Abstract
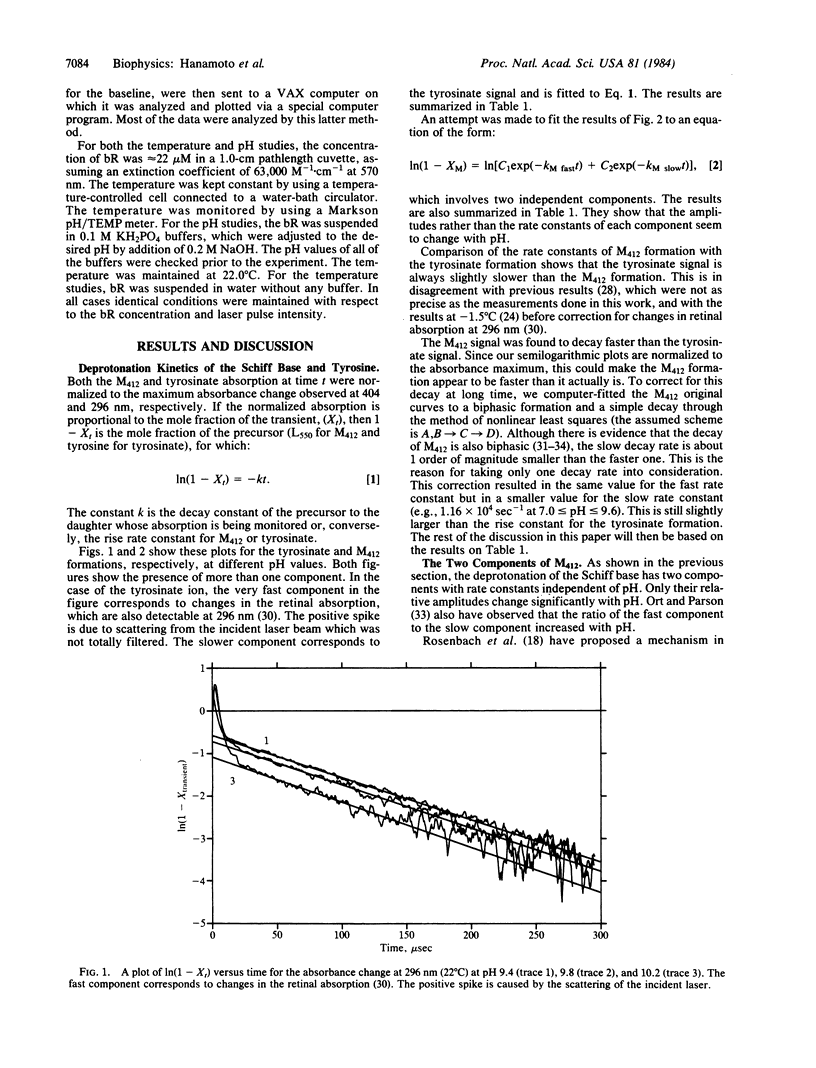
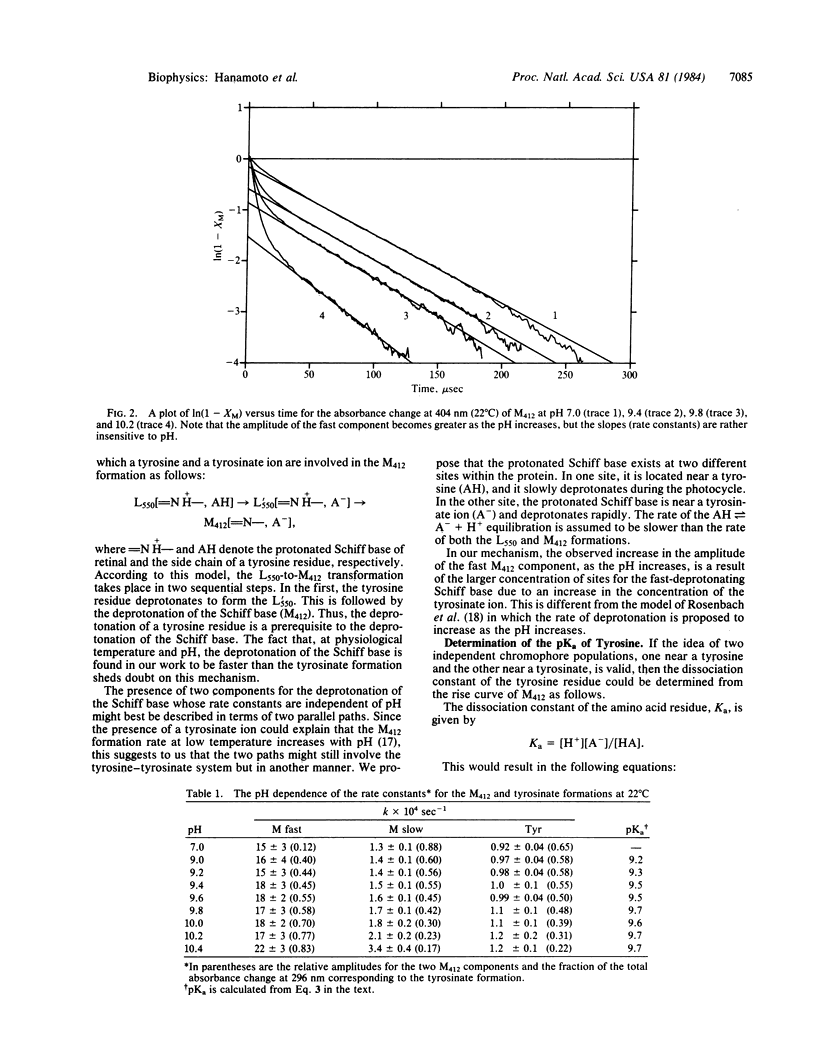
The kinetics of formation of both the tyrosinate ion (from its absorption at 296 nm) and the deprotonated Schiff base (M412) (from its absorption at 404 nm) are studied simultaneously at different pH values (7-11) and temperatures (5-25 degrees C). Two formation rates are observed for M412 in agreement with previous observations. The slow one is dominant under physiological conditions and is found to be slightly faster than that for the tyrosinate formation. This is in disagreement with the proposal that the tyrosinate formation is a prerequisite to the deprotonation of the Schiff base (M412). The ratio of the amplitudes of the fast and slow components is found to be sensitive to pH and, at any pH, it can be used to calculate an amino acid pKa value of 9.6. This is explained by proposing the existence of two sites for the protonated Schiff base within the protein. In one site, the Schiff base is near the neutral form of an amino acid residue with a pKa value of 9.6 (giving rise to the slow component), while in the other, it is near its conjugate base. The formation of the tyrosinate ion as well as the formation of the slow and fast components of M412 all have activation energies that are comparable to H-bond energies. A model is suggested to account for this and the comparable deprotonation rates of tyrosine and the slow component of the protonated Schiff base. It involves the reduction of their pKa by their exposure to a positively charged species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aton B., Doukas A. G., Callender R. H., Becher B., Ebrey T. G. Resonance Raman studies of the purple membrane. Biochemistry. 1977 Jun 28;16(13):2995–2999. doi: 10.1021/bi00632a029. [DOI] [PubMed] [Google Scholar]
