments increases the risk of certain infections but is generally well tolerated. The first patient with a B-cell malignancy successfully treated using an anti-CD19 CAR was reported by Kochenderfer et al. (94). This patient with extensive lymphoma received two treatments and has an ongoing response four years later. In expanded experience with this protocol, six of eight patients with B-cell lymphoma or chronic lymphocytic leukemia (CLL) experienced clinical responses, one a complete response (94, 95). At the time of publication, three partial responses were ongoing with seven to 18 months follow up, and the complete response was ongoing with 15 months of follow up. Patients experienced prolonged B-cell depletion but the more difficult to manage toxicities were related to elevated serum cytokine levels and included hypotension, fevers, fatigue, renal failure, and obtundation. These toxicities were transient but required protocol amendments discontinuing adjuvant interleukin-2 and reducing the cell dose. Others have also observed tumor regression and cytokine-related toxicities following administration of anti-CD19 CARs. Kalos et al. (96, 97) reported treatment of three patients with CLL; all experienced objective tumor responses, and two were complete and ongoing at 10 and 11 months. Elevated serum cytokine levels and a febrile syndrome associated with dyspnea or hypotension was noted in two patients. One patient developed fevers, constitutional symptoms, and cardiac dysfunction requiring corticosteroid administration. All of these toxicities resolved over time. CARs against CD19 may also have activity in adult and pediatric acute lymphoblastic leukemia (ALL). In one study of adult ALL, two patients with bone marrow blasts and two with minimal residual disease (MRD) were treated with infusion of T cells transduced with an anti-CD19 CAR; all experienced MRD− status and went on to allo-HSCT (98). Elevated cytokine levels and cytokine-related toxicities were noted consistently, and two patients developed relative hypotension and mental status changes requiring high-dose corticosteroids. Two pediatric patients with ALL were also treated with CD19-CAR therapy (99). Complete disease remission occurred in both patients. One response was ongoing after 11 months. However the other lasted only two months and was associated with recurrence of CD19-negative disease. Both patients experienced B-cell aplasia and cytokine release syndrome; one required cytokine blockade with etanercept (anti-TNF-α) and tocilizumab (anti-IL-6) to reverse the cytokine-related toxicities. Thus, in early clinical studies, T-cell therapy with anti-CD19 CARs appears to have robust clinical activity against a variety of B-cell malignancies. Treatment is associated with transient but frequently severe adverse events related to elevated serum cytokine levels. However, given the significant morbidity and mortality of alternative treatments – including allogeneic HSCT – and of refractory lymphoma/leukemia, these transient toxicities may be justified, particularly in the setting of more aggressive cancers (i.e. diffuse large B-cell lymphoma).
Other cell surface antigens might be targeted with CAR therapy, but few are specific to tumors. Mesothelin is a cell surface glycoprotein that is highly expressed by mesothelioma and pancreatic and ovarian cancers (100, 101). It is also present in mesothelial cells of the pleura, pericardium, and peritoneum, as well as the basal cells of the trachea, tonsil epithelium, and Fallopian tubes (100). However, it is absent from the parenchyma of vital organs including heart, liver, lung, kidney, and nervous tissue (100). Mesothelin has been targeted with the SSP1 immunotoxin, which was well-tolerated and dose-limited only by pleuritic chest pain (100). A CAR based on the SS1 antibody is presently being studied in a phase I clinical trial with careful dose escalation in the Surgery Branch, NCI.
Another cell surface antigen that is a particularly attractive target is epidermal growth factor variant III (EGFRvIII). EGFR is a transmembrane tyrosine kinase receptor that when activated signals to promote cell proliferation, invasion, motility, and adhesion (102, 103). EGFR overexpression results in unregulated cell growth and malignant transformation and is implicated in the progression of various malignancies. The most common EGFR mutant is EGFRvIII, which contains an in-frame deletion of exons 2–7 resulting in a constitutively activated signaling protein (104). EGFRvIII appears to be absent from normal tissues but expressed by certain cancers including 24 to 67% of glioblastomas and 42% of head and neck squamous cell carcinomas (105–107). Morgan et al. (108) have described a CAR against EGFRvIII based on human monoclonal antibody 139 and utilizing CD28 and CD3-ζ signaling domains. Transduction with the gene encoding this receptor confers specific recognition of glioma stem cells to open repertoire T cells. A clinical trial of anti-EGFRvIII-CAR gene therapy for glioblastoma is presently active in the Surgery Branch, NCI.
