Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel inhabits the apical membrane of airway epithelia, where its function is essential for mucus hydration, mucociliary clearance, and airway defense. Chronic obstructive pulmonary disease (COPD), most often a consequence of cigarette smoke (CS) exposure, affects 15 million persons in the US. Clinically, COPD is characterized by many of the salient features of cystic fibrosis lung disease, where CFTR is either absent or reduced in function. CS is an acidic aerosol (pH 5.3 to 6.3) reported to contain over 4,000 constituents. Acute CS exposure has been reported to decrease airway transepithelial voltage in vivo and short-circuit current in vitro; however, the mechanistic basis of these effects is uncertain. The goal of the studies described here was to develop a bioassay to characterize the effects of aqueous CS preparations on the channel function of CFTR. We studied aqueous CS extract (CSE) prepared in our laboratory, as well as commercial cigarette smoke condensate (CSC) in Xenopus oocytes expressing human CFTR. Application of CSE at pH 5.3 produced a reversible, voltage-dependent inhibition of CFTR conductance. CSE neutralized to pH 7.3 produced less inhibition of CFTR conductance. Serial dilution of CSE revealed a dose-dependent effect at acidic and neutral pH. In contrast, CSC did not inhibit CFTR conductance in oocytes. We conclude that one or more components of CSE inhibits CFTR in a manner similar to diphenylamine-2-carboxylate, a negatively charged, open-channel blocker.
Keywords: cigarette smoke, CFTR, pH, ClC-2
chronic obstructive pulmonary disease (COPD) comprises a constellation of progressive, debilitating respiratory conditions, which include emphysema and chronic bronchitis. It affects an estimated 15 million adults in the United States (6). COPD is most often associated with cigarette smoking (CS) and is the primary contributor to mortality caused by chronic lower respiratory diseases, which became the third leading cause of death in the United States in 2008 (6). The chronic bronchitis phenotype is associated with excess mucus production, impaired mucociliary clearance, and increased bacterial infections in the lung (26). A 2011 survey reports that 39% of adults with physician-diagnosed COPD continue to smoke following diagnosis (6).
The cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride channel located in the apical membrane of airway epithelia. Its function is essential for proper mucus hydration and lung health through mucociliary clearance (14). CFTR dysfunction is the hallmark of the disease cystic fibrosis (CF), which is characterized by thick mucus, frequent bacterial lung infections, and bronchiectasis (24). Cigarette smoke has been shown to impair mucociliary clearance, in part due to diminished airway ciliary activity and increased mucin production (12, 28). In 1983, Welsh showed that application of CS to canine trachea impaired chloride transport as indicated by diminished transepithelial potential difference in vivo and by reduced short-circuit current (Isc) measured in tissues mounted in Ussing chambers (28). In these experiments, ion transport appeared to recover rapidly after removal of CS. Human bronchial epithelial cells exposed to cigarette smoke extract (CSE) demonstrated an initial rapid decrease in cAMP-dependent chloride secretion when assayed by Isc (16). In vivo, CS exposure reduced the CFTR-dependent response to the cAMP agonist, isoproterenol, in human nasal potential difference studies (7). These observations suggest the possibility of a direct action by the components of CS on the CFTR chloride channel.
Cigarette smoke is a complex cloud aerosol, containing gases, particulates, and water. More than 4,000 chemical compounds have been identified in mainstream smoke, including arylamines, benzoic acid, and benzamide-like derivatives (23). The structurally related arylaminobenzoates reversibly block CFTR by entering the anion-conducting pore from the cytoplasmic side (19). The pH of mainstream CS is acidic, reportedly ranging from 4.5 to 6.3, depending on the measurement technique (5, 29, 30). Moreover, CO2 produced in mainstream CS is readily solubilized in aqueous solutions, adding to the acid burden of CS (30).
At the airway epithelial surface anion channels other than CFTR are thought to contribute to airway chloride secretion. These include the purinergic receptor-linked, calcium-activated chloride channels, such as TMEM16A, which are critical to mucus clearance in mouse airways (22). Another non-CFTR anion channel reported at the airway surface is the voltage-gated, pH-activated ClC-2 chloride channel (17, 20). Although acidic pH activation of ClC-2 in airway cells has been described (2), no evaluation of CS as a possible inhibitor of ClC-2 chloride transport has been reported.
The goal of the experiments reported here was to develop a bioassay that would be useful for identifying components of smoke that could alter the function of the CFTR channel. We chose the Xenopus laevis oocyte because of its functional stability over long periods (18), structural simplicity, and very low background conductance, which make it ideal for detecting changes in CFTR function. We anticipated that the oocyte would be superior to airway cell monolayers for discerning functional effects on CFTR channels. We employed two aqueous CS solutions: 1) the water-soluble fraction of whole smoke bubbled through an aqueous solution (CSE) or 2) a commercially available DMSO-solubilized, tar-particulate fraction captured on filter pads [cigarette smoke condensate (CSC)]. Because whole CS is known to be acidic, we studied CFTR channel function at both acidic and neutral pH. Isc measured in human airway cell layers exposed to CSE was examined for comparison to previously published results. Lastly, we examined the effect of CSE on the voltage-gated, pH-activated chloride channel ClC-2.
METHODS
Ethical approval.
Approval for harvest of Xenopus laevis oocytes was granted by the Animal Care and Use Committee of the Oregon Health & Sciences University.
Reagents.
