Abstract
The endoplasmic reticulum adapts to fluctuations in demand and copes with stress through an adaptive signaling cascade called the unfolded protein response (UPR). Accumulating evidence indicates that the canonical UPR is critical to the survival and function of insulin-producing pancreatic β-cells, and alterations in the UPR may contribute to the pathogenesis of type 2 diabetes. However, the dynamic regulation of UPR molecules in the islets of animal models and humans with type 2 diabetes remains to be elucidated. Here, we analyzed the expression of activating factor 6 (ATF6α) and spliced X-box binding protein 1 (sXBP1), and phosphorylation of eukaryotic initiation factor 2 (eIF2α), to evaluate the three distinct branches of the UPR in the pancreatic islets of mice with diet- or genetic-induced obesity and insulin resistance. ATF6 and sXBP1 expression was predominantly found in the β-cells, where hyperglycemia coincided with a decline in expression in both experimental models and in humans with type 2 diabetes. These data suggest alterations in the expression of UPR mediators may contribute to the decline in islet function in type 2 diabetes in mice and humans.
The endoplasmic reticulum (ER) is highly sensitive to the microenvironment and alters its functional capacity to meet the changing demands of the cell. Perturbations of the folding environment of the ER trigger an adaptive signaling pathway known as the UPR. Activation of the UPR involves the engagement of three ER membrane-resident proteins: PKR-like ER kinase (PERK), inositol requiring 1α (IRE1α), and activating transcription factor 6α (ATF6α)1. These proteins act as sensors of the ER microenvironment and initiate adaptive responses to improve the functional capacity of the ER in response to stress. These adaptive responses include translational inhibition, which is achieved by the PERK/eIF2α (eukaryotic initiation factor 2) arm of the UPR pathway, and chaperone expression, which is supported by the induction of transcription factors ATF6α and the spliced isoform of X-box-binding protein 1 (sXBP1), which is generated downstream of IRE1 activation. Together with protein degradation pathways such as ER-associated degradation (ERAD) and autophagy, the adaptive UPR supports recovery from stress1,2,3, which can be critical for maintaining cell function4. However, prolonged and unresolved stress can lead to a switch from adaptive to maladaptive or pro-apoptotic responses that are often associated with pathological states1,2,3.
Pancreatic β-cells are specialized secretory cells responsible for the production and secretion of insulin in response to glucose fluctuations. Insulin biosynthesis and proper folding require healthy ER function and an intact UPR5,6,7,8. β-cell loss and the development of diabetes have been observed in multiple experimental models where the UPR is compromised9,10,11,12,13,14,15,16,17,18,19,20 and in humans with mutations in genes involved in ER homeostasis11,12,15,16,19,20.
Although genetically impaired UPR function has been linked to β-cell death and diabetes, the regulation of the UPR components and the role of the UPR within β-cells at different stages of type 2 diabetes in animal models and humans has not been examined. Recent studies have demonstrated expression of some of the downstream mediators of the UPR in β-cell lines or in isolated primary islets from type 2 diabetes animal models and human patients, mainly at the transcript level21,22,23,24. However, questions in the field have remained in part because isolating and culturing primary cells could induce stress responses that are not reflective of the in vivo context and mRNA levels may not necessarily reflect the protein levels or provide insight into the posttranslational modifications of UPR components that are required for their activity. Here, in order to gain further understanding of β-cell UPR activation, we analyzed the protein levels of the main proximal regulators of the UPR- ATF6α, sXBP1, and phosphorylated eIF2α- in situ during different stages of diabetes progression. We detected marked modulation of these pathways in pancreas sections from diabetic mouse models and human patients.