- Bagley K., Dollinger G., Eisenstein L., Singh A. K., Zimányi L. Fourier transform infrared difference spectroscopy of bacteriorhodopsin and its photoproducts. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4972–4976. doi: 10.1073/pnas.79.16.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Lanyi J. K. Analogies between respiration and a light-driven proton pump as sources of energy for active glutamate transport in Halobacterium holobium. Arch Biochem Biophys. 1977 Jan 15;178(1):308–314. doi: 10.1016/0003-9861(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman evidence for an all-trans to 13-cis isomerization in the proton-pumping cycle of bacteriorhodopsin. Biochemistry. 1980 Nov 11;19(23):5421–5428. doi: 10.1021/bi00564a042. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Dencher N., Wilms M. Flash photometric experiments on the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1975 May 30;1(3):259–271. doi: 10.1007/BF00535760. [DOI] [PubMed] [Google Scholar]
- Edgerton M. E., Moore T. A., Greenwood C. Salt reversal of the acid-induced changes in purple membrane from Halobacterium halobium. FEBS Lett. 1978 Nov 1;95(1):35–39. doi: 10.1016/0014-5793(78)80046-5. [DOI] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. Kinetic interaction between aromatic residues and the retinal chromophore of bacteriorhodopsin during the photocycle. FEBS Lett. 1979 Apr 15;100(2):334–340. doi: 10.1016/0014-5793(79)80364-6. [DOI] [PubMed] [Google Scholar]
- Kalisky O., Ottolenghi M., Honig B., Korenstein R. Environmental effects on formation and photoreaction of the M412 photoproduct of bacteriorhodopsin: implications for the mechanism of proton pumping. Biochemistry. 1981 Feb 3;20(3):649–655. doi: 10.1021/bi00506a031. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on the photocycle of bacteriorhodopsin in thin layers of purple membrane. Nature. 1977 Nov 10;270(5633):184–186. doi: 10.1038/270184a0. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B., Kuschmitz D. Branching reactions in the photocycle of bacteriorhodopsin. FEBS Lett. 1978 Sep 15;93(2):266–270. doi: 10.1016/0014-5793(78)81118-1. [DOI] [PubMed] [Google Scholar]