Ca2+-free solution: 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES (pH 7.5), and 0.2 Wünsch units/ml Liberase Blendzyme (Roche Molecular Biochemicals, Indianapolis, IN). Modified Barth's solution (MBSH): 88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 2.4 mM NaHCO3, 10 mM HEPES-Hemi-Na, and 250 mg/l amikacin with 150 mg/l gentamicin (pH 7.5). Frog Ringer solution: 98 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES (hemi-Na) (pH 7.3∼7.4). Isoproterenol + IBMX (I+I): 10 μM isoproterenol (a β-adrenergic agonist) (Sigma, St. Louis, MO) and 1 mM 3-isobutyl-1-methylxanthine (IBMX) (Sigma) were added to Frog Ringer to create the I+I solution in two-electrode voltage-clamp (TEVC) experiments. HEPES-buffered Ringer: 145 mM NaCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 0.8 mM KH2PO4, 3.3 mM K2HPO4, 10 mM HEPES, and 10 mM dextrose (pH 7.4). Low Cl− HEPES-buffered Ringer: 145 mM Na gluconate, 1.2 mM MgCl2, 1.2 mM Ca gluconate, 0.8 mM KH2PO4, 3.3 mM K2HPO4, 10 mM HEPES, and 10 mM dextrose (pH 7.4). Diphenylamine-2-carboxylate (DPC) was obtained from Sigma and stocks concentrations prepared. CFTRinh-172 inhibitor was a gift of the Cystic Fibrosis Therapeutics Development Network, Bethesda, MD. The pH 5.5 solutions were made with the addition of 1 M HCl (Sigma). CSC: CSC was purchased from Murty Pharmaceuticals (Lexington, KY). Per the manufacturer, it was prepared by smoking University of Kentucky's 1R3F standard research cigarettes on a Phipps-Bird 20-channel smoking machine. Smoke particulate was collected on a Cambridge glass fiber filter and dissolved in dimethylsulfoxide (DMSO) at a concentration of 40 mg/ml.
CSE preparation.
Cigarette smoke extract (CSE) was prepared, in a manner similar to that described by Kreindler et al. (16) and Yasuda (31), by drawing the effluent of 50 lit University of Kentucky 3R4F research cigarettes at a flow rate of 0.33 liter per second (tidal breathing midpoint flow rate) through a flask containing glass beads and 50 ml of Frog Ringer solution or low Cl− HEPES-buffered Ringer with a constant vacuum. A final concentration of 1 cigarette per ml was determined experimentally as it produced consistent inhibition of CFTR expressed in oocytes. The solutions were kept at 37°C while the smoke was bubbled through. The pH of the solution was measured after each addition of 10 cigarettes. After 50 cigarettes were bubbled through the solution, the optical density at 280 nm was measured (Bio-Rad, Hercules, CA), and the solution was either used that day or immediately stored at −20°C.
Preparation and microinjection of oocytes.
Female Xenopus laevis were anesthetized by immersion in cold water containing tricaine, 3 mg/ml (Sigma). The oocytes were removed through a small abdominal incision that was then closed by 4-0 nylon suture. Frogs were recovered in their tanks. The follicular membranes were removed by mechanical agitation (1–2 h) in a Ca2+-free solution. Stage V and VI defolliculated oocytes were selected, then washed and incubated at 18°C in MBSH until injection the next day. Oocytes were injected with 0.1–15 ng of cRNA (50-nl volume) of human CFTR or rabbit ClC-2 cRNA (gift of N. McCarty), and CFTR oocytes were coinjected with cRNA encoding the human β2-adrenergic receptor (βAR) by use of a microinjector (Drummond Scientific, Broomhall, PA). Injected oocytes were incubated at 18°C in 12-well plates containing MBSH. Injection pipettes were pulled from filamented glass capillary tubes (Sutter Instrument, Novato, CA) on a P-97 Flaming-Brown micropipette puller (Sutter Instrument). Typically, CFTR and βAR expressing oocytes were used 3–4 days after injection, while ClC-2 expressing oocytes were used 5–6 days after injection.
Whole cell recordings.
Individual oocytes were placed in a 200 μl RC-1Z recording chamber (Warner Instruments, Hamden, CT) and continuously perfused with Frog Ringer solution (1.5 ml/min) with a syringe pump. Membrane currents were recorded from oocytes by use of a TEVC amplifier (TEV-200; Dagan, Minneapolis, MN) at room temperature. Current-injecting and potential-measuring electrodes had resistances of ∼0.5–2.0 and ∼1.0–3.0 MΩ, respectively, when filled with 3 M KCl. The bath solution was connected to the ground through a low-resistance agarose bridge containing 2% agarose in 3 M KCl. A second reference electrode was used to avoid polarization errors. Current measurements were low-pass filtered at 0.5 kHz. Data acquisition and analysis were done on a Pentium-based microcomputer using pCLAMP software and an analog-to-digital converter (Axon Instruments, Foster City, CA).
Oocytes were continuously perfused with the experimental solutions at a flow rate of 90 ml/h. Oocytes were initially maintained in the experimental chamber under open circuit conditions and experiments began when the transmembrane voltage was between −25 mV and −40 mV. For CFTR oocyte experiments, the membrane potential was ramped from −120 mV to +60 mV over a period of 1.8 s to construct whole-cell current-voltage (I-V) plots for each of the perfusate solutions tested. Conductance was then calculated from the slope of the I-V plot at the reversal potential (Vm = Erev) by using a voltage range from Vm = Erev −10 mV to Vm = Erev + 10 mV. In CFTR + βAR expressing oocytes, CFTR was activated with perfusion of I+I. Then, oocytes were perfused with CS solutions containing I+I for 5 or 10 min. Lastly, 20 μM CFTRinh-172 in I+I was perfused to confirm CFTR as the primary conductance channel. A subset of CFTR + βAR expressing oocytes were studied as above, however, DPC was substituted for CSE. For ClC-2 expressing oocytes the membrane potential was held from −160 mV to +40 mV with steps at 25-mV intervals for a period of 9 s. At least two replicates of the voltage-step protocol were performed for each of the perfusing solutions per oocyte.
