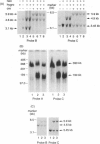
Abstract
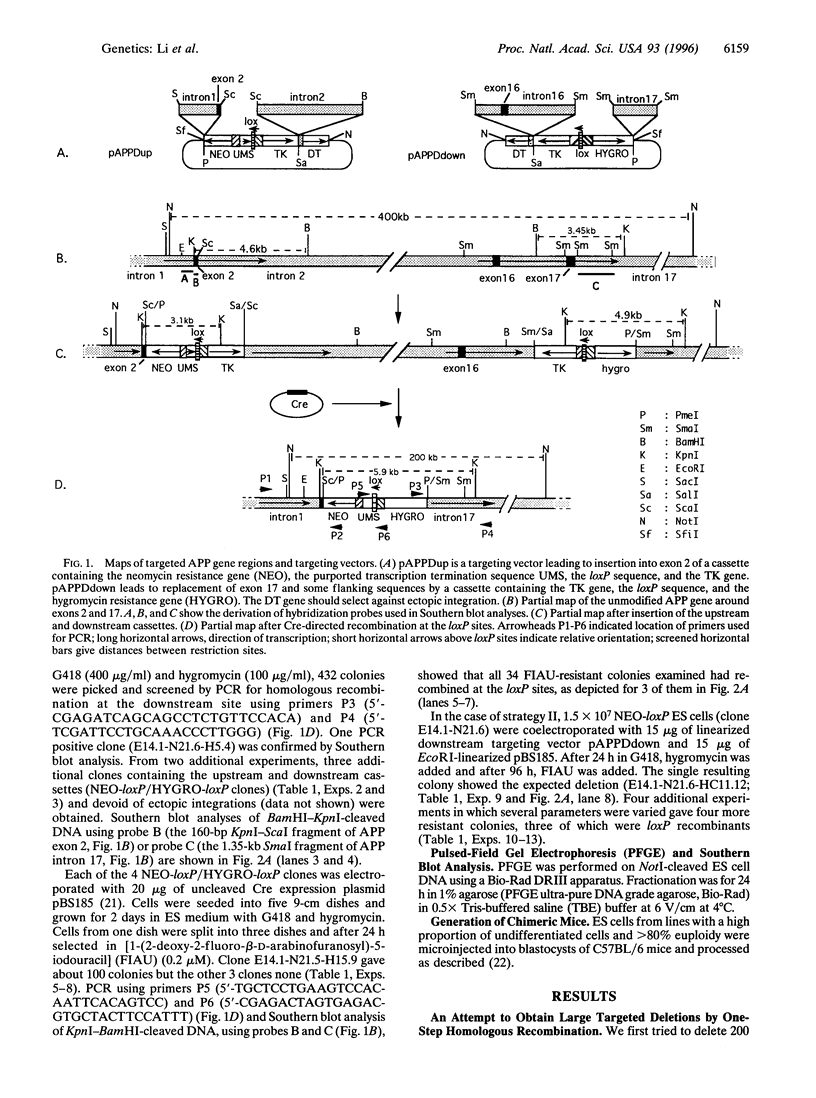
Gene disruptions and deletions of up to 20kb have been generated by homologous recombination with appropriate targeting vectors in murine embryonic stem (ES) cells. Because we could not obtain a deletion of about 200 kb in the mouse amyloid precursor protein gene by the classical technique, we employed strategies involving the insertion of loxP sites upstream and downstream of the region to be deleted by homologous recombination and elicited excision of the loxP-flanked region by introduction of a Cre expression vector into the ES cells. In the first approach, the loxP sequences were inserted in two successive steps and after each step, ES cell clones were isolated and characterized. Deletion of the loxP-flanked sequence was accomplished by introducing the cre gene in a third step. In the second approach, ES cells containing the upstream loxP cassette were electroporated simultaneously with the downstream loxP targeting vector and the Cre expression plasmid. ES cells were obtained that gave rise to chimeric mice capable of germ-line transmission of the deleted amyloid precursor protein allele.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandon E. P., Idzerda R. L., McKnight G. S. Targeting the mouse genome: a compendium of knockouts (Part III) Curr Biol. 1995 Aug 1;5(8):873–881. doi: 10.1016/s0960-9822(95)00177-1. [DOI] [PubMed] [Google Scholar]
- Braun R. E., Lo D., Pinkert C. A., Widera G., Flavell R. A., Palmiter R. D., Brinster R. L. Infertility in male transgenic mice: disruption of sperm development by HSV-tk expression in postmeiotic germ cells. Biol Reprod. 1990 Oct;43(4):684–693. doi: 10.1095/biolreprod43.4.684. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989 Mar;5(3):70–76. doi: 10.1016/0168-9525(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Dumont D. J., Gradwohl G., Fong G. H., Puri M. C., Gertsenstein M., Auerbach A., Breitman M. L. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994 Aug 15;8(16):1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Heard J. M., Herbomel P., Ott M. O., Mottura-Rollier A., Weiss M., Yaniv M. Determinants of rat albumin promoter tissue specificity analyzed by an improved transient expression system. Mol Cell Biol. 1987 Jul;7(7):2425–2434. doi: 10.1128/mcb.7.7.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess R. H., Abremski K. Interaction of the bacteriophage P1 recombinase Cre with the recombining site loxP. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1026–1029. doi: 10.1073/pnas.81.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner K. M., Pudney J., Milstone D. S., Ladd D., Seidman J. G. Flagellar and acrosomal abnormalities associated with testicular HSV-tk expression in the mouse. Biol Reprod. 1993 Aug;49(2):251–261. doi: 10.1095/biolreprod49.2.251. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Kühn R., Schwenk F., Aguet M., Rajewsky K. Inducible gene targeting in mice. Science. 1995 Sep 8;269(5229):1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Lamb B. T., Sisodia S. S., Lawler A. M., Slunt H. H., Kitt C. A., Kearns W. G., Pearson P. L., Price D. L., Gearhart J. D. Introduction and expression of the 400 kilobase amyloid precursor protein gene in transgenic mice [corrected]. Nat Genet. 1993 Sep;5(1):22–30. doi: 10.1038/ng0993-22. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989 Mar;9(3):1128–1136. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson B., Kenwrick S., Lakich D., Hammonds G., Jr, Gitschier J. A transcribed gene in an intron of the human factor VIII gene. Genomics. 1990 May;7(1):1–11. doi: 10.1016/0888-7543(90)90512-s. [DOI] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Müller U., Cristina N., Li Z. W., Wolfer D. P., Lipp H. P., Rülicke T., Brandner S., Aguzzi A., Weissmann C. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell. 1994 Dec 2;79(5):755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Ohno H., Goto S., Taki S., Shirasawa T., Nakano H., Miyatake S., Aoe T., Ishida Y., Maeda H., Shirai T. Targeted disruption of the CD3 eta locus causes high lethality in mice: modulation of Oct-1 transcription on the opposite strand. EMBO J. 1994 Mar 1;13(5):1157–1165. doi: 10.1002/j.1460-2075.1994.tb06365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Behringer R. R., Quaife C. J., Maxwell F., Maxwell I. H., Brinster R. L. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987 Jul 31;50(3):435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Qin M., Bayley C., Stockton T., Ow D. W. Cre recombinase-mediated site-specific recombination between plant chromosomes. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1706–1710. doi: 10.1073/pnas.91.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Solis R., Liu P., Bradley A. Chromosome engineering in mice. Nature. 1995 Dec 14;378(6558):720–724. doi: 10.1038/378720a0. [DOI] [PubMed] [Google Scholar]
- Reis L. F., Ruffner H., Stark G., Aguet M., Weissmann C. Mice devoid of interferon regulatory factor 1 (IRF-1) show normal expression of type I interferon genes. EMBO J. 1994 Oct 17;13(20):4798–4806. doi: 10.1002/j.1460-2075.1994.tb06805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffner H., Reis L. F., Näf D., Weissmann C. Induction of type I interferon genes and interferon-inducible genes in embryonal stem cells devoid of interferon regulatory factor 1. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11503–11507. doi: 10.1073/pnas.90.24.11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jun;7(6):2087–2096. doi: 10.1128/mcb.7.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Cre-stimulated recombination at loxP-containing DNA sequences placed into the mammalian genome. Nucleic Acids Res. 1989 Jan 11;17(1):147–161. doi: 10.1093/nar/17.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol. 1990 May;2(5):441–449. [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981 Aug 25;150(4):467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Schuetze S., Vanek M., Wagner E. F. Expression of retroviral vectors in transgenic mice obtained by embryo infection. EMBO J. 1987 Feb;6(2):383–388. doi: 10.1002/j.1460-2075.1987.tb04766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. L., Reis L. F., Pavlovic J., Aguzzi A., Schäfer R., Kumar A., Williams B. R., Aguet M., Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995 Dec 15;14(24):6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Jiang M., Trumbauer M. E., Sirinathsinghji D. J., Hopkins R., Smith D. W., Heavens R. P., Dawson G. R., Boyce S., Conner M. W. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995 May 19;81(4):525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]