There continues to be strong interest in targeting B-lineage malignancies with CARs against lineage-specific antigens. Haso et al. (109) have reported CD22 to be consistently expressed by B-lineage cells and precursor B-cell acute lymphoblastic leukemia, and they have assembled a CAR against this target for clinical testing. Carpenter et al. (110) have identified B-cell maturation antigen (BCMA) as a protein specific to plasma cells and multiple myeloma cells. Interestingly, by polymerase chain reaction (PCR)-based testing this antigen appeared to be expressed by important normal organs particularly in the gastrointestinal tract (110). However, careful immunohistochemistry (IHC) studies revealed that expression in these organs is isolated to plasma cells and plasmacytoid cells and not gastrointestinal epithelium (110). A CAR against BCMA has been constructed and a phase I clinical trial is planned. No cell surface antigen that is specific for malignant rather than normal B-lineage cells has been reported. However, certain cancer testis antigens (e.g. MAGEB2, MAGEA1, MAGEC1, SSX1, SSX2, and CTAG1B) appear to be expressed specifically by malignant cells and may be valuable targets for TCR gene therapy, particularly for multiple myeloma (111).
TCRs in clinical trials
The first report of tumor regression following administration of autologous T cells genetically engineered to express a receptor against a tumor antigen described the treatment of metastatic melanoma using a TCR against MART1 (112). Two of 15 patients experienced objective tumor responses, and both of these patients also displayed high-level engraftment of engineered T cells. Notably, none of the patients receiving these genetically engineered T cells developed autoimmune toxicities. However, detailed characterization of the TCR used in this trial, which was isolated from the TILs of a patient who had a tumor response to TIL therapy, revealed that it possessed intermediate affinity for its target epitope (MART127–35) as compared to a panel of other TCRs against the same peptide (one of which was isolated from the same patient’s TIL). A second-generation, TCR with greater affinity for MART127–35 was tested in a subsequent clinical trial (44). Six of twenty patients experienced partial tumor responses, three of which were ongoing at 16 or 17 months after treatment. Tumor regression occurred at diverse sites of disease including lung and brain. However, patients also displayed autoimmune T-cell-mediated destruction of normal melanocytes resulting in transient toxicities to skin, eyes, and ears. Sixteen of 20 patients manifested a skin rash, uveitis, hearing loss, or a combination of these adverse events. Similar clinical results were obtained with TCR gene therapy targeting another MDA, gp100 (44). A TCR against gp100154–162 was generated from a HLA-A2 transgenic mouse. The human epitope (KTWGQYWQVL) differs from the mouse epitope (KTWGKYWQVL) by a single amino acid. Therefore, central tolerance mechanisms do not limit the avidity of murine T cells against this target. A high-affinity murine TCR was identified and tested in a clinical trial for metastatic melanoma. Three of 16 patients demonstrated tumor responses, one a complete response that was ongoing after 14 months. Site of responding tumors included lung, brain, liver, spleen, and lymph nodes. Skin, eye, or ear toxicity – although mostly grade 1/2, transient, and responsive to local steroid therapies – occurred in 15/16 patients. The results of this series of clinical trials targeting the MDAs MART1 or gp100 illustrates the ability of TCR genetically engineered T cells to mediate tumor regression in humans. Furthermore, they demonstrate the capacity of transferred cells to traffic to widespread sites of target antigen and to mediate cytolysis of these cells. Finally, consistent with the findings of a mouse model, they show that adoptively transferred T cells against self-antigens can mediate autoimmunity as well as tumor regression and that autoimmune injury to rare but important populations of cells (i.e. melanocytes in the eyes and inner ears) can cause significant toxicities (113, 114).