Cell culture.
CFBE41o− cells stably transfected with wild-type CFTR expressed under a cytomegalovirus promoter (2) (a kind gift of Dr. J. P. Clancy) were employed to assure adequate CFTR expression. Cells were grown under standard conditions using E-MEM medium (Hyclone, Rockford, IL) supplemented with 10% FBS (Hyclone), and 1% penicillin-streptomycin (Hyclone). After reaching 80% confluency, the cells were lifted and plated on Snapwell polyester inserts (Corning, Tewksbury, MA) that were previously coated with a solution containing human fibronectin (1 mg/ml) (BD Biosciences, San Jose, CA), collagen I (2.9 mg/ml) (BD Biosciences), and 1% BSA (Sigma) in LHC basal medium (Hyclone). The cells were grown at liquid-liquid interface for 21 to 28 days at 37°C. Transepithelial resistance was monitored with an EVOM epithelial voltohmmeter (World Precision Instruments, Sarasota, FL), and only inserts with a resistance greater than 300 Ω were transferred to the Ussing chamber for Isc experiments.
Isc measurements.
For Isc experiments, an EasyMount Ussing Chamber and voltage clamp were employed (Physiologic Instruments, San Diego, CA). Initial chamber bathing solutions contained HEPES-buffered Ringer solution warmed to 37°C and were circulated with bubbling air. The basolateral membrane was permeabilized with 400 μM nystatin for 20 min. Voltage was clamped to zero and a 3-mV biphasic pulse was applied every 10 s. After 20 min, the apical solution was replaced with low Cl− Ringer, an equal molar gluconate-substituted HEPES-buffered solution. To activate CFTR, 10 μM forskolin was added to the apical chamber. After activation, three sequential replacements of 1 ml of the warmed CSE or low Cl− Ringer both containing 10 μM forskolin were exchanged for 1 ml of the apical chamber solution. After 10 min in the CSE or low Cl− Ringer, an identical replacement with low Cl− Ringer with 10 μM forskolin was performed. After 5–10 min, 20 μM CFTRinh-172 was added to the apical chamber to confirm that the remaining current was CFTR mediated.
RESULTS
Aqueous CSE is acidic.
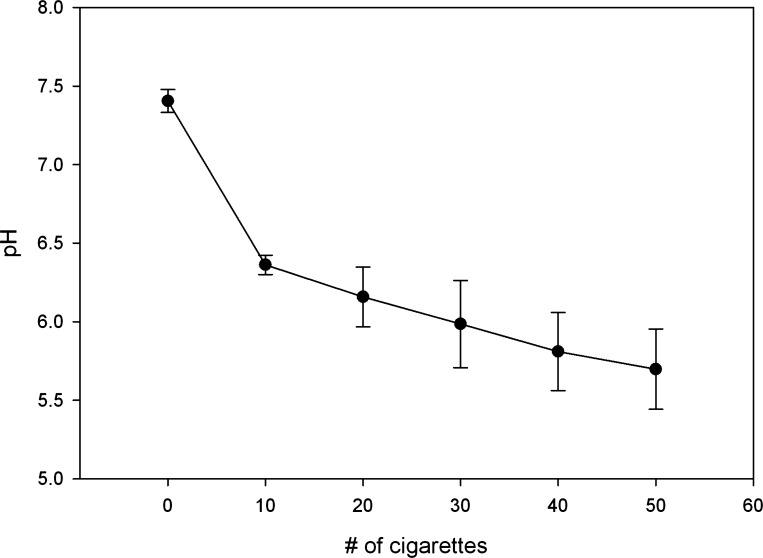
Figure 1 illustrates the change in pH as smoke from 50 cigarettes (3R4F) was drawn through 50 ml of warmed Frog Ringer (n = 4). The final optical density of the solution at 280 nm averaged 0.81 ± 0.10 absorbance units. After capturing the smoke of 50 cigarettes, the final pH of our CSE preparations ranged from 5.2 to 5.9, similar to that described previously for CSE (31), and in the range reported in direct measurement of whole CS (30). The addition of either 200 μM or 400 μM CSC to the perfusing solutions did not alter the pH.
Fig. 1.
Change in pH ± SD of Frog Ringer (FR) after each set of 10 cigarettes was drawn through at a flow rate of 0.33 liter per second (n = 4).
Acidic CSE inhibited CFTR conductance.
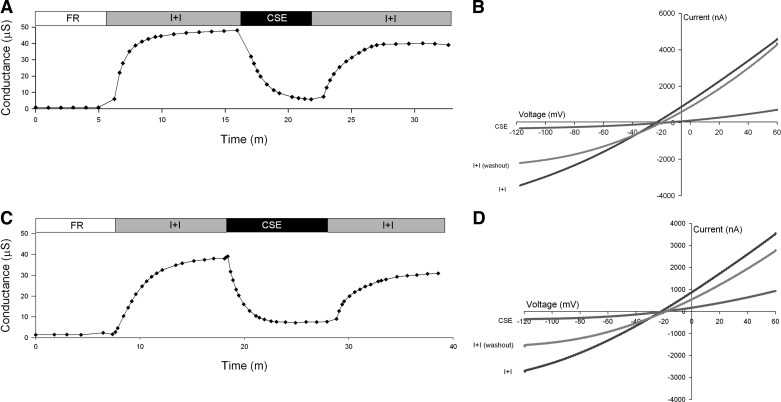
Following activation of CFTR, perfusion of CSE solution for 5–6 min at its native pH (5.2 to 5.9) reduced CFTR conductance (Fig. 2A). In 11 experiments the mean conductance fell from 34.17 μS (±8.6) following activation to 9.79 μS (±6.11) in the presence of CSE (P = 0.0005, paired t-test). Washout of CSE largely reversed the inhibition; the mean conductance rose to 31.94 μS (±7.9). The residual inhibition following washout averaged 7% (Table 1; P = 0.044 by paired t-test). Increasing perfusion time to 10 min did not result in further loss of conductance (Fig. 2C). In four oocytes, following 10-min perfusions of CSE, the mean conductance fell from 20.49 μS (±12.30) following activation to 4.54 μS (±2.36) (P = 0.03, paired t-test) in the presence of CSE. Following washout of CSE, however, the mean conductance only rose to 17.11 μS (±10.07), an average residual inhibition of 16% (Table 1; P = 0.03 by paired t-test). Table 1 summarizes the percent inhibition of CFTR conductance in the presence of CSE and after washout.
