Abstract
Objective
To investigate the levels of matrix metalloproteinases (MMPs), myeloperoxidase (MPO) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in tears of patients with Stevens-Johnson syndrome (SJS) and ocular cicatricial pemphigoid (OCP).
Design
Prospective non-interventional cohort study.
Participants
Four SJS patients (7 eyes), 19 OCP patients (37 eyes) and 20 post-phacoemulsification healthy controls (40 eyes).
Methods
Tear washes were collected from all patients and were analyzed for levels of MMP-2, -3, -7, -8, -9, -12, MPO and TIMP-1 using multi-analyte bead-based enzyme-linked immunosorbent assays (ELISA). Total MMP activity was determined using a fluorimetric assay. Correlation studies were performed between the various analytes within study groups.
Main Outcome Measures
Levels of MMP-2, -3, -7, -8, -9, -12, MPO and TIMP-1 (in ng/µg protein), total MMP activity (in relative fluorescent units/min/µg protein) in tears, MMP-8/TIMP-1, MMP-9/TIMP-1 ratios and the correlations between MMP-8 and MMP-9 and each MMP and MPO.
Results
MMP-8, MMP-9 and MPO levels were significantly elevated in SJS and OCP tears (SJS > OCP) when compared to controls. MMP activity was highest in SJS while OCP and controls showed lower and similar activities. TIMP-1 levels were decreased in SJS and OCP when compared to controls with OCP levels reaching significance. MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios were markedly elevated in SJS and OCP tears (SJS > OCP) when compared to controls. Across all study groups, MMP-9 levels correlated strongly with MMP-8 and MPO levels and MMP-8 correlated with MPO but did not reach significance in SJS. There was no relationship between MMP-7 and MPO.
Conclusions
Since MMP-8 and MPO are produced by inflammatory cells, particularly neutrophils, the correlation data indicate that they may be the common source of elevated enzymes including MMP-9 in SJS and OCP tears. Elevated MMP/TIMP ratios and MMP activity suggest an imbalance in tear MMP regulation that may explain the predisposition of these patients to develop corneal melting and chronic complications associated with persistent inflammation. MPO in tears may be a sensitive and specific marker for the quantification of ocular inflammation.
Introduction
Stevens-Johnson syndrome (SJS) and ocular cicatricial pemphigoid (OCP), a subset of mucous membrane pemphigoid*, are rare, yet severe, systemic autoimmune diseases associated with extensive bilateral persistent inflammation of the ocular surface and lid margins. Even though the term “mucous membrane pemphigoid (MMP)” has been widely accepted to describe this disease, in this manuscript, “ocular cicatricial pemphigoid (OCP)” has been used instead to minimize confusion between the acronym of mucous membrane pemphigoid (MMP) and that of the universally used acronym for matrix metalloproteinases (MMPs) assayed in this study.1–4 If left untreated or inadequately managed, chronic conjunctivitis can impair tear distribution and stability by loss of goblet cells, accessory glands and secretory ductules of the main lacrimal glands and meibomian gland orifices and glands. Chronic conjunctival inflammation eventually causes irreversible dry and cicatricial changes to the conjunctiva and cornea that can ultimately lead to blindness1–4. Due to extensive scar formation, neovascularization and keratinzation of the ocular surface, initiating treatments in the chronic stages of the disease is not very effective, and thus earlier intervention is initiated in an effort to control inflammation. Most surgical care is reserved for rehabilitation efforts after controlling the inflammation1,5–7. Therefore, judicious management rests on the severity and rapidity of disease progression to direct treatment. The approach that is clinically utilized relies on visually inspecting and grading conjunctival injection with a slit lamp microscope. Despite the advantages that come with an inexpensive easy-to-use method, its potential for inter-physician variability, failure to see minute changes and, worse, overlooking active cellular inflammation in the absence of clinically obvious ocular inflammation make such grading subjective. Thus, researchers have sought biomarkers and other alternative methods to evaluate ocular surface diseases. Identified biomarkers may also shed light on their possible roles in disease pathophysiology and the development of related complications.
