Abstract
Aim of the study
Ginseng has been used as a folk medicine for thousands of years in Asia, and has become a popular herbal medicine world-wide. Recent studies have revealed that ginseng, including American ginseng, exerts antioxidant effects in the cardiovascular system; however, the underlying mechanisms are not fully understood. Thus, we investigated role of Nrf2, a master transcription factor of endogenous anti-oxidative defense systems, in the regulation of American ginseng-mediated anti-oxidative actions in cardiomyocytes.
Materials and methods
A standardized crude extract of American ginseng was supplied by the National Research Council of Canada, Institute for National Measurement Standards. H9C2 cells, a rat cardiomyocyte cell line, were exposed to angiotensin II (Ang II) or tumor necrosis factor alpha (TNFα) to induce oxidative stress that was examined by measuring formation of reactive oxygen and nitrogen species. Oxidative stress-induced cell death was induced by exogenous addition of hydrogen peroxide (H2O2). Proteins were measured by Western blot and mRNA expression was determined by quantitative real time PCR. Nrf2-driven transcriptional activity was assessed by antioxidant response element (ARE)-luciferase reporter assay. Direct Nrf2 binding to its target gene promoters was determined by chromatin immunoprecipitation assay. Adenoviral overexpression of Nrf2 shRNA was utilized to knock down Nrf2 in H9C2 cells. Immunochemical staining was applied for Nrf2 expression in the heart.
Results
American ginseng induced dramatic increases in Nrf2 protein expression, Nrf2 nuclear translocation, Nrf2 transcriptional activity, direct Nrf2 binding to its target gene promoters, and expression of a group of anti-oxidative genes driven by Nrf2 in H9C2 cells. In addition, American ginseng inhibited Ang II- or TNFα-induced free radical formation and H2O2-induced cell death in H9C2 cells over-expressed with control shRNA but not in the cells over-expressed with Nrf2 shRNA. Finally, oral administration of American ginseng markedly increased Nrf2 activity in murine hearts.
Conclusion
These results demonstrate that American ginseng suppresses oxidative stress and oxidative stress-induced cell death in cardiomyocytes through activating the Nrf2 pathway, thereby providing cardioprotection against pathological cardiac remodeling.
Keywords: Panax quinquefolius, American ginseng, Nrf2, Oxidative stress, Cardiomyocytes, Cell death
1. Introduction
Ginseng is the root of genus Panax; including Panax ginseng (Chinese and Korean ginseng), P. notoginseng (Chinese Sanqi ginseng), and P. quinquefolius (American ginseng). The name ginseng comes from the Chinese words "Ren Sheng," meaning "man-herb," because of the humanoid shape of the root or rhizome of the plant. Carl Anton Meyer, a Russian botanist, named it Panax ginseng C. A. Meyer in 1843 (Gillis, 1997). The genus name Panax means “All-Healing” or “Cure All” in Greek, which describes the traditional belief that ginseng has properties to heal all aspects of the body. Ginseng has been used as a folk medicine for thousands of years in Asian countries, and has become a popular herbal medicine world-wide. It is estimated that ginseng might be the second top-selling herbal supplement in USA.
A growing body of evidence has revealed that regular use of ginseng is helpful in the treatment of cardiovascular diseases (Chan et al., 2002; Chen, 1996; Gillis, 1997; Wang et al., 2007; Zhou et al., 2004). Meanwhile, concerns on the potential adverse interactions between ginsengs and cardiovascular drugs have also been raised (Izzo et al., 2005). Nevertheless, the cellular and molecular mechanisms by which ginseng induces the pleiotropic cardiovascular actions are largely unknown, and the nature of ginseng-mediated cardiovascular protection is still enigma. The clinical efficacy of ginsengs on cardiovascular diseases has not yet established.
It has been demonstrated that regular ginseng consumption increases an antioxidant capacity of various tissues including the heart (Fu and Ji, 2003). Moreover, different ginseng extracts and individual components protect against cardomyocyte hypertrophy and death as well as maladaptive cardiac remodeling via a mechanism of suppressing oxidative stress remarkably in common although different content of ginseng-mediated cardiac protections might be coupled with other specific mechanisms (Jiang et al., 2007; Liu et al., 2004; Mehendale et al., 2006; Shao et al., 2004; Xie et al., 2006; You et al., 2005). These results suggest that activation of anti-oxidative signaling serves as a common pathway contributing to the ginseng-mediated cardiac protection. However, critical determinants for the ginseng-induced antioxidant activity in the heart remain unknown.
Nrf2 belongs to the Cap ‘n’ Collar (CNC) family of basic leucine zipper (bZip) transcription factors that include NF-E2, Nrf1-3 and Bach1-2 (Li et al., 2009a). Nrf2 is a pleiotropic protein that binds to a cis-acting enhancer sequence known as the antioxidant response element (ARE) with a core nucleotide sequence of 5‘-RTGACnnnGC-3‘ to control the basal and inducible expression of a battery of antioxidant genes and other cytoprotective phase II detoxifying enzymes, such as heme oxygenase-1 (HO-1), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-S-transferases (GST), NAD(P)H:quinone oxidoreductase (NQO1), NQO2, thioredoxin-1 (Txn-1), and thioredoxin reductase-1 (Txnrd-1). Several Nrf2 target genes, including HO-1 (Hu et al., 2004; Wiesel et al., 2001), SOD (Lu et al., 2008), and GPx (Matsushima et al., 2006), have been demonstrated to protect the heart against maladaptive remodeling. Moreover, emerging evidence has revealed that Nrf2/ARE signaling plays a key role in preventing oxidative cardiac cell injury in vitro (Zhu et al., 2005; Zhu et al., 2008). Importantly, we have recently demonstrated that Nrf2 coordinates a group of antioxidant genes, including GPx, HO-1, NQO-1, Txn-1, Txnrd-1, SOD-2, and SOD-3, to suppress oxidative stress in the heart, and serves as a negative regulator of maladaptive cardiac remodeling and dysfunction (Li et al., 2009b). These results indicate that Nrf2 plays a critical role in maintaining structural and functional integrity of the heart that is abnormally stressed. Of note, Nrf2 signaling has been shown to be involved in wild Panax ginseng-mediated protection against liver injury (Gum et al., 2007). Therefore, an intriguing question to be raised is whether or not ginseng activates Nrf2 to suppress cardiac oxidative stress, thereby protecting the heart against maladaptive cardiac remodeling and dysfunction.
Here, we demonstrate that American ginseng suppresses oxidative stress and oxidative stress-mediated cell death via activating Nrf2 signaling in cardiomyocytes. In addition, American ginseng up-regulates Nrf2 protein expression and enhances expression of its downstream anti-oxidative genes in the heart. Our data provides the first evidence that activation of Nrf2 signaling is critical for the ginseng-mediated cardiac protective actions.