Fig. 2.
Representative traces of conductance and current-voltage (I-V) plots of oocytes expressing CFTR and β2-adrenergic receptor (βAR). A: conductance of an oocyte perfused with FR, then activated with isoproterenol and IBMX (I+I), followed by a 5-min exposure to cigarette smoke extract (CSE) containing I+I, and lastly a washout with I+I. B: I-V plot at peak activation with I+I, 5-min exposure to CSE, and washout with I+I. C: conductance of an oocyte perfused with FR, then activated with I+I, followed by a 10-min exposure to CSE containing I+I, and lastly a washout with I+I. D: I-V plot at peak activation with I+I, 10-min exposure to CSE and washout with I+I.
Table 1.
Percent inhibition of CFTR conductance after 5 and 10 min exposures to CSE in CFTR-expressing oocytes
| % Inhibition |
||
|---|---|---|
| CSE | washout Ι+Ι | |
| 5 min CSE (n = 11) | 69 ± 21 | 7 ± 9 |
| 10 min CSE (n = 4) | 74 ± 12 | 16 ± 17 |
Values are means ± SD. All cigarette smoke extract (CSE) solutions contained isoproterenol and IBMX (Ι+Ι).
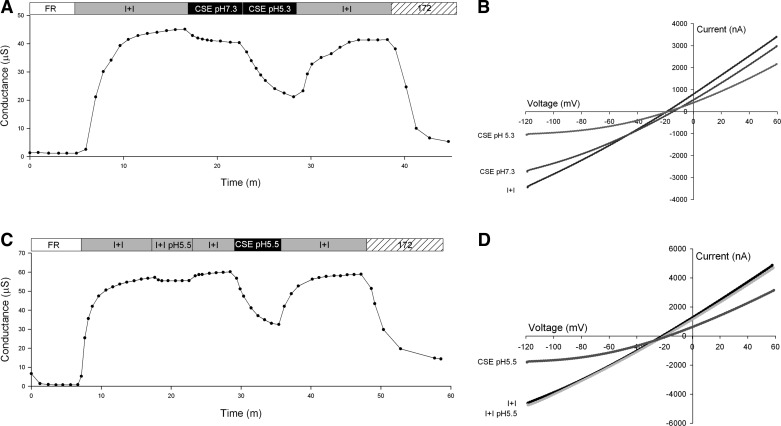
Examination of the I-V plots (Fig. 2, B and C) reveals a voltage-dependent inhibition of CFTR during CSE perfusion discernible as increased inhibition at negative membrane potentials. When the pH of the CSE solution was adjusted to 7.3 by addition of 10 N NaOH, inhibition of CFTR conductance was attenuated (Fig. 3A), but perfusion with CSE at native pH 5.3 produced the characteristic inhibition (Fig. 3A). The I-V plots captured in the presence of CSE consistently revealed a voltage-dependent inhibition (Fig. 3, B and D). Exposure to a pH 5.5 solution lacking CSE produced slight inhibition of CFTR conductance (Fig. 3C).
Fig. 3.
Representative traces of conductance and I-V plots of oocytes expressing CFTR and βAR. A: conductance of an oocyte perfused with FR, then activated with I+I pH 7.3, followed by a 5-min exposure to CSE at pH 7.3 containing I+I, then perfused for 5 min with CSE containing I+I at pH 5.5; the CSE was then washed out with I+I pH 7.3 and lastly 20 μM CFTRinh-172 (172) in I+I pH 7.3 was applied. B: I-V plot at peak activation with I+I, after 5-min exposure to CSE pH 7.3, and after 5-min exposure to CSE pH 5.5. C: conductance of an oocyte perfused with FR, then activated with I+I pH 7.3, followed by a 5-min exposure to I+I at pH 5.5, then a washout with I+I pH 7.3 and application of CSE containing I+I at pH 5.5 for 5 min, lastly an I+I pH 7.3 washout and application of 20 μM CFTRinh-172 in I+I pH 7.3 D: I-V plot at peak activation with I+I pH 7.3, after 5-min exposure to I+I pH 5.5 and after 5-min exposure to CSE pH 5.5.
To test the relationship between pH and CSE block of CFTR, a group of oocytes (n = 3) underwent perfusion of I+I at pH 5.5 for 5 min followed by a 5-min washout and subsequent exposure to CSE at pH 5.5. Only perfusate containing CSE produced the characteristic inhibition (Fig. 3C). To test the hypothesis that external pH (pHo) of the perfusate could rapidly lower the cytoplasmic pH (pHi), a subset of oocytes (n = 4) was injected with 10 nl of 100 mM HEPES buffer prior to experimentation. No difference in response to external acidification at pH 5.5 was seen between standard CFTR-injected oocytes and HEPES-prepared oocytes. Following acidic pH exposure, application of 20 μM CFTRinh-172 occasionally revealed residual non-CFTR conductance, which has been attributed to endogenous Ca2+-activated chloride conductance in the presence of acidic pH (15). Uninjected oocytes perfused with solutions at pH 6.0, 5.5, or 5.0 exhibited no change in basal conductance (not shown).