Results
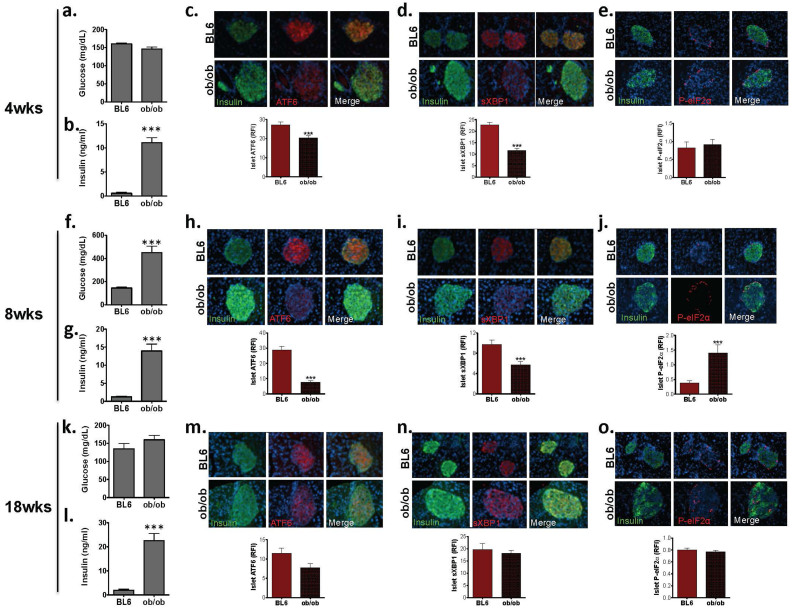
To evaluate the potential modulation of the three branches of the UPR in pancreatic islets during diabetes progression, we examined the expression patterns of ATF6α, sXBP1, and P-eIF2α- at the protein level in situ by immunofluorescence. As a first step, we interrogated leptin-deficient ob/ob mice at 4 weeks of age, a stage at which they are normoglycemic (Figure 1a.), but insulin resistant and hyperinsulinemic (Figure 1b.). Staining of pancreas sections revealed that expression of ATF6 and sXBP1 was markedly reduced in the islets of ob/ob mice, compared to age-matched wild type controls (Figure 1c., 1d). These data suggest that during this period of β-cell compensation to insulin resistance, β-cells of ob/ob mice already exhibit a significant reduction in the expression of critical UPR components. Interestingly, we observed P-eIF2α localized primarily to the non-β-cells of the islets, and in 4-week-old animals its expression was not different between ob/ob and lean controls (Figure 1e.). The P-eIF2α positive cells co-stained with glucagon (See Supplemental Fig. S1 online), suggesting that this branch of the UPR may be more highly expressed in α-cells than in other islet populations.
Figure 1. Expression of UPR mediators in the islets of ob/ob mice at different stages of the disease.
(a). Fasting blood glucose of 4-week-old C57/BL6 and ob/ob mice (b). Fasting serum insulin level of 4-week-old C57/BL6 and ob/ob mice. Immunofluorescence analysis was performed in pancreas sections of 4-week-old C57/BL6 and ob/ob mice (n = 4) by co-staining with (c). anti-ATF6α, (d). anti-sXBP1, or (e). anti-P-eIF2α (red) and anti-insulin (green) antibodies. Quantification of relative fluorescence intensity (RFI) was performed on 10–20 islets per mouse and calculated using MATLAB® (lower panels). (f–j). Analysis of fasting insulin and glucose levels, and UPR mediator expression in 8-week-old C57/BL6 and ob/ob mice. (k–o). Analysis of 18 week-old mice as described above. All data are presented as mean ± SEM, with statistical analysis performed by Student's t test (***p < 0.001, **p < 0.005, *p < 0.05).
Next, we stained pancreas sections from 8-week-old mice, an age at which the ob/ob were profoundly hyperglycemic (Figure 1f.) and hyperinsulinemic (Figure 1g.). At this stage, ob/ob mice continued to exhibit decreased ATF6α and sXBP1 levels (Figure 1h., 1i.). In contrast, P-eIF2α staining intensity was elevated in the α-cells of the islets of obese mice at this age (Figure 1j.). It has been previously demonstrated that ob/ob mice return to normoglycemia following a transient period of hyperglycemia lasting 6-8 weeks25,26. To examine the influence of these changes on islet stress responses, we evaluated ob/ob mice at 18 weeks of age, when they displayed normal glucose levels despite maintaining elevated serum insulin (Figure 1k., 1l.). Staining revealed that this improvement of glycemia was associated with normalization of islet ATF6 and sXBP1 level (Figure 1m., 1n.). Interestingly, the islet P-eIF2α level was no longer elevated in ob/ob mice following this transition (Figure 1o.).