2. Materials and methods
2.1. American ginseng extract
A standardized American ginseng extract was supplied by the National Research Council of Canada, Institute for National Measurement Standards (NRCC-INMS). This extract was derived from four year old, cultivated, ginseng roots grown by Chai-Na-Ta Farms Ltd. (Kamloops, British Columbia, Canada) and processed by Canadian Phytopharmaceuticals Corporation (Richmond, British Columbia, Canada). The identity of the roots was independently confirmed by Agriculture and Agri-Foods Canada and a voucher specimen of the Panax quinquefolius used in this study deposited with the University of Ottawa herbarium (UO 19908). Plant morphology conformed to that as previously described for P. quinquefolius (Small and Catling 1999) and the presence of a marker compound ginsenoside F11, unique to P. quinquefolius, confirmed by liquid chromatography - mass spectrometry. Following thorough homogenization, 4000 1g lots of the extract were bottled under argon, irradiated (5 kGy), and stored under cryogenic conditions (−80°C). Periodic analyses of the extract over 4 years has shown no significant change in ginsenoside content which is 10.5% w/w measured as the sum of: Rg1 3.5 (0.1), Re 21.2 (0.4), Rb1 45.1 (1.6), Rc 15.5 (0.7), Rb2 2.2 (0.1), Rd 17.8 (0.4) mg/g ± one standard deviation, respectively. The American ginseng crude extract was prepared as previously described (Ichikawa et al., 2009b). Solutions of American ginseng extract, at different concentrations, were freshly prepared before each experiment. Briefly, American ginseng extract was weighted and dissolved in Dulbecco’s modified Eagle’s media (DMEM) (Cat. No. 10313-039, Invitrogen Corp., Carlsbad, CA) to get a solution of American ginseng extract (2 mg/ml). This solution was passed through a 0.2 µm syringe filter (Cat. No. PN4192, Pall Life Science, Ann Arbor, MI) and then diluted using DMEM to get the working solutions of American ginseng extract with different concentrations for each experiment.
2.2. Cell culture, Nrf2 transcriptional reporter assay, and adenoviral knockdown of Nrf2
H9C2 (American Type Culture Collection, Rockville, MD, USA), a rat cardiomyocyte cell line, were cultured in DMEM supplemented with 10% fetal bovine serum. H9C2 cells at passages of 10 to 15 were used in the present study. Transcriptional activity of Nrf2 was determined by measurement of luciferase (luc) activities in H9C2 cells that were transfected with Nrf2 reporter gene ARE-luc (firefly luciferase) and internal control pRL-TK-luc (renilla luciferase) plasmids as previous described (Ichikawa et al., 2008). Adenoviral knockdown of Nrf2 was performed as previous described (Ichikawa et al., submitted manuscript). Cell death was measured using the Cytotoxicity Detection Kit PLUS (LDH, Cat. No. 04744926001. Roche Applied Science, Indianapolis, IN). Cell viabilitywas calculated as follows: Cell viability = (LDH activity of cell lyate in Exp.)/(LDH activity of cell lyate in Con.) × 100%. Exp. Indicates experimental groups except the control (Con.) of Ad-scramble (−).
2.3. Quantitative real-time PCR (Q-PCR)
Q-PCR was performed as previously described (Li et al., 2009b). Expression levels of interested genes were normalized by concurrent measurement of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels. Primers for Q-PCR were shown in supplementary table 1.
2.4. Chromatin immunoprecipitation (ChIP) assay
ChIP was performed as described previously with a minor modification (Ichikawa et al., 2008). Briefly, cells were fixed in 1% formaldehyde for 10 minutes at room temperature and cross-linking was stopped by addition of glycine at a final concentration of 0.125 M. The fixed cells were washed twice with ice-cold PBS and harvested in Lysis Buffer (sc-45000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA.) supplemented with protease inhibitors. Cells were pelleted by centrifugation, and washed once in ice-cold PBS. Cell pellet was resuspended in Lysis Buffer High Salt (sc-45001, Santa Cruz) supplemented with proteinase inhibitor, and then disrupted by sonication, yielding genomic DNA fragments with a bulk size of 200 – 1000 bp that was determined by ethidium bromide gel electrophoresis. For each immunoprecipitation, 0.3 ml of lysate was precleared by addition of 50 µl Protein A/G PlusAgarose (sc-2003, Santa Cruz). Aliquots (0.2 ml) of precleared suspension were reserved for input DNA. Immunoprecipitation was performed overnight at 4 °C with an antibody for Nrf2 (sc13032, H-300, Santa Cruz). The antibody, protein, and DNA immune complex was captured by centrifugation, washed once with Lysis Buffer High Salt (sc-45001, Santa Cruz) and 4 times with Wash Buffer (sc-45002, Santa Cruz), then resuspended in 400 µl Elution Buffer (sc-45003, Santa Cruz). Precipitated and input chromatins were subjected to reverse cross-links by incubating tubes in a 67 °C water bath overnight. The DNA was concentrated with PrepEase DNA Clean-up Kit (Cat. No. 78758, USB Corp., Cleveland, Ohio). The precipitated DNA and input DNA were used for measurement of the presence of the selected ARE sequences by Q-PCR utilizing promoter-specific primers (Supplementary table 2). Nrf2 binding to ARE on the promoters was assessed by the relative presence of selective DNA sequences in the precipitated DNA to that in the corresponding input DNA.
2.5. Measurement of reactive oxygen species (ROS) and reactive nitrogen species (RNS)
H9C2 cells infected with adenovirus of control scramble shRNA (20 MOI) or Nrf2 shRNA (20 MOI) were stimulated with angiotensin II (Ang II) and/or American ginseng extract (Am. g.) for 1 hour as indicated. Formation of reactive free radicals of superoxide (O2•−) or peroxynitrite (ONOO•−) was measured using the chemical probes of dihydroethidium (DHE, Cat. No. D23107, Invitrogen) or dihydrorhodamine 123 (DHR 123, Cat. No. D23806, Invitrogen) as previously described (Li et al., 2009b). DHE, a nonfluorescent membrane-permeant probe, interacts with O2•−, leading to the liberation of membrane-impermeant ethidium cations that fluoresce on intercalating with nuclear DNA (Rothe and Valet, 1990). DHR-123 is oxidized by ONOO •− to the highly fluorescent product rhodamine in vitro (Gagnon et al., 1998). Experiments for the detection of O2•− or ONOO •− were repeated for 3 times with duplicate wells for each group. The culture slides were photographed immediately after mounted with coverslips using Nikon ECLIPSE E600 (Nikon Inc., Melville, NY). For every well, 8 fields were randomly chosen to photograph and integrated optical density (IOD)s of the images were quantified with Image-Pro Plus 6.0 (Media Cybernetics, Inc., Bethesda, MD) as previously described (Li et al., 2009b).