CSE inhibition of CFTR was dose dependent.
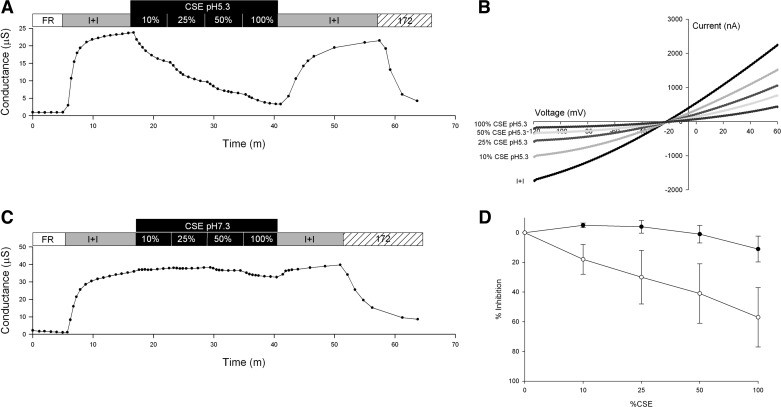
To further characterize CFTR response to CSE, a dose-response relation was determined by dilution of CSE to 10, 25, and 50% of its original concentration. Two groups of oocytes (n = 4) were studied at either pH 5.3 or 7.3. At its native pH of 5.3, CSE inhibition of conductance increased with CSE concentration (Fig. 4A). The I-V plots associated with increasing CSE concentrations revealed increasing, voltage-dependent inhibition (Fig. 4B). At neutral pH (7.3), inhibition of conductance by CSE was barely detectable at 50% and remained modest (11%) at 100% CSE (Fig. 4C). Figure 4D contains a dose-response curve showing percent inhibition of CFTR conductance in the presence of increasing concentrations of CSE at pH 7.3 or 5.3.
Fig. 4.
Representative traces of conductance and I-V plots of oocytes expressing CFTR and βAR. A: conductance of an oocyte perfused with FR, then activated with I+I, followed by sequential 5-min exposures to pH 5.3 CSE containing I+I at 10, 25, 50, and 100% concentrations, the CSE was then washed out with I+I pH 7.3, and lastly 20 μM CFTRinh-172 in I+I was applied. B: I-V plot of peak activation with I+I and after 5-min exposure to CSE pH 5.3 at 10, 25, 50, and 100% concentrations C: conductance of an oocyte perfused with FR, then activated with I+I, followed by 5-min exposures to pH 7.3 CSE containing I+I at 10, 25, 50, and 100% concentrations. The CSE was then washed out with I+I and lastly 20 μM CFTRinh-172 in I+I was applied. D: dose-response curve of CFTR % inhibition ± SD with increasing concentrations of CSE at pH 7.3 (●) and 5.3 (○).
CSE inhibition of CFTR in oocytes resembles that seen with DPC.
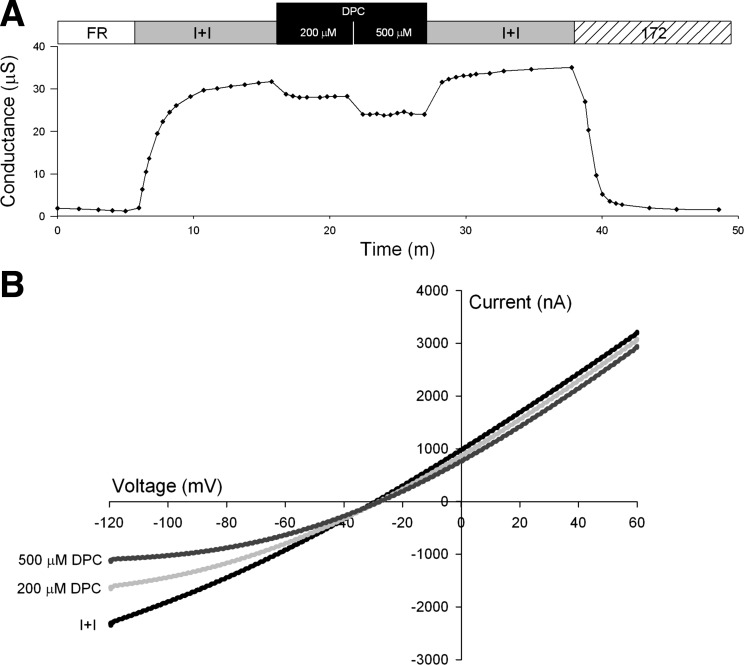
The voltage-dependent, pH-enhanced CSE inhibition of CFTR resembles that of the well-characterized CFTR pore blocker, DPC. Exposure to DPC (200 and 500 μM) at pH 7.3 (n = 3) produced voltage-dependent inhibition of CFTR-mediated conductance (Fig. 5, A and B). DPC blockade was reversible upon washout (Fig. 5A). The I-V plot shows that DPC inhibition was readily discernible at negative membrane potentials, consistent with the notion that the negatively charged species blocks the CFTR pore, entering from the cytoplasmic side as suggested by McCarty et al. (19) (Fig. 5B).
Fig. 5.
Representative traces of conductance and I-V plots of oocytes expressing CFTR and βAR. A: conductance of an oocyte perfused with FR, then activated with I+I pH 7.3, followed by a 5-min exposure to 200 μM DPC in I+I pH 7.3, then perfused for 5 min with 500 μM DPC in I+I pH 7.3, followed by a washout with I+I pH 7.3 and lastly exposure to 20 μM CFTRinh-172 in I+I. B: I-V plot of peak activation with I+I pH 7.3, after 5-min exposure to 200 μM DPC pH 7.3 and after 5-min exposure to 500 μM DPC pH 7.3.