Matrix metalloproteinases (MMPs) are zinc- and calcium-dependent endopeptidases that can degrade virtually all components of the extracellular matrix including the basement membrane. Released as inactive zymogens, these enzymes are activated by various proteases to perform their specific functions and are primarily regulated by endogenous inhibitors (i.e. tissue inhibitors of metalloproteinases (TIMPs))8. Studies on the cornea in vitro and in vivo have underscored the role of MMPs in normal and pathological epithelial wound healing, stromal remodeling and corneal ulceration9,10. Furthermore, studies of human tears showed elevated levels and activities of MMPs in inflammatory ocular surface diseases such as dry eye11, infective keratitis12, active peripheral ulcerative keratitis13, atopic blepharoconjunctivitis14, ocular rosacea15, vernal conjunctivitis16 and non-allergic eosinophilic conjunctivitis17. Inflammatory cells, particularly neutrophils, are major sources of neutrophil collagenase (MMP-8) and gelatinase B (MMP-9), both of which have been associated with the extent of cellular inflammation12,14,18,19.
Neutrophils also produce myeloperoxidase (MPO), a heme-protein that is stored within primary azurophilic granules that are released only upon neutrophilic activation (respiratory burst) and degranulation20,21. In addition to its function as a microbicidal enzyme, MPO has been studied as an inflammatory marker for a number of systemic disease processes21 and in tears of patients with ocular allergy22. There are no tear studies that evaluated MMPs and MPO on the ocular surface of patients with chronic Stevens-Johnson syndrome and mucous membrane pemphigoid. The purpose of the study was to assess the MMP profiles and activities and MPO levels in tears of patients with these ocular surface diseases and to correlate their levels as an indication of levels of inflammation on the ocular surface.
Patients and Methods
Study population
This study was a prospective, non-interventional, consecutive cohort study conducted at 2 independent sites. All study participants were recruited and tear washes were performed at the Massachusetts Eye Research and Surgery Institution (MERSI), Cambridge, Massachusetts. The tear and statistical analyses were performed at the Schepens Eye Research Institute-Massachusetts Eye and Ear (SERI-MEE), Harvard Medical School, Boston, Massachusetts. All procedures were HIPAA(Health Insurance Portability and Accountability Act)-compliant and conformed to the tenets of the Declaration of Helsinki. The Institutional Review Board (IRB)/Ethics Committee approval was obtained at MERSI and SERI-MEE and informed consents were obtained from all participants.
Study participants were divided into three groups: SJS, OCP and control groups. Tear washes were obtained between October 2011 and January 2012.
Inclusion criteria for SJS patients were clinical systemic, dermatological and ocular histories consistent with SJS having ocular involvement. Inclusion criteria for OCP patients were conjunctival biopsy results consistent with OCP (presence of linear deposition of immunoglobulins at the basement membrane zone by direct immunofluorescence). Both SJS and OCP participants were in the chronic stages of the disease. The control group included patients that underwent cataract surgery by phacoemulsification at least 4 weeks prior to tear collection with otherwise healthy ocular surfaces. Exclusion criteria for the SJS and OCP groups were prior recent ocular surgeries within the past 6 months (except for cataract), history of allergic ocular diseases, concurrent ocular infection, severe meibomian gland dysfunction or blepharitis. For the control groups, subjects with a current history of dry eye syndrome, ocular or systemic autoimmune background or prior ocular histories other than cataract surgery were excluded from the study.
All participants were contacted by phone 24 hours prior to their visit during which the risks, benefits and tear collection method were explained in detail. Upon verbal agreement to participate, the participants that were on topical ocular hypertensive and/or anti-inflammatory medications were asked to stop those medications 12 hours prior to the tear collection then resume them after the collection had been completed. For SJS and OCP patients, tears were collected at least 9–12 months following diagnosis. All participants underwent detailed assessment before inclusion in the study, including clinical history and slit lamp examination. Medical records of the enrolled subjects were reviewed for gender, age, race, ocular surgical history and existing systemic and ocular conditions and medications if any.
On the day of the tear collection, participants were examined for evidence of clinical conjunctival inflammation with a slit lamp examination by a single investigator (CSF). A standard numerical grading system from 0 to 4 was used as previously described23 to record conjunctival clinical inflammation: (0) quiet (no inflammation), (1) mild inflammation, (2) moderate inflammation, (3) severe and (4) extreme inflammation and eyes were categorized as either being in clinical remission or not. For OCP patients, disease severity was staged according to Foster classification23. SJS patients could not be staged due to the lack of a validated and accepted staging system.