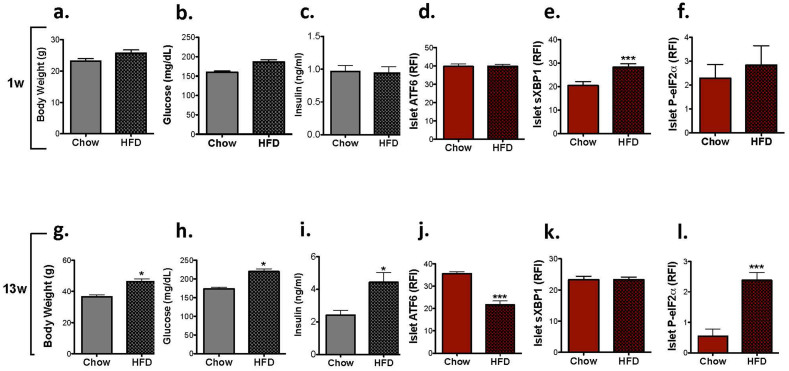
To investigate the modulation of the islet UPR during the development of diet-induced insulin resistance, we placed male C57/BL6 mice on a high fat diet (HFD) that contained 60% kcal from fat. Interestingly, although 1 week of HFD feeding was not sufficient to induce changes in body weight, blood glucose, serum insulin (Figure 2a.–c.), or ATF6 expression (Figure 2d.), the level of β-cell sXBP1 was elevated at this stage (Figure 2e.), suggesting that this brief intervention induced β-cell stress. Similar to our observation in young normoglycemic ob/ob mice, the level of P-eIF2α in α-cells was not altered by HFD at this stage (Figure 2f.). After 13 weeks of the diet manipulation, we observed significant increases in body weight, blood glucose and serum insulin in HFD-fed mice (Figure 2g.–i.). This was accompanied by a significant decline in ATF6 staining intensity in β-cells (Figure 2j.), but a normalization of the level of sXBP1 (Figure 2k.). P-eIF2α expression was significantly increased in the islets at this stage (Figure 2l.).
Figure 2. Expression of UPR markers in the islets of a diet induced animal model of obesity and insulin resistance.
Wild type male mice (n = 4 for each group) were placed either on regular (chow) or high fat diet (HFD). (a–c). Analysis of body weight, fasting glucose, and serum insulin after one week on diet. Immunofluorescence analysis was performed using (d). anti-ATF6 (red), (e). anti-sXBP1 (red), or (f). anti-P-eIF2α (red), and anti-insulin antibodies (green). Quantification was performed on 10–20 islets per mouse. (g–l). Mice and pancreas sections were analyzed as above after 13 weeks on diet. Data are presented as mean ± SEM, with statistical analysis performed by Student's t test (***p < 0.001, *p < 0.05).
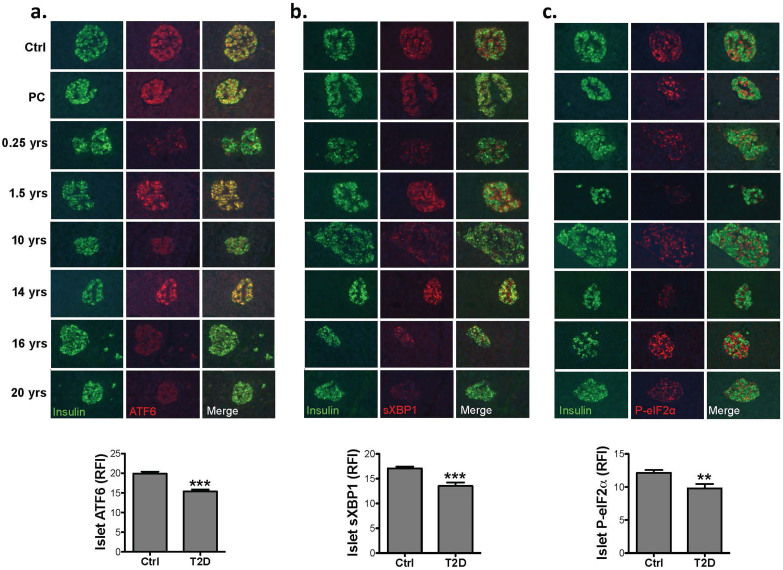
The striking regulation of UPR components in the islets of mouse models prompted us to examine these parameters in the pancreatic islets of type 2 diabetics. We obtained pancreatic sections from 5 control and 8 diabetic donors (Table 1) and assessed the UPR using our immunofluorescence approach. In these samples, ATF6α, sXBP1, and P-eIF2α staining was readily detectable in control sections as well as those from patients with preclinical (PC) or early stage disease. Although the expression of these UPR components was variable in the T2D patients, cumulatively we observed a significant decrease in ATF6α and sXBP1 expression in the islets of diabetic subjects (Figure 3a., 3b.). This pattern is highly reminiscent of our observations in genetically obese mice, and supports the concept that a deficient β-cell UPR response coincides with the emergence of frank disease in both mouse and human. Interestingly, the level of P-eIF2α was reduced in the islets of most diabetic patients (Figure 3c.).