CSE acutely inhibits cAMP-stimulated Isc in CFBE41o− airway cells expressing CFTR.
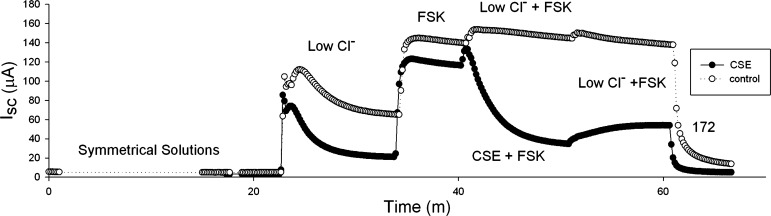
Following permeabilization of the basolateral membrane, creating an apical-to-basolateral chloride gradient, the addition of 10 μM forskolin to the apical bath increased Isc as shown in Fig. 6. Exchanges of the apical bath with CSE containing solutions at their native pH resulted in a reduction in Isc (62% ± 10; n = 4). The rate of inhibition was comparable to that seen in oocytes, but the residual inhibition following washout was greater (37 vs. 16%). Addition of 20 μM CFTRinh-172 to the apical chamber confirmed that this current was CFTR mediated.
Fig. 6.
Short-circuit current (Isc) plot of CFBE41o− cells expressing CFTR. The basolateral membrane was permeabilized with 400 μM nystatin while the monolayer was bathed in symmetrical Ringer solutions. An apical-to-basolateral chloride gradient was imposed by replacing the apical bath with gluconate-substituted Ringer (low Cl−). Then 10 μM forskolin (FSK) was added to the apical chamber to activate CFTR. The apical bath was then replaced with CSE in the low Cl− solution (CSE+FSK), or low Cl− solution (low Cl−+FSK), both containing 10 μM forskolin. After 10 min the apical bath was again replaced with low Cl− solution containing 10 μM forskolin (low Cl−+FSK), resulting in partial restoration of Isc, which was then inhibited by the addition of 20 μM CFTRinh-172 (172) to the apical chamber.
Commercial CSC does not significantly inhibit CFTR in oocytes.
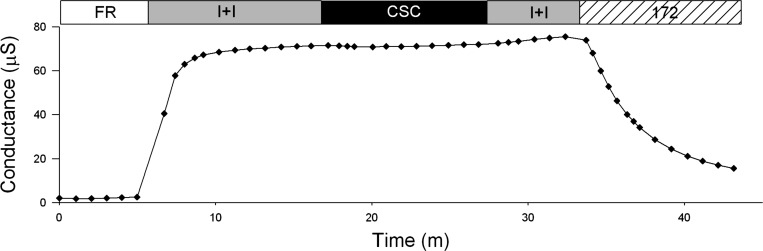
As shown in the conductance plot in Fig. 7, CSC (200 μM) did not inhibit CFTR conductance in oocytes (n = 3). Increasing the CSC concentration to 400 μm was also without effect (not shown).
Fig. 7.
Representative trace of conductance plot of oocyte expressing CFTR and βAR. Conductance of an oocyte perfused with FR, then activated with I+I, followed by a 5-min exposure to 200 μM cigarette smoke condensate (CSC) in I+I solution, the CSC was then washed out with I+I and lastly 20 μM CFTRinh-172 in I+I solution was applied.
The chloride channel ClC-2 activates in the presence of acidic CSE.
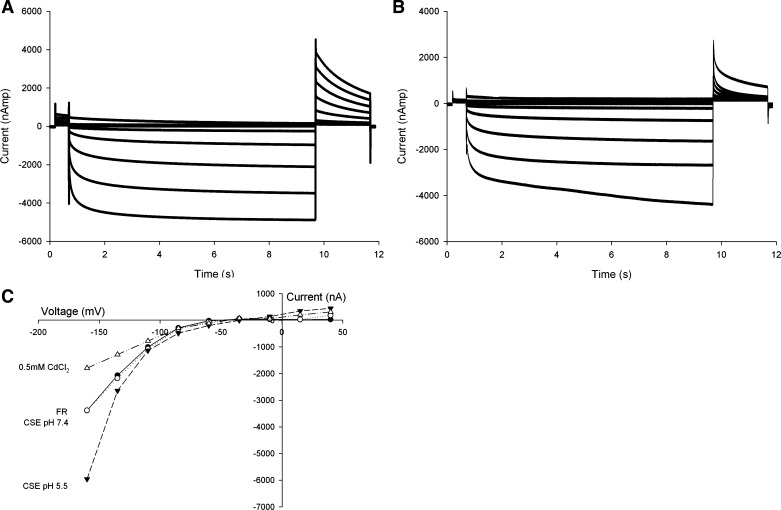
To determine whether the CSE effects seen were specific to CFTR, oocytes expressing ClC-2 (n = 3) were tested for their ability to activate in the presence of CSE at pH 7.4 and pH 5.5. With use of a voltage-step protocol timed to allow complete activation of the channel, ClC-2 exhibited the expected voltage-dependent activation in Frog Ringer at pH 7.4 (Fig. 8A). Perfusion of CSE at pH 7.4 altered the voltage-dependent activation (Fig. 8B), an effect that was readily detectable in the magnitude of the tail current. Perfusion of CSE at pH 5.5 produced an increase in current, consistent with previously demonstrated activation of the ClC-2 channel by acidic pH (Fig. 8C) (21). Lastly, perfusion of 0.5 mM CdCl2, a nonspecific inhibitor of ClC-2, resulted in inhibition of current.
Fig. 8.