Another tissue differentiation antigen than has been targeted with TCR gene therapy is carcinoembryonic antigen (CEA). CEA is an attractive therapeutic target because of its consistent and high levels of expression by epithelial cancers, most notably colorectal adenocarcinoma (115). It is expressed by normal epithelial cells throughout the gastrointestinal tract, but autoimmune toxicities have not been observed in clinical trials of antibodies or vaccines against this antigen (116–118). A TCR against CEA was generated by immunizing HLA-A2 transgenic mice with CEA691–699. The affinity of this receptor was further enhanced by a single amino acid substitution in the CDR3α (119, 120). Three patients were treated with small numbers of cells (2–4 × 108) (121). All three patient developed transient but dose-limiting colitis. One patient demonstrated a partial tumor response. The results of this trial again illustrate the potency of TCR gene therapy and its ability to break immunological tolerance to mediate autoimmunity that does not occur with weaker therapies such as cancer vaccines and antibodies. The objective tumor regression in a patient with colorectal cancer suggests that epithelial malignancies may be susceptible to T-cell-based approaches. However, the dose-limiting T-cell-mediated autoimmunity incurred by targeting a tissue differentiation antigen that is present in normal tissues again points to the importance of identifying targets with expression restricted to tumors or possibly to tumors and non-essential normal tissues.
One family of antigens with restricted tissue expression is the cancer-testis (CT) antigens. These proteins are expressed predominantly by germ cells rather than other normal adult tissues. They are, however, also activated in a significant subset of certain malignancies. Germ cells are protected from TCR-based T-cell recognition by the absence of MHC molecules. Hence, in T-cell therapy certain CT antigens can function as tumor specific proteins. Based on their tissue restriction patterns, CT antigens can be classified as testis-restricted, testis/brain-restricted, or testis-selective. Testis/brain-restricted CT antigens are not viable targets for ACT because of their expression in the brain. However, testis-restricted and some testis-selective CT antigens potentially can be targeted. The testis-restricted CT antigen, NY-ESO-1, is expressed by a subset of certain malignancies including melanoma, synovial sarcoma, non-small cell lung cancer, cholangiocarcinoma, and breast cancer (111). A TCR against NY-ESO-1157–165 was generated and dual amino acid substitutions in the CDR3α chain made to enhance affinity (120). Patients with metastatic melanoma or synovial cell sarcoma were treated with adoptively transferred cells expressing this TCR (122). Five of 11 patients with melanoma displayed objective tumor responses, two of which were complete and ongoing at 20 and 22 months after treatment. Four of six patients with synovial cell sarcoma also experienced tumor regression (all partial responses). No autoimmune toxicities occurred. This experience demonstrates the potential for ACT targeting a CT antigen to mediate tumor regression without inducing autoimmune toxicities to normal tissues.
Seeking to expand on the success of TCR gene therapy against NY-ESO-1, researchers have targeted another CT antigen, MAGEA3. MAGEA3 is a testis-selective CT antigen that is expressed in a variety of cancers and has been targeted with therapeutic cancer vaccines without clear clinical benefit or autoimmune toxicities (123–125). A high affinity TCR against MAGEA3112–120 was generated by peptide vaccination of an HLA-A2 transgenic mouse (126). Five of nine patients receiving autologous T cells transduced to express this receptor experienced tumor responses; two were ongoing after more than 12 months (one a complete response). However, four patients manifested severe neurological toxicities. Extensive studies of TCR cross-reactivity revealed recognition of a non-identical HLA-A2-restricted epitope present in MAGEA12. The combined analysis of real-time quantitative-polymerase chain reaction, Nanostring quantitation, and deep-sequencing indicated that MAGE-A12 (and possibly MAGEA9, which shares the targeted epitope with MAGEA3) were in normal brain tissue. This cross-reactivity against a non-identical epitope with low levels of expression in vital normal tissues suggests two potential hazards to consider in TCR gene therapy. First, targeting of epigenetically regulated human gene products, such as cancer testis antigens or lineage-specific antigens, leaves open the possibility of unexpected target expression by normal tissues. Therefore, in assessing potential targets sensitive techniques should be used to test for expression by vital normal tissues. The significance of low levels of expression may be difficult to interpret but should be viewed cautiously. Second, generation of TCRs generated in HLA-A2-transgeneic mice have not been selected in the thymus against the full repertoire of normal human proteins. Therefore, these TCRs can have high affinity for normal human proteins, and unintended cross-reactivity can occur. These receptors should be assessed for cross-reactivity, and their clinical testing should be approached cautiously.