- Lam E., Seltzer S., Katsura T., Packer L. Light-dependent nitration of bacteriorhodopsin. Arch Biochem Biophys. 1983 Nov;227(1):321–328. doi: 10.1016/0003-9861(83)90376-4. [DOI] [PubMed] [Google Scholar]
- Lemke H. D., Oesterhelt D. The role of tyrosine residues in the function of bacteriorhodopsin. Specific nitration of tyrosine 26. Eur J Biochem. 1981 Apr;115(3):595–604. doi: 10.1111/j.1432-1033.1981.tb06244.x. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Iwasa T., Yoshizawa T. Formation of 9-cis- and 11-cis-retinal pigments from bacteriorhodopsin by irradiating purple membrane in acid. Biochemistry. 1980 Aug 5;19(16):3825–3831. doi: 10.1021/bi00557a027. [DOI] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A. Kinetic resonance Raman spectroscopy: dynamics of deprotonation of the Schiff base of bacteriorhodopsin. Science. 1977 Mar 25;195(4284):1328–1330. doi: 10.1126/science.841330. [DOI] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Muccio D. D., Cassim J. Y. Interpretations of the effects of pH on the spectra of purple membrane. J Mol Biol. 1979 Dec 15;135(3):595–609. doi: 10.1016/0022-2836(79)90166-9. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Tristram-Nagle S. Hydrogen bonded chain mechanisms for proton conduction and proton pumping. J Membr Biol. 1983;74(1):1–14. doi: 10.1007/BF01870590. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Parson W. W. Flash-induced volume changes of bacteriorhodopsin-containing membrane fragments and their relationship to proton movements and absorbance transients. J Biol Chem. 1978 Sep 10;253(17):6158–6164. [PubMed] [Google Scholar]
- Scherrer P., Packer L., Seltzer S. Effect of iodination of the purple membrane on the photocycle of bacteriorhodopsin. Arch Biochem Biophys. 1981 Dec;212(2):589–601. doi: 10.1016/0003-9861(81)90402-1. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Rosenheck K. The low pH species of bacteriorhodopsin. Structure and proton pump activity. FEBS Lett. 1979 Feb 15;98(2):368–372. doi: 10.1016/0014-5793(79)80219-7. [DOI] [PubMed] [Google Scholar]