Representative traces of ClC-2 step protocol and current-voltage plot. A: current plot for ClC-2 activation in oocytes during a voltage-step protocol with 9-s membrane voltage step. B: same oocyte during CSE perfusion at pH 7.4. C: I-V plot of ClC-2 expressing oocyte. FR (solid line) and CSE (dotted line) at 7.4 show similar voltage-dependent activation. Exposure to CSE at pH 5.5 resulted in activation of ClC-2. Addition of 0.5 mM CdCl2 inhibited ClC-2 activation.
DISCUSSION
Aqueous CSE causes voltage-dependent block of CFTR.
The primary finding of this study is the inhibition of human CFTR channels expressed in Xenopus oocytes by one or more components of CSE. This mostly reversible inhibition is characterized by a voltage dependence that increases with concentration and is less pronounced at neutral pH. CFTR inhibition seen with CSE exposure was similar to the voltage-dependent, acidic pH-enhanced, reversible block described for the arylaminobenzoate family of chloride channel blockers, such as DPC. The characteristics of DPC inhibition of CFTR have been well described (19, 32). The voltage dependence is consistent with the notion that the protonated form of DPC enters the cell and binds within the CFTR pore, entering from the cytoplasmic side. DPC (pKa ∼4.2) is expected to enter the cell more readily at relatively acidic pH (32). Some oocytes subjected to 10-min exposures to CSE demonstrated residual inhibition, which could suggest a cytoplasmic accumulation of the responsible agent. Given the large number of compounds in CSE, it is possible that more than one agent is acting on CFTR-mediated conductance, either directly as suggested here or perhaps indirectly by inhibiting phosphorylation of the channel.
The similarity of CFTR inhibition by CSE to that seen with the arylaminobenzoate, DPC, suggests that arylamines and similar aromatic compounds produced when organic compounds undergo incomplete combustion may directly affect CFTR, possibly by blocking the anion-selective pore. Examples of incomplete combustion of organic materials include tobacco smoking, diesel combustion, and wood burning.
The CSE preparation employed here was chosen to facilitate reproducible, aqueous extraction and is not directly comparable to whole CS or human smoking. This represents a limitation in applying our results to the human condition. Our CSE represents the stable water soluble components of whole CS and confirms Welsh's observations that the acute inhibition of Isc in airway tissues was produced by the water-soluble fraction (28). The CSE employed here lacks the volatile components of freshly inhaled smoke. By using a constant vacuum, components created during variable rates of pyrolysis in human smoking are also unaccounted for in this CSE. We did, however, preserve the native range of CS pH, a factor often overlooked in cell culture models. pH is a useful tool in identifying the behavior of compounds in model assay systems.
One complementary explanation for the reduced CFTR-mediated conductance seen in some oocytes and in CFBE41o− cell layers after CSE exposures is reduced membrane expression. Previous surface labeling of CFTR in oocytes revealed little, if any, retrieval of the channel from the surface over several hours at 23°C (18). However, the reduced recovery of CFTR-mediated conductance by apical membranes of CFBE41o− cells following washout may reflect, in part, the loss of CFTR membrane expression reported by Clunes et al. (9). It remains to be determined whether the acute CFTR inhibition seen here is causally related to the internalization of CFTR.
Acidic pH of CSE enhances inhibition of CFTR.
This study demonstrates that acidic pH greatly enhances CSE-induced inhibition of CFTR, whereas acidic pH alone did not produce appreciable CFTR inhibition. Arrested, late-stage Xenopus oocytes, like those we employed, have been reported to have a cytoplasmic pH of ∼7.4. As reported by Broer et al. (4), in response to acidic perfusate (pH 5.0), naive or water-injected oocytes' cytoplasmic pH changed by 0.1. CFTR has been reported to be sensitive to changes in cytoplasmic pH. Open probability was reportedly enhanced at mildly acidic pHi (pH 6.8) and reduced at more acidic pHi (pH 5.8) (8), but we detected no difference in conductance in oocytes injected with HEPES buffer prior to exposure to acidic pH.
Whether the acidic nature of CS leads to a decrease in the pH of the airway surface liquid (ASL) in the lung is unclear. In a review of mechanisms of acid and base secretion by airway epithelium, the average in vivo pH of the ASL was reported to be pH 6.6 (13), suggesting that the ASL is mildly acidic. However, in vivo estimates of airway pH made using exhaled breath condensate (EBC) in healthy smokers and nonsmokers were not statistically different (7.16 vs. 7.39), although the authors noted 5 of 17 healthy smokers had an EBC pH below 6 (3). Tobacco company documents released by the Tobacco Master Settlement Agreement show experimental evidence for acidification of synthetic airway mucus following CS exposure (11). The pH of the ASL during cigarette smoking warrants investigation as it could influence the action of toxicants as we have shown here.
Commercial CS condensate does not acutely inhibit CFTR.
CSC obtained from Murty Pharmaceuticals contains the DMSO soluble CS particulate captured on Cambridge filter pads by use of a cigarette smoking machine. In Xenopus oocytes, CSC at concentrations previously reported to inhibit CFTR in primary nasal epithelial cells (10) had no effect. However, we have not directly compared the chemical composition of our CSE to commercially available CSC; thus multiple factors could account for the differing observations.
CSE does not inhibit acidic pH activation of ClC-2 expressed in Xenopus oocytes.
To test whether the inhibitory effect of CSE is specific to CFTR, we examined the voltage-gated, pH-activated chloride channel ClC-2 (27). Immunofluorescent techniques suggest ClC-2 might be expressed in the apical membranes of ciliated rat and human nasal and airway cells (17). Previous experiments in ClC-2 overexpressing IB-3 CF airway cells and newborn rat tracheal cells demonstrated that acidic pH activation that was sensitive to Cd2+ and was presumed to represent ClC-2 activity (2, 25). Our preliminary results indicate possible differences in both the activation current and the tail current of ClC-2 in the presence of CSE at neutral pH and significant activation in the presence of CSE at pH 5.5, but no inhibition comparable to that seen with CFTR. These initial observations suggest detailed investigations of ClC-2 and Ca2+-activated chloride channels expressed in oocytes in the presence of CSE would be informative.