The problem of potential cross-reactivity of TCRs isolated from T cells that were not subjected to thymic selection in a human is illustrated by second trial targeting MAGEA3 (127). This trial used an affinity-enhanced human TCR with four amino acid substitutions in CDR2α to target a HLA-A1-restricted epitope of MAGEA3. Two patients, one with melanoma and the other with multiple myeloma, received autologous T cells transduced to express the anti-MAGEA3 TCR. Both experienced fatal cardiac toxicity associated with myocardial T-cell infiltration and elevated levels of genetically engineered cells in myocardial tissue. Additional studies revealed recognition of beating cardiac myocytes by the affinity-enhanced anti-MAGEA3 TCR but not by the parental TCR. Furthermore, normal resting cardiac myocytes were not recognized by the affinity-enhanced TCR suggesting that expression of the target protein is context-dependent. Further studies revealed cross-reactivity of the MAGEA3 TCR against an epitope of titin, a striated muscle-specific protein expressed by actively beating rather than resting cardiac myocytes (127, 128). These findings demonstrate again the potential for unexpected, avid recognition of self-antigens by TCRs that were not subjected to thymic selection in humans. This cross-reactivity can be against apparently unrelated proteins, and expression of these proteins can be turned on and off making detection more difficult. Therefore, as with TCRs isolated from mice, receptors that have undergone CDR manipulations may have an increased risk of cross-reactivity and should be tested for recognition of unintended targets prior to initiating clinical trials.
Increasing T-cell potency
Identification of antigens that are restricted to tumors/non-essential tissues and isolation of highly avid and specific receptors against these targets is important for ongoing advances in adoptive immunotherapy. New discoveries that might increase the potency of therapeutic T cells are of limited utility in settings where the strength of the treatment is constrained by toxicities to normal tissues. Several ways to increase the proliferative potential and therapeutic efficacy of T cells for adoptive immunotherapy have been elucidated in animal models. One crucial finding is that the T-cell subset from which effector CD8+ T cells are derived influences their fate following infusion into host animals. Effector cells generated from effector memory (Tem) cells rapidly perish following infusion into macaques (129). In contrast, those generated from the central memory (Tcm) pool are capable of long-term persistence and prolonged function (129). Extending these findings, in a murine tumor model T cells derived from naive (Tn) rather than Tcm precursors display greater proliferative potential and cytokine responses as well as enhanced ability to mediate tumor regression (130). That effector CD8+ T cells generated from Tn rather than Tcm or Tem cells might be more effective for ACT is also suggested by a study of human T cells in which Tn-derived cells displayed higher expression of CD27 and longer telomeres, traits positively associated with tumor responses in studies of patients receiving TILs for metastatic melanoma (as discussed above) (37). Another T-cell subset of interest in adoptive immunotherapy is the memory stem T cell (Tscm) (36). In mouse models, these antigen-experienced cells have greater replicative capacity and anti-tumor efficacy than traditional memory T-cell subsets (131, 132). A final manner of generating more effective cells for ACT may be to induce their differentiation into functionally distinct T-cell subsets using polarizing cytokines. Type 17 T cells are distinguished by their expression of the transcriptional regulator retinoic acid–related orphan receptor γ thymus and by production of IL-17A and IL-17F. Both CD4+ and CD8+ T cells induced to differentiate into type 17 cells demonstrate enhanced capacity to mediate tumor regression in a mouse model of ACT (133, 134). Insights into the role of inducible costimulator (ICOS) and other signals in the polarization of human T cells to a type 17 phenotype is bringing these potentially highly effective cells closer to clinical application (135). Thus, an increased appreciation of the influence of T-cell ontogeny and subset plasticity is opening the way to more effective therapies. However, clinical model systems in which to test these principles are evolving more slowly, limited largely by the few targets with tumor-restricted expression and by the challenges in isolating high affinity receptors against these antigens.