Table 1. Description of pancreatic donors.
| Patient | Age | Sex | Ethnicity | Duration of Diabetes | BMI | C-peptide (ng/ml) | Histology |
|---|---|---|---|---|---|---|---|
| Ctrl | 6.3 | Female | Hispanic | N/A | 18.4 | 5.11 | Normal- excellent islet density. No inflammation or fatty infiltrates |
| Ctrl | 7.5 | Female | Hispanic | N/A | 16.3 | 1.7 | Ins+/Gluc+ islets, normal |
| Ctrl | 8.9 | Female | Hispanic | N/A | 24.2 | 12.13 | Ins+ islets. Mild fatty infiltrate. No inflammatory infiltrates |
| Ctrl | 24 | Female | Hispanic | N/A | 22.6 | No serum | Normal islets. Mild fatty infiltrate. Low Ki67 |
| Ctrl | 72 | Female | Hispanic | N/A | 24.5 | 22.92 | Ins+ normal islets. Low Ki67. Amyloid. Exocrine atrophy w/fatty infiltrates |
| T2D | 55.8 | Female | Hispanic | Pre-clinical | 44.6 | 0.8 | Ins+ (reduced) islets. Low Ki67 |
| T2D | 48.8 | Female | Hispanic | Pre-clinical | 32.5 | <0.05 | Ins+/Gluc+ islets (reduced). Amyloid. Low Ki67. No fatty infiltrate despite high BMI. |
| T2D | 18.8 | Female | Hispanic | 0.25 yrs | 39.1 | 10.68 | Ins+ islets, single cells plentiful. Mild acinar atrophy and pancreatic adipose tissue. |
| T2D | 37.2 | Female | Hispanic | 1.5 yrs | 45.4 | 0.6 | Ins+ islets plentiful with nuclear polymorphism. Minimal fibrosis, no pancreatitis. |
| T2D | 62 | Female | Caucasian | 10 yrs | 19.9 | 6.14 | Ins+/Gluc+ plentiful islets, several with amyloid. Severe, multifocal exocrine atrophy. |
| T2D | 29.8 | Female | Hispanic | 14 yrs | 34.4 | 0.19 | Ins+ islets (reduced), also as clusters and single cells. Multifocal amyloid. Low Ki67. |
| T2D | 39.3 | Female | African American | 16 yrs | 29.1 | 11.55 | Ins+/Gluc+ islets numerous, some hypertrophied. Islet amyloidosis. |
| T2D | 45.8 | Female | Caucasian | 20 yrs | 40.2 | 0.84 | Ins+ islets, many w/amyloid. Mild, interstitial CD3+ infiltrate. Severe exocrine atrophy. |
Pancreatic sections were obtained from nPOD from these donors for immunofluorescence analysis of UPR mediators. Ins: Insulin, Gluc: Glucagon, Ab: Antibody.
Figure 3. Expression of UPR mediators in the islets of type 2 diabetic humans.
(a). Pancreas sections from non-diabetic and diabetic patients were obtained from nPOD and co-stained with anti-ATF6α (red), and anti-insulin (green) antibodies (upper panel) and 10–20 islets per sample were quantified by MATLAB® (lower panel). (b). Pancreas sections from non-diabetic and diabetic subjects co-stained with anti-sXBP1 (red) and anti-insulin (green) antibodies (upper panel) and 10–20 islets per sample were quantified by MATLAB® (lower panel). (c). Pancreas sections from non-diabetic and diabetic subjects co-stained with anti-P-eIF2α (red) and anti-insulin (green) antibodies (upper panel) and 10–20 islets per sample per time point were quantified by MATLAB® (lower panel). All data are presented as mean ± SEM, with statistical analysis performed by one-way ANOVA (***p < 0.001, **p < 0.005, *p < 0.05).