2.6. Animals
Male C57BL/6J mice at age of 8 weeks were administrated with American ginseng extract (30 mg/kg) or vehicle (saline) by single gavage. Hearts were harvested at different time after the administration as indicated. Myocardial expression of Nrf2 was examined by immunochemical staining. Myocardial expression of Nrf2 downstream genes was determined by Q-PCR. All of the animal procedures were approved by the University’s Institutional Animal Care and Use Committee.
2.7. Immunochemical staining and confocal microscopic analysis
Cells cultured on Lab-Tek™ Chamber Slides (Cat. No. 12-565-22, Thermo Fisher Scientific, Rochester, NY) and left ventricle tissue sections of the mice fed with or without American ginseng were stained using rabbit polyclonal anti-Nrf2 (sc-13032, H300, Santa Cruz) as previously described (Li et al., 2009b). Nuclei were labeled using blue dye of 4',6-Diamidino-2-phenylindole (DAPI, Cat. No. D9542, Sigma-Aldrich, St. Louis, MO). F-actin was labeled using green dye of Alexa Fluor 488 phalloidin (Cat. No. A12379, Invitrogen). Images were acquired using a confocal microscope (LSM510META, Carl Zeiss Inc., Maple Grove, MN). For semi-quantification of Nrf2 protein expression in the heart, 8 fields of one Nrf2 stained section (picked up by chance from each heart) were randomly chosen to photograph and integrated optical density (IOD)s of Nrf2 staining were quantified with Image Pro Plus software as previously described (Li et al., 2009b).
2.8. Statistics
Values are expressed as mean ± SEM in the text and figures. The data were analyzed using ANOVA with the LSD test unless specified. Values of P < 0.05 were considered to be statistically significant.
3. Results
American ginseng activates Nrf2 pathway, thereby suppressing oxidative stress in H9C2 cardiomyocytes
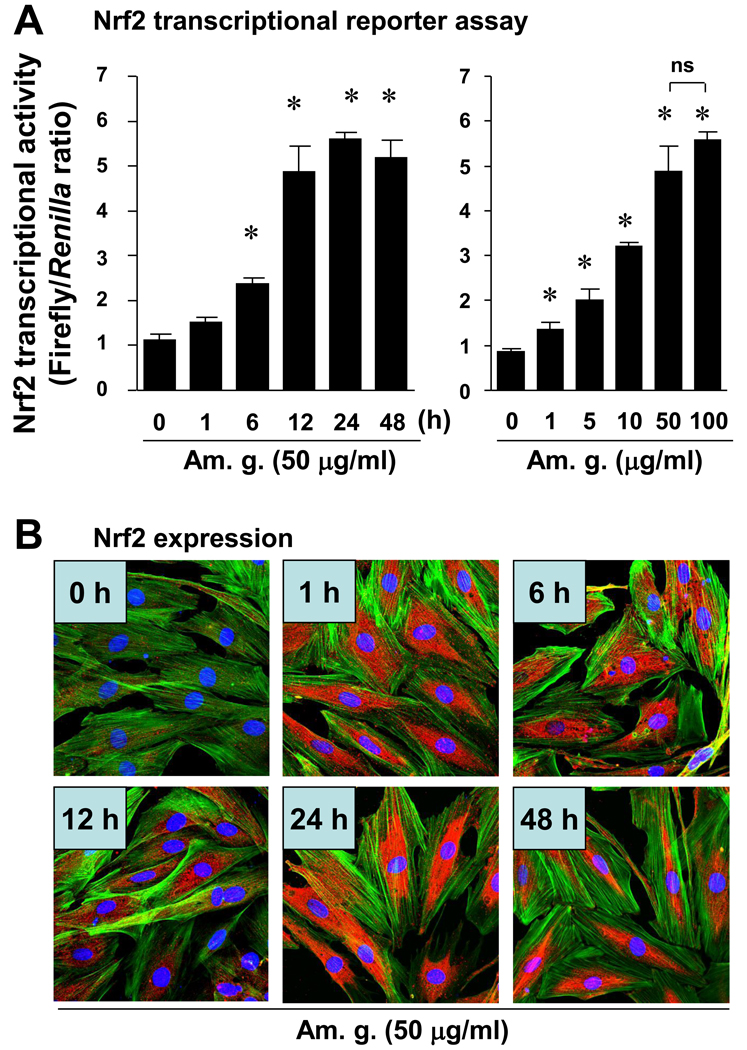
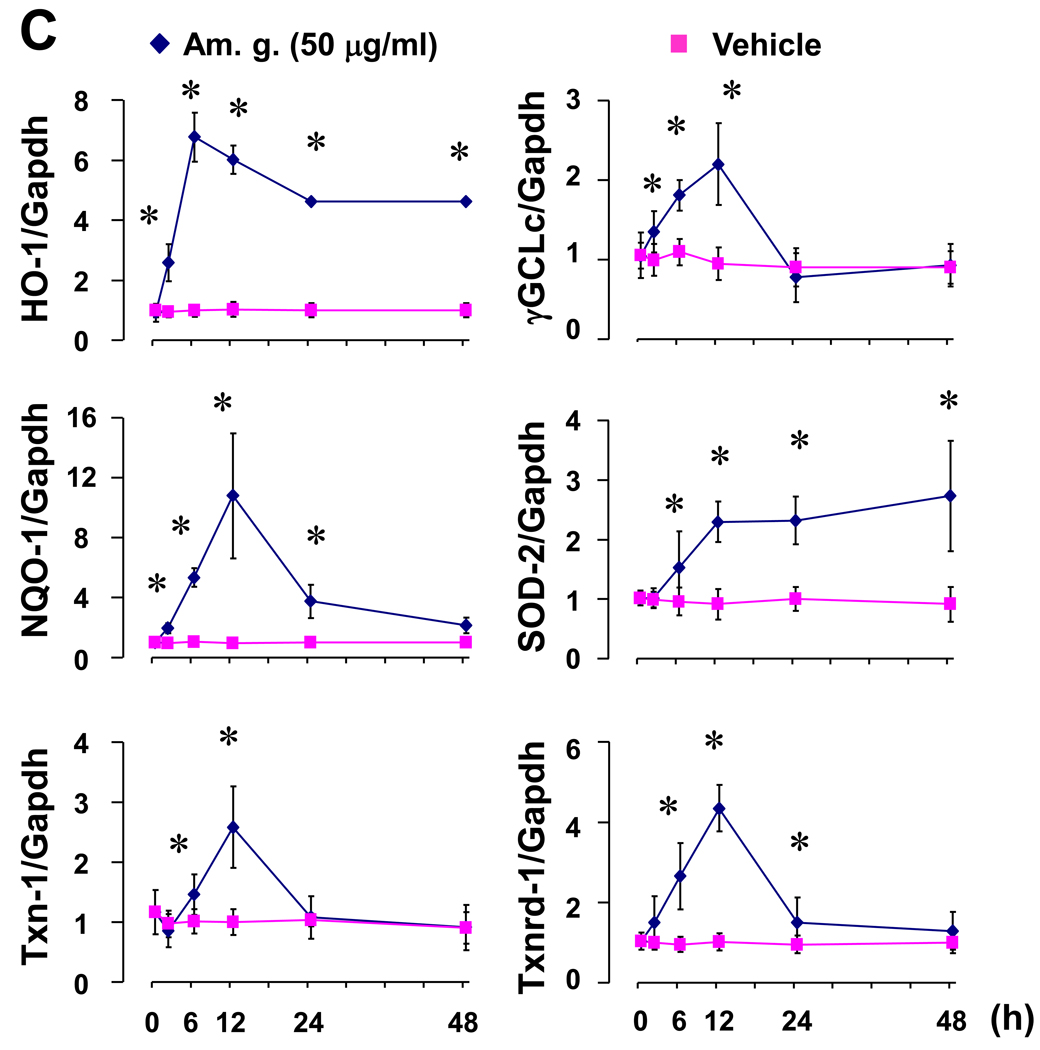
To exclude any cytotoxic effect of the American ginseng extract supplied by NRCC-INMS, we carefully determined American ginseng extract-mediated cytotoxicity in H9C2 cells - a rat cardiomyocyte cell line. In quiescent H9C2 cells that were induced by serum starvation for 24 hours, American ginseng extract did not exhibit any cytotoxic effects at concentrations up to 200 µg/ml (Supplementary Fig. 1). Thus, we used American ginseng extract within a range of non-cytotoxic doses in the present study. We then mechanistically analyzed the American ginseng-mediated inhibition of oxidative stress with a focus on Nrf2 signaling in H9C2 cells. American ginseng extract (50 µg/ml) time-dependently activated Nrf2 transcriptional activity in H9C2 cells (Fig. 1A). American ginseng extract was able to activate Nrf2 transcriptional activity at a dose as low as 1 µg/ml and induced the maximal activation of Nrf2 at a dose of 50 µg/ml (Fig. 1A). Therefore, we used American ginseng crude extract at the dose of 50 µg/ml for the other experiments in vitro. Immunochemical staining analysis showed that American ginseng extract (50 µg/ml) up-regulated Nrf2 protein expression (red) and enhanced Nrf2 nuclear translocation (pink) (Fig. 1B). The Nrf2 