Conclusions
ACT is a potent treatment platform capable of inducing complete and durable regression of widespread, chemotherapy refractory cancers. The greatest experience in solid tumors is with metastatic melanoma, where it increasingly appears that the primary target antigens are mutated gene products. Efforts to extend TIL therapy to other types of cancers are ongoing and may be facilitated by new technologies that permit rapid identification of candidate mutational epitopes and expedient isolation of T cells against these targets. Advances in gene transfer techniques have opened the door to targeting any tumor antigen for which an antibody or TCR gene sequence can be identified. In clinical trials, T cells engineered to express high affinity antigen receptors based on these antibodies or TCRs demonstrate the capacity to localize to essentially any site of antigen expression and mediate destruction of target tissues – both tumor and normal cells. The strength of this antigen targeting exceeds that observed with cancer vaccines and antibodies and has revealed autoimmune consequences of targeting self-antigens that have not been seen with weaker approaches. However, with carefully selected targets such as CD19 and NY-ESO-1 diverse types of cancer can be treated with this approach and the early results of small clinical trials are encouraging. In an era of many marginally effective cancer treatments, ACT is emerging as a potent and flexible treatment modality with increasingly broad application.
Acknowledgments
The research is funded by the NIH intramural research program.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4:127ps8–127ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much? Clin Cancer Res. 2010;16:5972–5980. doi: 10.1158/1078-0432.CCR-10-1277. [DOI] [PubMed] [Google Scholar]
- 3.Klapper JA, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith FO, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radvanyi LG, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18:6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itzhaki O, et al. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;34:212–220. doi: 10.1097/CJI.0b013e318209c94c. [DOI] [PubMed] [Google Scholar]
- 10.Ellebaek EI, et al. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med. 2012;10:169. doi: 10.1186/1479-5876-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallina G, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA. 1995;92:6254–6258. doi: 10.1073/pnas.92.14.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res. 2012;18:5212–5223. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antony PA, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmadzadeh M, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao X, et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–5696. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley ME, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected yumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31:2152–2159. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrzesinski C, et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrzesinski C, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulos CM, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerkar SP, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerkar SP, et al. Collapse of the tumor stroma is triggered by IL-12 induction of Fas. Mol Ther J Am Soc Gene Ther. 2013;21:1369–1377. doi: 10.1038/mt.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner H-J, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin’s disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 2004;11:81–91. doi: 10.1038/sj.cgt.7700664. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, et al. Evaluation of γ-retroviral vectors that mediate the inducible expression of IL-12 for clinical application. J Immunother. 2012;35:430–439. doi: 10.1097/CJI.0b013e31825898e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosman JA, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flaherty KT, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw C-NJ, Sloss CM, Ferrone CR, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 32.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesmann A, et al. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 35.Nolte MA, Arens R, van Os R, van Oosterwijk M, Hooibrink B, van Lier RAW, van Oers MHJ. Immune activation modulates hematopoiesis through interactions between CD27 and CD70. Nat Immunol. 2005;6:412–418. doi: 10.1038/ni1174. [DOI] [PubMed] [Google Scholar]
- 36.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinrichs CS, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang J, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg SA. The development of new cancer therapies based on the molecular identification of cancer regression antigens. Cancer J Sci Am. 1995;1:90–100. [PubMed] [Google Scholar]