Discussion
Sustaining β-cell secretory function in the face of obesity and insulin resistance is assumed to place a heavy demand on the β-cell ER27. ER stress has been implicated in β-cell failure in diet-induced and genetic mouse models of diabetes21,28,29,30, and in human patients22. However, the dynamic responses of β-cells to increased insulin demand during the development of insulin resistance is not well understood, in part due to limitations in the reagents required to study ATF6α, sXBP1, and P-eIF2α in vivo.
Here, we used recently developed antibodies and staining techniques31 to investigate UPR modulation in mice with genetic and diet-induced obesity and insulin resistance. We found that in the genetic model (ob/ob), disruption of the UPR was detectable in β-cells prior to the onset of hyperglycemia, likely due to the increased demand for insulin production. In this model, the UPR response was insufficient to sustain β-cell functional capacity, and deficiency of the ATF6 and sXBP1 arms was pronounced in 8-week-old mice with insulin resistance. Remarkably, expression of these UPR markers resolved following the transition of ob/ob mice to a euglycemic state, likely indicating a lower level of ER stress within individual β-cells due to the expansion of β-cell mass at this age. In contrast, the pre-diabetic stage of the HFD model was marked by an upregulation of sXBP1, perhaps indicating that β-cells were capable of responding to mild dietary stress by activating UPR pathways. However, as in the genetic model, once HFD-fed mice progressed to hyperglycemia, there was a significant decrease in the level of ATF6, indicating failure of the β-cell UPR to compensate for the ongoing insulin demand. A similar general trend was observed in our analysis of human tissue samples, in which deficiency of UPR component expression was associated with the diabetic phenotype.
In contrast to the primarily β-cell-specific localization of ATF6 and sXBP-1, we observed relatively high expression of the third UPR branch (P-eIF2α) in α-cells of mouse islets, and states of hyperglycemia were associated with increased eIF2α phosphorylation. Models of hyperglycemica including ob/ob mice and HFD-fed rats have previously been shown to be hyperglucagonemic32,33, as have human subjects with impaired glucose tolerance34. Thus our observation of increased eIF2α phosphorylation in the setting of hyperglycemia may reflect an adaptive response within α-cells to the stress associated with increased production of glucagon. Alternatively, it may be possible that different islet cell types that are in contact within the same microenvironment transfer stress signals, and that β-cell stress induces adaptive responses in neighboring cells. The level of eIF2α phosphorylation was decreased in human diabetic samples, which may highlight a difference in the adaptive capacity of this lineage between mice and humans, although there are limitations in these human studies that limit our interpretation and will require further investigation.
A recent study from Chan et al.35 investigated the expression of UPR markers in islets isolated from db/db and ob/ob mice at 6 and 16 weeks of age, demonstrating that ER chaperones and the distal mediators of UPR declined with time in db/db islets but were maintained or increased at 16 weeks in ob/ob islets. In contrast, we observed decreased expression of UPR mediators within ob/ob islets during the hyperglycemic stage, and normalization in euglycemic 18 week-old animals. These differences may be related to the timing of analysis in our study, or may reflect differential regulation of these mediators at the RNA and protein level. In addition, it may be difficult to reconcile our in situ analysis with measurements in islets ex vivo, because the islet isolation process may itself alter stress responses. However, taken together with previously reported studies, the data presented here support a model in which ER stress is evident in beta cells at earlier stages of disease and continuous beta cell stress that cannot be met with a continuous and productive UPR in the long term may lead to the demise of these cells. Finally, the defective patterns of some UPR branches appears to be a shared pathology between type 1 and type 2 diabetes31.
We believe that this work represents an important advance in the understanding of the dynamic responses of islet cells to the stress associated with the development of insulin resistance and diabetes. However, further investigation and interventional studies will be required to understand how alterations in specific branches of the UPR affect islet function. In addition, due to the small sample size available for the analysis, in our human studies we are not currently able to determine whether UPR component expression declines as a marker or driver of β-cell failure. This notwithstanding, the broad agreement between our observations in animal models and in human samples support the hypothesis that ER stress plays an important role in type 2 diabetes in humans, and that a decline in adaptive responses within β-cells underlies the progression of the disease. In addition to its role in β-cells, ER stress also underlies the development of obesity-induced insulin resistance in other tissues2,36,37,38. Thus, it will be interesting to determine if chemical chaperone treatment is an effective strategy to manage both β function and peripheral insulin sensitivity in diabetes, both type 1 and type 231,39,40.