protein expression and nuclear translocation appeared apparent as earlier as at 1 hour, reached a peak at 6 hour and maintained high levels till 48 hour after American ginseng treatment (Fig. 1B). Western blot analysis confirmed that American ginseng extract increased Nrf2 nuclear translocation in H9C2 cells (Supplementary Fig. 2). Several Nrf2 downstream genes, including NQO-1, Txn-1, Txnrd-1 and SOD-2 that are involved in Nrf2-mediated cardiac protection (Li et al., 2009b), were dramatically up-regulated by American ginseng extract (Fig. 1C). In contrast, adenoviral knockdown of Nrf2 blocked the American ginseng-mediated up-regulation of these anti-oxidative gene expressions (Table 1). To further confirm the American ginseng-activated Nrf2 transcriptional activity, effect of American ginseng on a directing binding of Nrf2 to ARE on promoters of its downstream genes was determined by ChIP assay. Whole non-coding sequences (all upstream of transcriptional start site) of NQO-1, Txn-1 and SOD-2 were obtained by UCSC Genome Browser and aligned with the core ARE motifs of RTGACnnnGC or its reverse using Lasergene (DNASTAR, Inc., Madison, WI). Based on the sequences of 300 bp upstream and downstream of each ARE on each gene promoter, primers were designed with Lasergene (Supplementary table 2). ChIP assay showed that American ginseng induced direct binding of Nrf2 to ARE on the promoters of NQO-1, Txn-1 and SOD-2 in H9C2 cells (Fig. 2 and supplementary table 3). Overall, these results clearly demonstrate that American ginseng could up-regulate Nrf2 protein levels, enhance Nrf2 nuclear translocation, and activate Nrf2-driven expression of a group of anti-oxidative genes in cardiomyocytes.
Fig. 1.
Effect of American ginseng on Nrf2 transcriptional activity in H9C2 cells. (A) Cells transfected with Nrf2 transcription reporter gene of ARE-luc and internal control gene of pRL-TK-luc were cultured in serum-free DMEM for 24 hours to induce a quiescent status, and then treated with or without American ginseng extract (Am. g. ) as indicated. Nrf2 transcriptional activity was assessed by dual luciferase assay as described in “Methods”. Left panel was the time-course analysis (Am. g. 50 µg/ml), *p < 0.05 vs non-treated group (Am. g., 0), n=4. Right panel was the dose-response analysis (American ginseng treatment for 12 hours). *p < 0.05 vs non-treated group (Am.g. 0), n=4. (B) Quiescent cells were treated with or without American ginseng extract (Am. g., 50 µg/ml) as indicated. Nrf2 protein expression as well as nuclear translocation was examined by immunochemical staining of Nrf2 and confocal microscopic analysis. Red is Nrf2. Green is F-actin. Blue is nuclei. Results were representatives of three separated experiments. (C) Quiescent cells were treated with or without American ginseng extract (Am. g., 50 µg/ml) as indicated. Expression of Nrf2 downstream genes including HO-1, Txn-1, Txnrd-1, NQO-1, SOD-2, and γGCLc was quantified by Q-PCR. *p < 0.05 vs Vehicle (non-treated), n=4.
Table 1. Effect of Nrf2 knockdown on American ginseng-induced activation of Nrf2 downstream genes in H9C2 cells.