Tear Wash Collection and Processing
A tear wash method was chosen for collecting tear proteins24 as previous work demonstrated that the method allows harvest of tear fluid from patients that have little tear production, and it yields a greater amount of protein than Schirmer strip or microcapillary tear collection allowing multiple analyte assays. On the day of the visit, the tear washes were collected within a narrow time frame (between 10am to 12pm) to reduce potential diurnal variations in MMP levels in the tears25. The tear washes were collected before any scheduled manipulation (e.g. intraocular pressure assessment) or instillation of any diagnostic drops (e.g. fluorescein) to avoid artifactual washout of the tear components that might interfere with interpretation of results. The tear wash method was done as previously described24. Briefly, 60 µL of sterile physiological saline was applied onto the ocular surface using a sterile micropipette. Patients were then asked to move their eyes up, down, nasally and temporally without blinking. The tear washes were collected from the inferior fornix by micropipette, transferred into sterile polypropylene tubes and centrifuged for 30 minutes at 4°C at 13500 × g to remove cellular debris and supernatants were transferred into new sterile polypropylene tubes and stored at −80°C.
Protein Quantitation
After all tear washes were collected, protein concentrations were determined using the Micro BCA™ Protein Assay Kit (Pierce, Rockford, IL) according to the manufacturer’s instructions, using a 1:50 dilution of tear sample to diluent. The samples were then aliquoted and stored at −80°C for further analysis.
Three types of assays (two enzyme-linked immunosorbent assay (ELISA)-based and one MMP fluorimetric activity assay) were performed to optimize quantitation of multiple analytes from a single eye. Accommodating for variable sample yield of protein content and/or volume precluded the ability to quantify all analytes in some specimens.
Measurement of Matrix Metalloproteinases (MMPs) and Total MMP Activity
Total (pro-form and active) levels of six MMPs: gelatinase A (MMP-2), stromelysin-1 (MMP-3), matrilysin (MMP-7), neutrophil collagenase (MMP-8), gelatinase B (MMP-9) and macrophage metalloelastase (MMP-12) were measured simultaneously using the Fluorokine® Human MMP MultiAnalyte Profiling (MAP) Kit (R&D Systems, Minneapolis, MN) using the manufacturer’s instructions. Samples were analyzed on a Bio-Rad Bio-Plex analyzer powered by Luminex® 100™ ×MAP technology (Luminex Corp., Austin, TX). Resultant median fluorescence intensities were used to calculate the final concentrations from a standard curve (in ng/mL) and were standardized to µg protein loaded in the assay. The values were transformed to logarithmic data points for correlation studies between the analytes.
The total MMP activity was measured using a modified protocol of the MMP-9 Fluorimetric Drug Discovery Kit (Enzo Life Sciences, Plymouth Meeting, PA). The samples were diluted in MMP assay buffer containing 50mM HEPES, 10mM CaCl2, 0.05% Brij-35, pH 7.5 into a sensoplate, black 96 well glass bottom plate (Greiner Bio-One North America Inc., Monroe, NC). A quenched OmniMMP™ RED fluorogenic substrate (Enzo Life Sciences, Plymouth Meeting, PA) was added to each well. The substrate is cleaved by active MMPs and, upon cleavage, emits at the red end of the spectrum to avoid any interference of lower wavelengths exhibited by unwanted substances in the tear samples. Fluorescence was measured at 1-minute intervals for 45 minutes at 540 nm (excitation) and 590 nm (emission) using the Synergy Mx monochromator-based multi-mode microplate reader (Biotek U.S., Winooski, VT). For data analysis, slopes were derived from the linear portion of the curves and background slopes were subtracted from all samples. The net slopes corresponded to the fluorescent intensities (in relative fluorescent units per minute (RFU/min)) that were directly proportional to the total MMP activity present in the samples. The values were standardized to µg protein loaded in the assay. The values were transformed to logarithmic data points for correlation studies.
Measurement of Myeloperoxidase (MPO) and Tissue Inhibitor of Metalloproteinase-1 (TIMP-1)
Tear samples were assayed for levels of MPO and TIMP-1 using the Fluorokine® Human Cardiac MAP Kit (R&D Systems, Minneapolis, MN) and Luminex® technology using the manufacturer’s instructions. Resultant median fluorescence intensities were used to calculate the final concentrations from the standard curve (in ng/mL) and were standardized to µg protein loaded in the assay. The values were transformed to logarithmic data points for correlation studies between the analytes.