Methods
Human pancreatic tissue
Paraffin embedded pancreatic sections from female cadaveric donors including non-diabetic controls and type 2 diabetes patients were obtained from the Juvenile Diabetes Research Foundation (JDRF)-sponsored Network for Pancreatic Organ Donors with Diabetes (nPOD) program (http://www.jdrfnpod.org/for-investigators/online-pathology-information/). Insulin positive (Ins+) β-cells were used for staining and quantification. Information regarding the human tissue donors is in Table 1.
Mouse models
The animal care and experimental procedures were performed in accordance and with approval from animal care committees of Harvard University. Male C57/BL6 and homozygous B6.V-Lepob/J (ob/ob) mice were purchased from Jackson Labs. C57BL/6J mice used in the diet-induced obesity model were placed on high fat diet (Research Diets: D12492) at 10 weeks of age for the indicated period of time.
Histological analyses
Pancreata from C57/BL6 and ob/ob mice were dissected, formalin fixed, and paraffin embedded. Antigen retrieval was performed on 5 μm serial sections of the pancreata by heating the sections in citrate buffer (pH: 6) for 20 mins. Sections were blocked with 5% normal goat serum in phosphate buffered saline (PBS) for 30 mins and incubated with primary antibodies: anti-insulin (1:200, Linco) 2 h at room temperature, anti-ATF6α (1:20, Santa Cruz Biotechnology), anti-sXBP1 (1:20, in house, rabbit poly-clonal), and anti- P-eIF2α (1:20, Invitrogen) at 4°C overnight. Sections were then incubated for 2 h at room temperature with goat anti-guinea Alexa 488 or with goat anti-rabbit Alexa 568 (1:200, Invitrogen Molecular Probes) in PBS. Samples were mounted in Vectashield medium with DAPI (Vector Laboratories) for viewing with a Zeiss Apotome system. ATF6 and sXBP1 immunostaining was validated using β-cell deletion models for both genes as previously described31.
Image analysis
Image analysis was performed by using custom software developed by MATLAB® (The Mathworks, Inc.). Briefly, islet regions were identified as contiguous areas (connected pixels) of insulin staining (green channel) at or above a threshold intensity value optimized across multiple images. Data acquisition was automated, and mean fluorescence intensity for insulin (green channel) and for either sXBP1, ATF6 or P-eIF2α (red channel) was calculated as the sum of intensities for all pixels divided by the number of pixels within the islet.
Statistical analysis
Analysis of data was performed using the program GraphPad Prism. Data are represented as the means ± SEM and analyzed using Student's t test and one-way ANOVA with Tukey's post test. P < 0.05 was considered statistically significant.
Author Contributions
The hypothesis and research concept was by F.E. and G.S.H. F.E. designed and performed experiments, analyzed the data, and wrote the first draft of the article, G.S.H. supervised research, analyzed the data, and wrote the article. T.N. and A.Y. contributed to experiments.
Supplementary Material
Supplemental Figure 1
Acknowledgments
We thank Dr. Kathryn C. Claiborn for critical reading of the manuscript. This work was supported by a grant from the Juvenile Diabetes Research Foundation to G.S.H.