H9C2 cells infected with adenovirus of control scramble shRNA or Nrf2 shRNA for 48 hours, and then stimulated with or without American ginseng extract (Am. g. 50 µg/ml) for 12 hours. Gene expression was quantified by Q-PCR.
| Genes | Ad-scramble shRNA | Ad-Nrf2 shRNA | ||||
|---|---|---|---|---|---|---|
| Vehicle | Am. g. | p | Vehicle | Am. g. | p | |
| HO-1 | 0.92+0.180 | 4.14+0.142 | A | 0.26+0.028 | 0.39+0.073 | C,D |
| NQO-1 | 0.97+0.191 | 3.86+0.281 | A | 0.16+0.012 | 0.23+0.033 | C, D |
| Txn-1 | 0.95+0.250 | 1.75+0.325 | A | 0.94+0.134 | 1.13+0.250 | C,D |
| Txnrd-1 | 0.94+0.125 | 1.96+0.184 | A | 0.28+0.024 | 0.33+0.070 | C, D |
| γGCLc | 0.96+0.098 | 1.84+0.161 | A | 0.30+0.073 | 0.34+0.052 | C, D |
| SOD-2 | 1.012+0.165 | 1.71+0.128 | A | 2.43+0.629 | 2.84+0.788 | C,D |
p < 0.05, Am. g. vs Vehicle;
p < 0.05, Vehicle of Ad-Nrf2 shRNA vs Vehicle of Ad-scramble;
p < 0.05, Am. g. of Ad-scramble vs Am. g. of Ad-Nrf2 shRNA; n=4.
Fig. 2.
Effect of American ginseng on Nrf2 binding to ARE on the promoters of its target genes in H9C2 cells. Cells were stimulated with or without American ginseng extract (Am. g., 50 µg/ml) for 12 hours and subjected to Chip assay as described in “Methods”. *p < 0.05 vs vehicle, n=4. TSS, transcriptional start site.
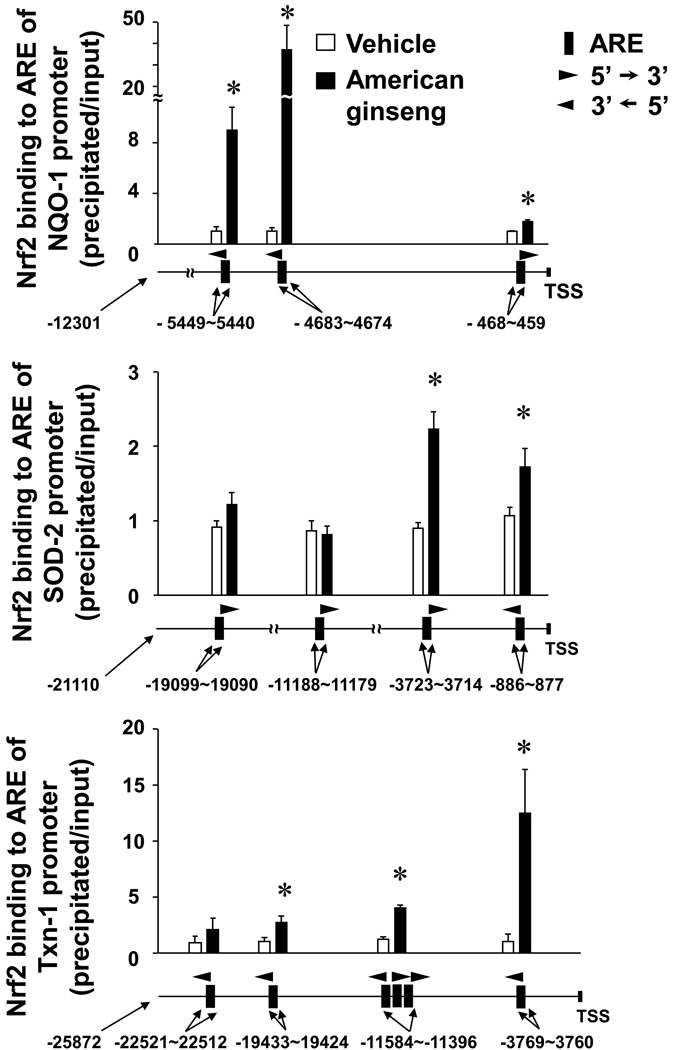
To further study role of Nrf2 in American ginseng-mediated suppression of oxidative stress in cardiomyocytes, we applied Nrf2 loss-of-function approach utilizing adenovirus of Nrf2 shRNA. Adenoviral knockdown of Nrf2 led to increases in formation of ROS and RNS that was evidenced by robust increases in formation of O2•− and ONOO •− in H9C2 cells (Fig. 3). Co-administration of American ginseng extract with Ang II dramatically suppressed Ang II-induced formation of O2 •− and ONOO •− in the cells infected with adenovirus of control scramble shRNA, whereas the anti-oxidant effects of American ginseng were largely impaired in the cells infected with adenovirus of Nrf2 shRNA (Fig. 3). American ginseng exerted similar effects on the formation of these free radicals in TNFα-inflamed H9C2 cells infected with adenovirus of control shRNA or Nrf2 shRNA (Supplementary Fig. 3). These results demonstrate that activation of Nrf2 signaling is essential for American ginseng-induced suppression of oxidative stress in cardiomyocytes.
Fig. 3.
Effect of Nrf2 knockdown on American ginseng-mediated suppression of ROS and RNS formation in H9C2 cells. Cells infected with adenovirus of control scramble shRNA or Nrf2 shRNA for 48 hours, and then treated with Ang II (0.5 µM) and American ginseng extract (Am. g., 200 µg/ml) for 1 hour as indicated. Free radical formation was determined as described in “Methods”. *p < 0.05 vs Ad-scramble (−); #p < 0.05 vs Ang II (+) of Ad-scramble;§p < 0.05 vs Ang (+) of Ad-Nrf2 shRNA; †p < 0.05 vs Ad-Nrf2 shRNA (−); n=6.