Statistical Analysis
Differences in the levels of all analytes and total MMP activity between OCP, SJS and control patients were examined for statistical significance using the Mann-Whitney U and Kruskal-Wallis tests for non-parametric and non-matched groups. Correlations between the amounts of analytes including total MMP activity of all patients and within each study group were determined using the Spearman rank correlation test. Statistical analysis was performed using Instat 3.10 statistical software (GraphPad Software, San Diego, CA). Results were presented with a 2-tailed p value, set at 0.05.
Results
Study Population
A total of 45 patients (85 eyes) met our inclusion criteria and were enrolled into the study. These included 5 SJS (9 eyes), 20 OCP (40 eyes) and 20 control (40 eyes) patients. All SJS (100%) and 37 OCP (92.5%) eyes were diagnosed to be in clinical remission prior to the tear wash collections. From those eyes, tear samples from two SJS eyes (1 patient) and 1 OCP eye were excluded from tear analysis due to low protein content recovered from the tear wash (<1µg/µL). Samples from two OCP eyes (2 patients) were excluded due to failure to collect sufficient tears as a result of severe ankyloblepharon. The mean ages and standard errors of the study groups were as follows: SJS (21.0 ± 3.2 years, range: 14 – 27 years), OCP (66.3 ± 2.5 years, range: 48 – 86 years) and control (50.1 ± 3.5 years, range: 25 – 77 years). There were 3 males and 1 female in the SJS group and 7 males and 13 females in each of the OCP and control groups. With respect to ocular history, the SJS group had 5 eyes (3 patients) on lubricating drops, 2 eyes (1 patient) on steroids and 1 eye on antibiotics. In the OCP group, 14 eyes (7 patients) were on lubricant drops where 2 eyes (1 patient) were treated with a mild steroid and 2 other eyes (1 patient) were also treated for glaucoma. In the control group, 6 eyes (3 patients) were treated with one or more anti-glaucoma drugs for diagnosed or suspected glaucoma and 2 eyes (1 patient) had a history of a non-penetrating ocular trauma (bilateral traumatic uveitis) on no topical treatment and required no surgical intervention. Surgical history included one SJS eye that had undergone amniotic membrane grafting 5 years prior to tear wash collection. Two OCP eyes (1 patient) had undergone fornix reconstruction and 1 OCP eye had undergone a fornix reconstruction and an amniotic membrane grafting 6 years prior to the tear wash collection. One OCP eye had undergone blepharoplasty 7 years prior to the tear wash collection.