References
- Walter P. & Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011). [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13, 89–102 (2012). [DOI] [PubMed] [Google Scholar]
- Gregor M. F. et al. The role of adipocyte XBP1 in metabolic regulation during lactation. Cell Rep 3, 1430–1439 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M. & Nolan C. J. Islet beta cell failure in type 2 diabetes. J Clin Invest 116, 1802–1812 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A. E. et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003). [DOI] [PubMed] [Google Scholar]
- Eizirik D. L. & Cnop M. ER stress in pancreatic beta cells: the thin red line between adaptation and failure. Sci Signal 3, pe7 (2010). [DOI] [PubMed] [Google Scholar]
- Fonseca S. G., Gromada J. & Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab 22, 266–274 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 103, 27–37 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. A. Proinsulin and the genetics of diabetes mellitus. J Biol Chem 284, 19159–19163 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepine M. et al. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 25, 406–409 (2000). [DOI] [PubMed] [Google Scholar]
- Senee V. et al. Wolcott-Rallison Syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes 53, 1876–1883 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang P. et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22, 3864–3874 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D., Wei J., Gupta S., McGrath B. C. & Cavener D. R. Acute ablation of PERK results in ER dysfunctions followed by reduced insulin secretion and cell proliferation. BMC Cell Biol 10, 61 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H. et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 20, 143–148 (1998). [DOI] [PubMed] [Google Scholar]
- Strom T. M. et al. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet 7, 2021–2028 (1998). [DOI] [PubMed] [Google Scholar]
- Ishihara H. et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet 13, 1159–1170 (2004). [DOI] [PubMed] [Google Scholar]
- Riggs A. C. et al. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia 48, 2313–2321 (2005). [DOI] [PubMed] [Google Scholar]
- Thameem F., Farook V. S., Bogardus C. & Prochazka M. Association of amino acid variants in the activating transcription factor 6 gene (ATF6) on 1q21-q23 with type 2 diabetes in Pima Indians. Diabetes 55, 839–842 (2006). [DOI] [PubMed] [Google Scholar]
- Meex S. J. et al. Activating transcription factor 6 polymorphisms and haplotypes are associated with impaired glucose homeostasis and type 2 diabetes in Dutch Caucasians. J Clin Endocrinol Metab 92, 2720–2725 (2007). [DOI] [PubMed] [Google Scholar]
- Laybutt D. R. et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50, 752–763 (2007). [DOI] [PubMed] [Google Scholar]
- Marchetti P. et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50, 2486–2494 (2007). [DOI] [PubMed] [Google Scholar]
- Seo H. Y. et al. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases insulin gene expression via up-regulation of orphan nuclear receptor small heterodimer partner. Endocrinology 149, 3832–3841 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. J. et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56, 2016–2027 (2007). [DOI] [PubMed] [Google Scholar]
- Garthwaite T. L., Martinson D. R., Tseng L. F., Hagen T. C. & Menahan L. A. A longitudinal hormonal profile of the genetically obese mouse. Endocrinology 107, 671–676 (1980). [DOI] [PubMed] [Google Scholar]
- Menahan L. A. Age-related changes in lipid and carbohydrate metabolism of the genetically obese mouse. Metabolism 32, 172–178 (1983). [DOI] [PubMed] [Google Scholar]
- Back S. H., Kang S. W., Han J. & Chung H. T. Endoplasmic reticulum stress in the beta-cell pathogenesis of type 2 diabetes. Exp Diabetes Res 2012, 618396 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T. et al. Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice. J Clin Invest 120, 115–126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez G. et al. Adaptation and failure of pancreatic beta cells in murine models with different degrees of metabolic syndrome. Dis Model Mech 2, 582–592 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva M. M. et al. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci USA 106, 19090–19095 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F. et al. Restoration of the Unfolded Protein Response in Pancreatic beta Cells Protects Mice Against Type 1 Diabetes. Sci Transl Med 5, 211ra156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumrad N. A., Stearns S. B., Tepperman H. M. & Tepperman J. Studies on serum lipids, insulin, and glucagon and on muscle triglyceride in rats adapted to high-fat and high-carbohydrate diets. J Lipid Res 19, 423–432 (1978). [PubMed] [Google Scholar]
- Dubuc P. U., Mobley P. W., Mahler R. J. & Ensinck J. W. Immunoreactive glucagon levels in obese-hyperglycemic (ob/ob) mice. Diabetes 26, 841–846 (1977). [DOI] [PubMed] [Google Scholar]
- Larsson H., Berglund G. & Ahren B. Glucose modulation of insulin and glucagon secretion is altered in impaired glucose tolerance. J Clin Endocrinol Metab 80, 1778–1782 (1995). [DOI] [PubMed] [Google Scholar]
- Chan J. Y., Luzuriaga J., Bensellam M., Biden T. J. & Laybutt D. R. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in beta-cell gene expression and progression to diabetes. Diabetes 62, 1557–1568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004). [DOI] [PubMed] [Google Scholar]
- Gregor M. F. et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C., Giacca A. & Lewis G. F. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes 60, 918–924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kars M. et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59, 1899–1905 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1