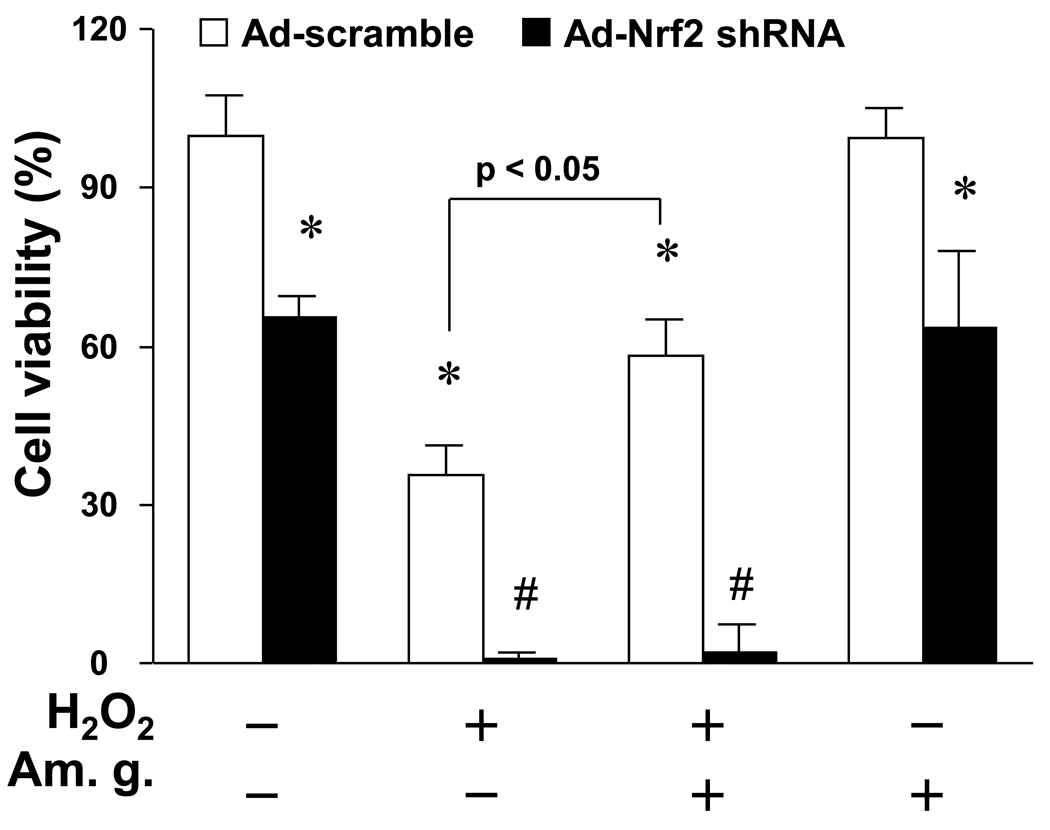
Activation of Nrf2 is essential for American ginseng-mediated inhibition of cardiomyocyte death caused by oxidative stress
It has been reported that American ginseng inhibits oxidative stress-induced cytotoxicity in chick embryo cardiomyocytes (Mehendale et al., 2006). Therefore, it raises a question whether or not American ginseng suppresses oxidative stress-induced cardiomyocyte death via activating Nrf2-driven anti-oxidant signaling. To test the notion, we determined effect of American ginseng on H2O2-induced cardiomyocyte death utilizing Nrf2 shRNA approach in H9C2 cardiomyocytes. As depicted in Fig. 4, H9C2 cells infected with adenovirus of Nrf2 shRNA were much more susceptible to cell injury induced H2O2, compared to the cells infected with adenovirus of control shRNA. In agreement with our observations, a recent study has demonstrated that knockout of Nrf2 dramatically enhances H2O2-induced cytotoxicity in primary culture of murine cardiomyocytes (Zhu et al., 2008). Thus, these results clearly demonstrate that Nrf2 is a critical negative regulator of oxidative stress-mediated cell death in cardiomyocytes including H9C2 cells. On the other hand, pretreatment of American ginseng reversed partly H2O2-induced cell death in H9C2 cardiomyocytes infected with control shRNA; however, the American ginseng-mediated protection was blocked by the knockdown of Nrf2 (Fig. 4), suggesting an essential role of Nrf2 in American ginseng-mediated protection against oxidative stress-induced cardiomyocyte death.
Fig. 4.
Effect of Nrf2 knockdown on American ginseng-mediated inhibition of H2O2-induced cell death in H9C2 cells. Cells infected with adenovirus of control scramble shRNA or Nrf2 shRNA for 48 hours, and then treated with H2O2 (50 µM) for 12 hours. American ginseng extract (Am. g., 50 µg/ml) was added 12 hours before H2O2 treatment. Cell viability was assessed by the Cytotoxicity Detection Kit PLUS (Roche) as described in “Methods”. *p < 0.05 vs Ad-scramble (−); #p < 0.05 vs H2O2 (+) of Ad-scramble; n=6.
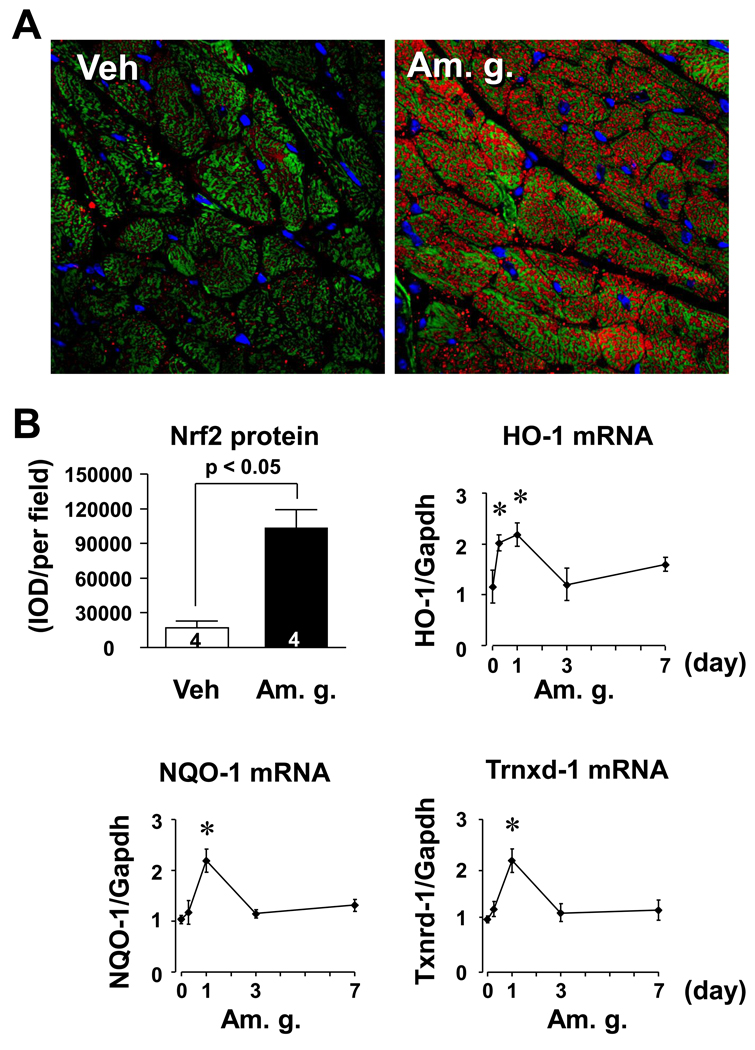
American ginseng activates Nrf2 in the heart
To explore physiological relevance of the American ginseng-operated Nrf2 signaling in the regulation of pathological cardiac remodeling, we examined effect of American ginseng on myocardial Nrf2 activity in vivo. Oral single administration of American ginseng extract at dose of 30 mg/kg for 6 hours dramatically enhanced Nrf2 protein expression the heart, and most of the Nrf2 proteins (red) were localized in cardiomyocytes (Fig. 5). Moreover, the oral administration of American ginseng activated Nrf2-driven transcriptional activity that was evidenced by increases in expression of several Nrf2 downstream genes including HO-1, Txnrd-1, and NQO-1 in the heart (Fig. 5B). In previous clinical trials, it has been demonstrated that there are no significant side effects with long-term consumption of 3 g American ginseng (Stavro et al., 2006). Based on the formula for dose translation from animal to human, i.e., the human equivalent dose (mg/kg) = animal dose (mg/kg) × (animal Km/human Km) (Reagan-Shaw et al., 2008), the dose of American ginseng (30 mg/kg) in mice is equivalent to 2.43 mg/kg [30 mg/kg × 3 (mouse Km)/37 (human Km)] in human. If an average human adult weighs 60 kg, this equates to 2.43 mg/kg × 60 kg = 146 mg daily for humans. Thus, the dose we used is far below an equivalent dose that is safe for human. These results demonstrate that American ginseng at a clinical relevant dose could activate Nrf2 signaling in the heart.