- 40.Wang RF, Robbins PF, Kawakami Y, Kang XQ, Rosenberg SA. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J Exp Med. 1995;181:799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawakami Y, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakker AB, et al. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kvistborg P, et al. TIL therapy broadens the tumor-reactive CD8+ T cell compartment in melanoma patients. Oncoimmunology. 2012;1:409–418. doi: 10.4161/onci.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson LA, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies MA, Samuels Y. Analysis of the genome to personalize therapy for melanoma. Oncogene. 2010;29:5545–5555. doi: 10.1038/onc.2010.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei X, et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat Genet. 2011;43:442–446. doi: 10.1038/ng.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prickett TD, et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet. 2011;43:1119–1126. doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei X, et al. Mutational and functional analysis reveals ADAMTS18 metalloproteinase as a novel driver in melanoma. Mol Cancer Res. 2010;8:1513–1525. doi: 10.1158/1541-7786.MCR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 50.Palavalli LH, et al. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet. 2009;41:518–520. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prickett TD, et al. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nat Genet. 2009;41:1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gartner JJ, et al. Comparative exome sequencing of metastatic lesions provides insights into the mutational progression of melanoma. BMC Genomics. 2012;13:505. doi: 10.1186/1471-2164-13-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;39:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walia V, Mu EW, Lin JC, Samuels Y. Delving into somatic variation in sporadic melanoma. Pigment Cell Melanoma Res. 2012;25:155–170. doi: 10.1111/j.1755-148X.2012.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu Y-C, et al. Mutated PPP1R3B is recognized by T cells used to treat a melanoma patient who experienced a durable complete tumor regression. J Immunol. 2013;190:6034–6042. doi: 10.4049/jimmunol.1202830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robbins PF, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–752. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yannelli JR, et al. Growth of tumor-infiltrating lymphocytes from human solid cancers: summary of a 5-year experience. Int J Cancer. 1996;65:413–421. doi: 10.1002/(SICI)1097-0215(19960208)65:4<413::AID-IJC3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 58.Haas GP, Solomon D, Rosenberg SA. Tumor-infiltrating lymphocytes from nonrenal urological malignancies. Cancer Immunol Immunother Cell. 1990;30:342–350. doi: 10.1007/BF01786883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inozume T, et al. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33:956–964. doi: 10.1097/CJI.0b013e3181fad2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chattopadhyay PK, Yu J, Roederer M. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat Protoc. 2006;1:1–6. doi: 10.1038/nprot.2006.1. [DOI] [PubMed] [Google Scholar]
- 63.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 64.Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 21;373:673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee W, et al. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465:473–477. doi: 10.1038/nature09004. [DOI] [PubMed] [Google Scholar]
- 67.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agrawal N, et al. Exome Sequencing of Head and Neck Squamous Cell Carcinoma Reveals Inactivating Mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Distel LV, et al. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009;45:e167–174. doi: 10.1016/j.oraloncology.2009.05.640. [DOI] [PubMed] [Google Scholar]
- 71.Badoual C, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 72.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 73.de Vos van Steenwijk PJ, et al. An unexpectedly large polyclonal repertoire of HPV-specific T cells is poised for action in patients with cervical cancer. Cancer Res. 2010;70:2707–2717. doi: 10.1158/0008-5472.CAN-09-4299. [DOI] [PubMed] [Google Scholar]
- 74.Piersma SJ, et al. Human papilloma virus specific T cells infiltrating cervical cancer and draining lymph nodes show remarkably frequent use of HLA-DQ and -DP as a restriction element. Int J Cancer. 2008;122:486–494. doi: 10.1002/ijc.23162. [DOI] [PubMed] [Google Scholar]
- 75.Piersma SJ, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 76.Bethwaite PB, Holloway LJ, Thornton A, Delahunt B. Infiltration by immunocompetent cells in early stage invasive carcinoma of the uterine cervix: a prognostic study. Pathology. 1996;28:321–327. doi: 10.1080/00313029600169274. [DOI] [PubMed] [Google Scholar]
- 77.Shah KM, Young LS. Epstein-Barr virus and carcinogenesis: beyond Burkitt’s lymphoma. Clin Microbiol Infect. 2009;15:982–988. doi: 10.1111/j.1469-0691.2009.03033.x. [DOI] [PubMed] [Google Scholar]
