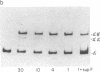
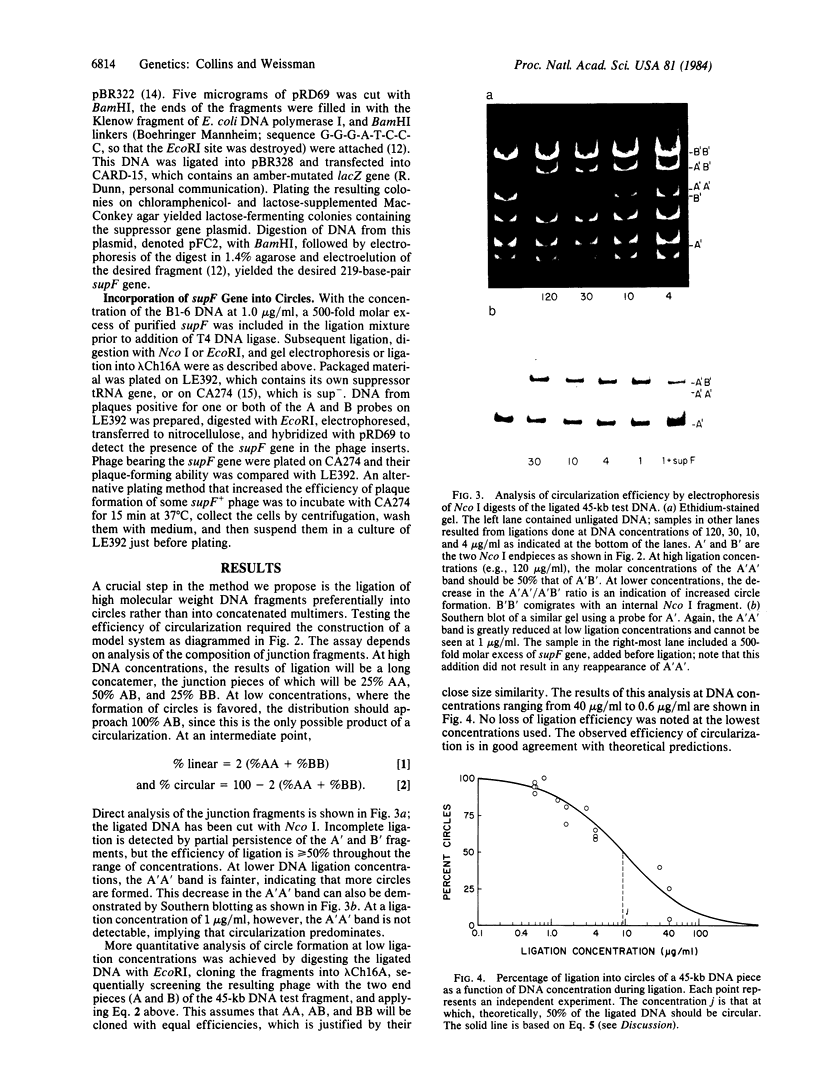
Abstract
The principle of a DNA cloning procedure that directionally generates genomic DNA fragments 50-2000 kilobases away from an initial probe is presented. The method depends on partial digestion of high molecular weight genomic DNA and subsequent ligation at very low concentration to generate covalent DNA circles. A library of the junction fragments from these circles can then be constructed. Biological or physical selection of the junction pieces can be achieved by incorporating a marker DNA fragment into the covalent circles. A 45-kilobase cosmid fragment has been successfully used to test the procedure. At appropriately low ligation concentrations (0.8 micrograms/ml), approximately equal to 90% of the ligated DNA is present as monomeric circles. Larger DNA fragments will require reducing the DNA concentration as the inverse square root of the DNA length. A suppressor tRNA gene has been tested as the selectable marker gene. Ligation of the digested circles into an amber-mutated lambda phage and propagation in a sup- host allows only the phage that contain junction fragments to produce plaques. Potential applications of this approach, such as mapping of complex genetic loci or moving from a linked gene toward a gene of interest, are presented and discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor C. R. Huntington's disease. Charting the path to the gene. 1984 Mar 29-Apr 4Nature. 308(5958):404–405. doi: 10.1038/308404a0. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Dunn R. J., Belagaje R., Brown E. L., Khorana H. G. The synthesis and cloning of two tyrosine suppressor tRNA genes with altered promoter sequences. J Biol Chem. 1981 Jun 25;256(12):6109–6118. [PubMed] [Google Scholar]
- Fangman W. L. Separation of very large DNA molecules by gel electrophoresis. Nucleic Acids Res. 1978 Mar;5(3):653–665. doi: 10.1093/nar/5.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Wexler N. S., Conneally P. M., Naylor S. L., Anderson M. A., Tanzi R. E., Watkins P. C., Ottina K., Wallace M. R., Sakaguchi A. Y. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983 Nov 17;306(5940):234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Versatile cosmid vectors for the isolation, expression, and rescue of gene sequences: studies with the human alpha-globin gene cluster. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5225–5229. doi: 10.1073/pnas.80.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Dressler D. Biochemical assay designed to detect formation of recombination intermediates in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1084–1088. doi: 10.1073/pnas.76.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Saffran W., Welsh J., Haas R., Goldenberg M., Cantor C. R. New techniques for purifying large DNAs and studying their properties and packaging. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):189–195. doi: 10.1101/sqb.1983.047.01.024. [DOI] [PubMed] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. doi: 10.1093/nar/11.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P. Electrophoresis of duplex deoxyribonucleic acid in multiple-concentration agarose gels: fractionation of molecules with molecular weights between 2 X 10(6) and 110 X 10(6). Biochemistry. 1980 Jun 24;19(13):3001–3004. doi: 10.1021/bi00554a026. [DOI] [PubMed] [Google Scholar]
- Serwer P. Improvements in procedures for electrophoresis in dilute agarose gels. Anal Biochem. 1981 Apr;112(2):351–356. doi: 10.1016/0003-2697(81)90304-3. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Sakaguchi A. Y., Naylor S. L. Mapping the human genome, cloned genes, DNA polymorphisms, and inherited disease. Adv Hum Genet. 1982;12:341–452. doi: 10.1007/978-1-4615-8315-8_5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Davidson N. On the probability of ring closure of lambda DNA. J Mol Biol. 1966 Aug;19(2):469–482. doi: 10.1016/s0022-2836(66)80017-7. [DOI] [PubMed] [Google Scholar]