Fig. 5.
American ginseng-induced activation of Nrf2 in the murine heart. (A) Representatives of immunochemical staining of Nrf2 from C57BL/6J mice (n=3) 6 hours after single gavages with American ginseng extract (Am. g., 50 mg/kg). Vehicle (Veh) is saline. Red is Nrf2. Green is F-actin. Blue is nuclei. (B) Semi-quantification of Nrf2 protein expression and Q-PCR analysis of Nrf2 downstream gene expression. Semi-quantification was performed using Image-Pro Plus software as described in “Methods”. Expression of HO-1, NQO-1 and Txn-1 mRNAs was used as biomarkers to indicate Nrf2 activation in the heart. Results are fold changes relative to the control treated with vehicle. *p < 0.05 vs basal (0), n=4.
4. Discussion
The public enthusiasm for ginseng has been inspired at least partly by the growing evidence of ginseng-mediated cardiovascular protection (Chan et al., 2002; Chen, 1996; Gillis, 1997; Wang et al., 2007; Zhou et al., 2004). It has been demonstrated that the major active components in ginseng are ginsenosides, derivatives of triterpene dammarane structures, and the minor components include amino acids, peptides, and minerals (Gillis, 1997). In addition, the content of various ginseng roots and root extracts of the same species of Panax could differ depending on the method of extraction, geographic locations of cultivation, or the season of collection. So far, approximately 40 ginsenosides have been identified (Gillis, 1997; Lu et al., 2009). Indeed, ginsenosides have been shown to induce diverse cardiovascular actions via modulating multiple signaling cascades. Therefore, it is most likely that the putative ginseng-mediated therapeutic effects on cardiovascular diseases are achieved by a net effect orchestrated by multiple components of ginseng rather than by any single ginsenoside alone. Indeed, it has been demonstrated that whole ginseng extract appears to give a better protection against radiation-induced DNA damage than isolated ginsenoside fractions (Lee et al., 2005). Accordingly, a common mechanism by which the whole extracts of different ginseng species exert cardiovascular protection remains unresolved. In the present study, we have demonstrated that a standardized American ginseng extract of NRCC-INMS activates Nrf2 signaling to suppress oxidative stress and oxidative stress-induced cell death in cardiomyocytes, presumably ameliorating maladaptive myocardial remodeling and dysfunction. Our results have revealed for the first time that activation of Nrf2 signaling is likely to be a crucial common pathway for the ginseng-mediated cardiac protective actions.
It has been firmly established that oxidative stress plays a causative role in the pathogenesis of cardiovascular diseases including pathological cardiac remodeling and dysfunction (Murdoch et al., 2006; Takimoto and Kass, 2007). Because Nrf2 is a critical negative regulator of maladaptive cardiac remodeling and dysfunction via suppressing oxidative stress (Li et al., 2009b), we have proposed that targeting the Nrf2 pathway might provide a novel therapeutic approach for the cardiac diseases (Li et al., 2009a). In this context, our finding that American ginseng activates the Nrf2-driven anti-oxidative pathway thereby inhibiting oxidative stress-mediated cell death in cardiomyocytes establishes a direct mechanistic link between the ginseng-mediated cardioprotective effects and anti-oxidative activity (Jiang et al., 2007; Liu et al., 2004; Mehendale et al., 2006; Shao et al., 2004; Xie et al., 2006; You et al., 2005). Additionally, we observed that pharmacological activation of Nrf2 suppressed oxidative stress in cardiomyocytes (Ichikawa et al., 2009a) and protected against pathological pressure overload-induced cardiac hypertrophy and dysfunction (unpublished data). Collectively, these results support a notion that American ginseng-operated Nrf2 signaling represents a common mechanism of ginseng-mediated cardiac protection.
It is worthy to note that inflammatory response plays an important role in the regulation of cardiac homeostasis (Heymans et al., 2009; Nian et al., 2004). While precise mechanisms by which inflammatory cytokines regulate cardiac remodeling and function need to be further dissected, an intimate link between inflammatory cytokines and oxidative stress has lately been established (Nian et al., 2004; Sekiguchi et al., 2004). Inflammatory cytokines including TNFα-interact with other hypertrophic factors such as Ang II to induce oxidative stress, contributing to cardiac pathophysiology. In the present study, we have demonstrated that American ginseng suppresses Ang II and TNFα-induced oxidative stress in cardiomyocytes via activating Nrf2 signaling, suggesting that ginsengs at least American ginseng could activate Nrf2 pathway to inhibit the detrimental interaction between inflammatory cytokines and hypertrophic agonists to induce oxidative stress in the heart. Thus, the American ginseng-operated Nrf2 anti-oxidative signaling might represent a unique mechanism of an appropriate control of inflammation and oxidative stress against maladaptive cardiac remodeling and heart failure.
Although Nrf2 appears to orchestrate a group of antioxidant and other cytoprotective genes providing a protective mechanism against detrimental stress-induced cellular and tissue damage in the cardiovascular system, a therapeutic approach targeting Nrf2 pathway for the cardiovascular disease is not yet established (Li et al., 2009a). It is likely that Nrf2/ARE signaling is tissue or cell type specific. Noteworthy is the fact that a recent report demonstrated Keap1-Nrf2 system to contain discrete sensor sites, including Keap1 cysteines Cys-151 and Cys-275, for a variety of Nrf2-activating compounds and special “cysteine codes” that are linked with different Nrf2-mediated biological actions (Kobayashi et al., 2009). Since our data clearly demonstrated that American ginseng-activated Nrf2 signaling is cardiac protective, it is possible that American ginseng extract (NRCC-INMS) hits special “cysteine codes” of Keap-Nrf2 system to activate preferentially the Nrf2-driven cardiovascular protective signaling. A further dissection of the American ginseng-mediated Nrf2 signaling might not only raises the veil of ginseng-mediated mystical salvation in cardiovascular system but also provide guidance in ginseng usage for the public.