- 78.Young LS, Rickinson AB. Epstein–Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 79.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878–889. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9:510–519. doi: 10.1038/nrclinonc.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heslop HE, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haque T, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 83.Comoli P, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 84.Louis CU, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33:983–990. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bollard CM, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr Virus+ Hodgkin’s disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2011;39:D913–D919. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Do no harm. Nat Biotechnol. 2013;31:365–365. doi: 10.1038/nbt.2587. [DOI] [PubMed] [Google Scholar]
- 88.Leibovich BC, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25:4757–4764. doi: 10.1200/JCO.2007.12.1087. [DOI] [PubMed] [Google Scholar]
- 89.Brouwers AH, Mulders PFA, Oyen WJG. Carbonic anhydrase IX expression in clear cell renal cell carcinoma and normal tissues: experiences from (radio) immunotherapy. J Clin Oncol. 2008;26:3808–3809. doi: 10.1200/JCO.2008.17.6073. author reply 3811–3812. [DOI] [PubMed] [Google Scholar]
- 90.Ivanov S, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lamers CHJ, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 92.Lamers CH, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther. 2012;11:517–525. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lal A, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–3339. [PubMed] [Google Scholar]
- 103.Boockvar JA, et al. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol Cell Neurosci. 2003;24:1116–1130. doi: 10.1016/j.mcn.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 104.Chu CT, Everiss KD, Wikstrand CJ, Batra SK, Kung HJ, Bigner DD. Receptor dimerization is not a factor in the signalling activity of a transforming variant epidermal growth factor receptor (EGFRvIII) Biochem J. 1997;324:855–861. doi: 10.1042/bj3240855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong AJ, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Humphrey PA, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci USA. 1990;87:4207–4211. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sok JC, et al. Mutant Epidermal Growth Factor Receptor (EGFRvIII) Contributes to Head and Neck Cancer Growth and Resistance to EGFR Targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 108.Morgan RA, et al. Recognition of Glioma Stem Cells by Genetically Modified T Cells Targeting EGFRvIII and Development of Adoptive Cell Therapy for Glioma. Hum Gene Ther. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haso W, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carpenter RO, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.CTpedia.
- 112.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palmer DC, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci USA. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 116.Gulley JL, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marshall JL, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 118.Ullenhag GJ, et al. Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factor. Clin Cancer Res. 2004;10:3273–3281. doi: 10.1158/1078-0432.CCR-03-0706. [DOI] [PubMed] [Google Scholar]
- 119.Parkhurst MR, Joo J, Riley JP, Yu Z, Li Y, Robbins PF, Rosenberg SA. Characterization of genetically modified T-cell receptors that recognize the CEA:691–699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin Cancer Res. 2009;15:169–180. doi: 10.1158/1078-0432.CCR-08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robbins PF, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parkhurst MR, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2010;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robbins PFM, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hofmann O, et al. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci USA. 2008;105:20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Atanackovic D, et al. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Baren N, et al. Tumoral and immunologic response after vaccination of melanoma patients with an ALVAC virus encoding MAGE antigens recognized by T cells. J Clin Oncol. 2005;23:9008–9021. doi: 10.1200/JCO.2005.08.375. [DOI] [PubMed] [Google Scholar]
- 126.Morgan RA, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cameron BJ, et al. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Linette GP, et al. Cardiovascular toxicity and titin cross-reactivity of affinity enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hinrichs CS, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cieri N, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 133.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hinrichs CS, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paulo CM, et al. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci Transl Med. 2010;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]