GRANTS
This work was supported by the National Institutes of Health Grants K12 HD057588 02 (K. D. MacDonald) and DK045880 (D. C. Dawson), the Cystic Fibrosis Foundation Dawson08GO (D. C. Dawson), and Parker B. Francis Fellowship (K. D. MacDonald). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.R.M., Y.N., D.C.D., and K.D.M. conception and design of research; A.R.M., Y.N., and K.D.M. performed experiments; A.R.M., D.C.D., and K.D.M. analyzed data; A.R.M., Y.N., D.C.D., and K.D.M. interpreted results of experiments; A.R.M. and K.D.M. prepared figures; A.R.M. and K.D.M. drafted manuscript; A.R.M., Y.N., D.C.D., and K.D.M. edited and revised manuscript; A.R.M., Y.N., D.C.D., and K.D.M. approved final version of manuscript.
REFERENCES
- 1.Bebok Z, Collawn JF, Wakefield J, Parker W, Li Y, Varga K, Sorscher EJ, Clancy JP. Failure of cAMP agonists to activate rescued ΔF508 CFTR in CFBE41o− airway epithelial monolayers. J Physiol 569: 601–615, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaisdell CJ, Edmonds RD, Wang XT, Guggino S, Zeitlin PL. pH-regulated chloride secretion in fetal lung epithelia. Am J Physiol Lung Cell Mol Physiol 278: L1248–L1255, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Borrill ZL, Roy K, Vessey RS, Woodcock AA, Singh D. Non-invasive biomarkers and pulmonary function in smokers. Int J Chron Obstruct Pulmon Dis 3: 171–183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broer S, Schneider HP, Broer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J 333: 167–174, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunnemann KD, Hoffmann D. The pH of tobacco smoke. Food Cosmet Toxicol 12: 115–124, 1974 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep 46: 938–943, 2012 [PubMed] [Google Scholar]
- 7.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 173: 1139–1144, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chen JH, Cai Z, Sheppard DN. Direct sensing of intracellular pH by the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. J Biol Chem 284: 35495–35506, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, Bennett WD, Riordan JR, Boucher RC, Tarran R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 26: 533–545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen NA, Zhang S, Sharp DB, Tamashiro E, Chen B, Sorscher EJ, Woodworth BA. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope 119: 2269–2274, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Creighton D. The effect of cigarette smoke on the pH of mucus. In: Legacy Tobacco Documents Library, 1968. http://legacy.library.ucsf.edu/tid/ich38a99/pdf
- 12.Dalhamn T, Rylander R. Cigarette smoke and ciliastasis. Effect of varying composition of smoke. Arch Environ Health 13: 47–50, 1966 [DOI] [PubMed] [Google Scholar]
- 13.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol 211: 139–150, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. Eur Respir J 13: 1177–1188, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Kongsuphol P, Schreiber R, Kraidith K, Kunzelmann K. CFTR induces extracellular acid sensing in Xenopus oocytes which activates endogenous Ca2+-activated Cl− conductance. Pflügers Arch 462: 479–487, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol 288: L894–L902, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 282: C805–C816, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Smith SS, Sun F, Dawson DC. CFTR: covalent modification of cysteine-substituted channels expressed in Xenopus oocytes shows that activation is due to the opening of channels resident in the plasma membrane. J Gen Physiol 118: 433–446, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty NA, McDonough S, Cohen BN, Riordan JR, Davidson N, Lester HA. Voltage-dependent block of the cystic fibrosis transmembrane conductance regulator Cl− channel by two closely related arylaminobenzoates. J Gen Physiol 102: 1–23, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. CIC-2: a developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol 12: 597–604, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Norimatsu Y, Moran AR, MacDonald KD. Lubiprostone activates CFTR, but not ClC-2, via the prostaglandin receptor (EP4). Biochem Biophys Res Commun 426: 374–379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated chloride secretory channel in mouse airways. J Biol Chem 284: 14875–14880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodgman A, Perfetti TA. Introduction. In: The Chemical Components of Tobacco and Tobacco Smoke. Boca Raton, FL: CRC, 2009 [Google Scholar]
- 24.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Schwiebert EM, Cid-Soto LP, Stafford D, Carter M, Blaisdell CJ, Zeitlin PL, Guggino WB, Cutting GR. Analysis of ClC-2 channels as an alternative pathway for chloride conduction in cystic fibrosis airway cells. Proc Natl Acad Sci USA 95: 3879–3884, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med 26: 142–153, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356: 57–60, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Welsh MJ. Cigarette smoke inhibition of ion transport in canine tracheal epithelium. J Clin Invest 71: 1614–1623, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williard CS, McDaniel EB, Striegel RM, Walker RT, Sudholt MS. A comparison of two methods for determining the pH of mainstream cigarette smoke. Tobacco Science: 5–7, 2003 [Google Scholar]
- 30.Williard CS, McDaniel EB, Striegel RM, Walker RT, Sudholt MS. Puff-by-puff determination of the pH of water-extractables from mainstream particulate phase and whole mainstream smoke of reference and commercial cigarettes. Tobacco Science: 8–11, 2003 [Google Scholar]
- 31.Yasuda K, Takashima M, Sawaragi I. Influence of a cigarette smoke extract on the hormonal regulation of platelet-activating factor acetylhydrolase in rats. Biol Reprod 53: 244–252, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZR, Zeltwanger S, McCarty NA. Direct comparison of NPPB and DPC as probes of CFTR expressed in Xenopus oocytes. J Membr Biol 175: 35–52, 2000 [DOI] [PubMed] [Google Scholar]