In summary, we have demonstrated that activation of Nrf2 pathway is a novel mechanism by which American ginseng suppresses oxidative stress in cardiomyocytes, thereby providing a cardiac protection against maladaptive remodeling. Further characterization of American ginseng-mediated activation of Nrf2 pathway in the regulation of pathological cardiac remodeling and dysfunction will untwist the molecular bases by which ginseng acts as a folk medicine or perhaps as a drug for the treatment of cardiac diseases.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. John Arnason, University of Ottawa, for assistance with the botanical voucher specimens. This work was supported by AHA BGIA (0865101E) (T.C.) and NIH (1PO1AT003961) (P.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chan P, Thomas GN, Tomlinson B. Protective effects of trilinolein extracted from panax notoginseng against cardiovascular disease. Acta Pharmacol Sin. 2002;23:1157–1162. [PubMed] [Google Scholar]
- Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996;23:728–732. doi: 10.1111/j.1440-1681.1996.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Ji LL. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J Nutr. 2003;133:3603–3609. doi: 10.1093/jn/133.11.3603. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Leblond FA, Filep JG. Peroxynitrite production by human neutrophils, monocytes and lymphocytes challenged with lipopolysaccharide. FEBS Lett. 1998;431:107–110. doi: 10.1016/s0014-5793(98)00741-8. [DOI] [PubMed] [Google Scholar]
- Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Gum SI, Jo SJ, Ahn SH, Kim SG, Kim JT, Shin HM, Cho MK. The potent protective effect of wild ginseng (Panax ginseng C.A. Meyer) against benzo[alpha]pyrene-induced toxicity through metabolic regulation of CYP1A1 and GSTs. J Ethnopharmacol. 2007;112:568–576. doi: 10.1016/j.jep.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschope C, Van Bilsen M, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:119–129. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CM, Chen YH, Chiang MT, Chau LY. Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation. 2004;110:309–316. doi: 10.1161/01.CIR.0000135475.35758.23. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One. 2009a;(4):e8391. doi: 10.1371/journal.pone.0008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Li J, Nagarkatti P, Nagarkatti M, Hofseth LJ, Windust A, Cui T. American ginseng preferentially suppresses STAT/iNOS signaling in activated macrophages. J Ethnopharmacol. 2009b;125:145–150. doi: 10.1016/j.jep.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Zhang J, Chen K, Liu Y, Schopfer FJ, Baker PR, Freeman BA, Chen YE, Cui T. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology. 2008;149:4086–4094. doi: 10.1210/en.2007-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Di Carlo G, Borrelli F, Ernst E. Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. Int J Cardiol. 2005;98:1–14. doi: 10.1016/j.ijcard.2003.06.039. [DOI] [PubMed] [Google Scholar]
- Jiang QS, Huang XN, Dai ZK, Yang GZ, Zhou QX, Shi JS, Wu Q. Inhibitory effect of ginsenoside Rb1 on cardiac hypertrophy induced by monocrotaline in rat. J Ethnopharmacol. 2007;111:567–572. doi: 10.1016/j.jep.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Johnke RM, Allison RR, O'Brien KF, Dobbs LJ., Jr Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009a;13:785–794. doi: 10.1517/14728220903025762. [DOI] [PubMed] [Google Scholar]
- Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, Cui T. Nrf2 Protects Against Maladaptive Cardiac Responses to Hemodynamic Stress. Arterioscler Thromb Vasc Biol. 2009b doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- Liu JC, Cheng TH, Lee HM, Lee WS, Shih NL, Chen YL, Chen JJ, Chan P. Inhibitory effect of trilinolein on angiotensin II-induced cardiomyocyte hypertrophy. Eur J Pharmacol. 2004;484:1–8. doi: 10.1016/j.ejphar.2003.10.043. [DOI] [PubMed] [Google Scholar]
- Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima S, Kinugawa S, Ide T, Matsusaka H, Inoue N, Ohta Y, Yokota T, Sunagawa K, Tsutsui H. Overexpression of glutathione peroxidase attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Am J Physiol Heart Circ Physiol. 2006;291:H2237–H2245. doi: 10.1152/ajpheart.00427.2006. [DOI] [PubMed] [Google Scholar]
- Mehendale SR, Wang CZ, Shao ZH, Li CQ, Xie JT, Aung HH, Yuan CS. Chronic pretreatment with American ginseng berry and its polyphenolic constituents attenuate oxidant stress in cardiomyocytes. Eur J Pharmacol. 2006;553:209–214. doi: 10.1016/j.ejphar.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Faseb J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescin. J Leukoc Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- Sekiguchi K, Li X, Coker M, Flesch M, Barger PM, Sivasubramanian N, Mann DL. Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc Res. 2004;63:433–442. doi: 10.1016/j.cardiores.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Xie JT, Vanden Hoek TL, Mehendale S, Aung H, Li CQ, Qin Y, Schumacker PT, Becker LB, Yuan CS. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim Biophys Acta. 2004;1670:165–171. doi: 10.1016/j.bbagen.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Small E CPM. Canddian medicinal crops. Ottawa, Ontario, Canada: NRC Press; 1999. [Google Scholar]
- Stavro PM, Woo M, Leiter LA, Heim TF, Sievenpiper JL, Vuksan V. Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension. 2006;47:791–796. doi: 10.1161/01.HYP.0000205150.43169.2c. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel P, Patel AP, Carvajal IM, Wang ZY, Pellacani A, Maemura K, DiFonzo N, Rennke HG, Layne MD, Yet SF, Lee ME, Perrella MA. Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1-deficient mice. Circ Res. 2001;88:1088–1094. doi: 10.1161/hh1001.091521. [DOI] [PubMed] [Google Scholar]
- Xie JT, Shao ZH, Vanden Hoek TL, Chang WT, Li J, Mehendale S, Wang CZ, Hsu CW, Becker LB, Yin JJ, Yuan CS. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- You JS, Huang HF, Chang YL. Panax ginseng reduces adriamycin-induced heart failure in rats. Phytother Res. 2005;19:1018–1022. doi: 10.1002/ptr.1778. [DOI] [PubMed] [Google Scholar]
- Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen CJ. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit. 2004;10:RA187–RA192. [PubMed] [Google Scholar]
- Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- Zhu H, Jia Z, Misra BR, Zhang L, Cao Z, Yamamoto M, Trush MA, Misra HP, Li Y. Nuclear Factor E2-Related Factor 2-Dependent Myocardiac Cytoprotection Against Oxidative and Electrophilic Stress. Cardiovasc Toxicol. 2008 doi: 10.1007/s12012-008-9016-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.