The tear wash samples yielded 15 to 50 µL for patients with SJS, 5 to 45 µL for patients with OCP, and 9 to 45 µL for controls, with protein concentrations that ranged from 0.36 to 3.19 µg/µL (SJS patients), 0.10 to 2.34 µg/µL (OCP patients) and 0.14 to 1.81 µg/µL (control patients).
MMP-8, MMP-9 and MPO in the Tears
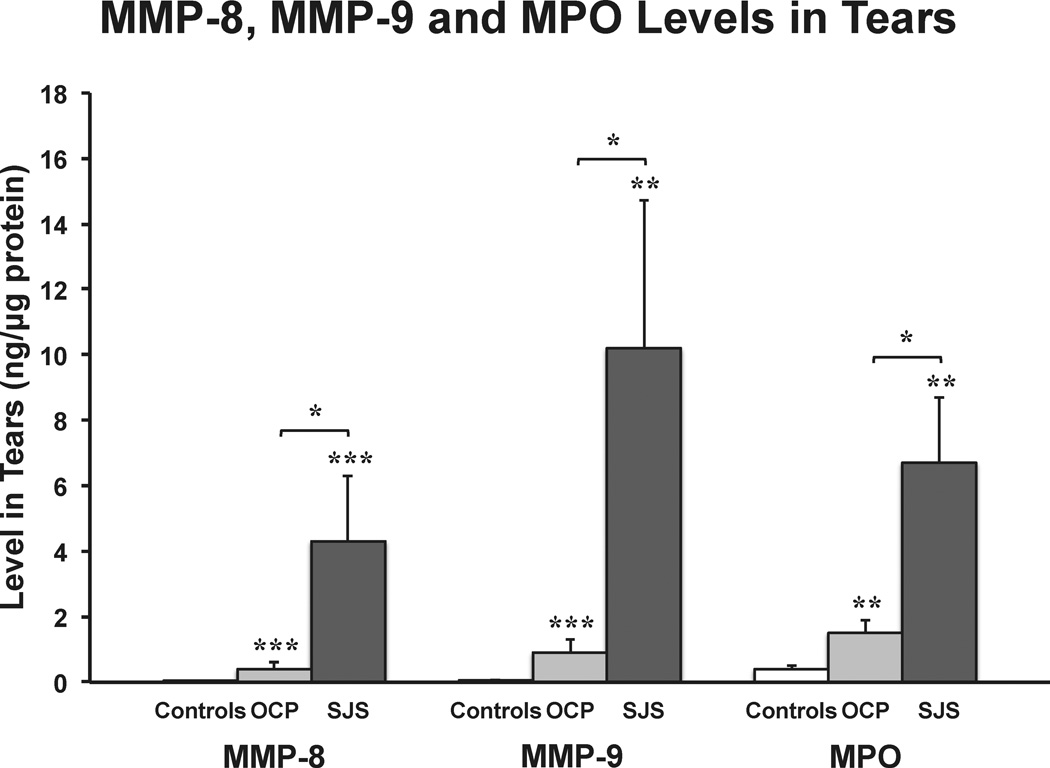
Levels of MMP-8 were 296-fold higher in SJS tears and 25-fold higher in OCP tears when compared with controls (mean ± standard error (in ng/µg protein), SJS: 4.3 ± 2.0, OCP: 0.4 ± 0.2 and controls: 0.01 ± 0.004; Fig 1). MMP-9 levels showed similar elevations with disease, in that they were 180-fold higher in SJS tears and 16-fold higher in OCP tears when compared with controls (SJS: 10.2 ± 4.5, OCP: 0.9 ± 0.4 and controls: 0.06 ± 0.01; Fig 1). The neutrophil enzyme, MPO, was 15-fold higher in SJS tears and 3.7-fold higher in OCP tears when compared with controls (SJS: 6.7 ± 2.0, OCP: 1.5 ± 0.4 and controls: 0.4 ± 0.1; Fig 1). The levels of MMP-8, MMP-9 and MPO in tears of SJS patients were statistically higher than in the OCP patients and both disease groups had statistically higher levels than controls.
Figure 1.
Bar graph showing the mean levels of neutrophil collagenase (MMP-8), gelatinase B (MMP-9) and myeloperoxidase (MPO) in Stevens-Johnson syndrome (SJS) (n = 7 eyes), ocular cicatricial pemphigoid (OCP) (n = 37 eyes) and healthy control (controls) (n = 38 eyes) tears using a fluorimetric bead-based enzyme-linked immunosorbent assay. All values were standardized to µg of protein loaded (ng/µg protein) and the MMP-8, MMP-9 and MPO levels in SJS and OCP tears were compared to controls and between disease groups (*P<0.05, **P<0.01, ***P<0.001, Kruskal-Wallis test). The error bars indicate the standard errors of the mean.
Other MMPs in the Tears
MMP-2 and MMP-3 levels were statistically elevated in SJS and OCP tears (SJS > OCP). However, their levels were at least a 100-fold lower than those observed with MMP-8, MMP-9 and MPO. For MMP-2 (pg/µg protein), levels were 9.9 ± 4.1 in SJS, 2.2 ± 1.0 in OCP and 0.2 ± 0.2 in controls. For MMP-3, levels were 95.9 ± 51.2 in SJS, 16.9 ± 4.3 in OCP and 2.4 ± 0.6 in controls.
MMP-7 and MMP-12 levels were also statistically higher in OCP tears when compared to controls. However, levels of both MMPs were not significantly different in SJS when compared to OCP or controls. MMP-12 was at least 300 fold lower than those observed with MMP-8, MMP-9 and MPO. The MMP-7 levels (ng/µg protein) were 0.4 ± 0.2 in SJS, 0.2 ± 0.03 in OCP and 0.1 ± 0.01 in controls. By comparison, MMP-12 levels (pg/µg protein) were not detectable in SJS, 0.2 ± 0.06 in OCP and 0.06 ± 0.03 in controls.
Total MMP Activity in the Tears
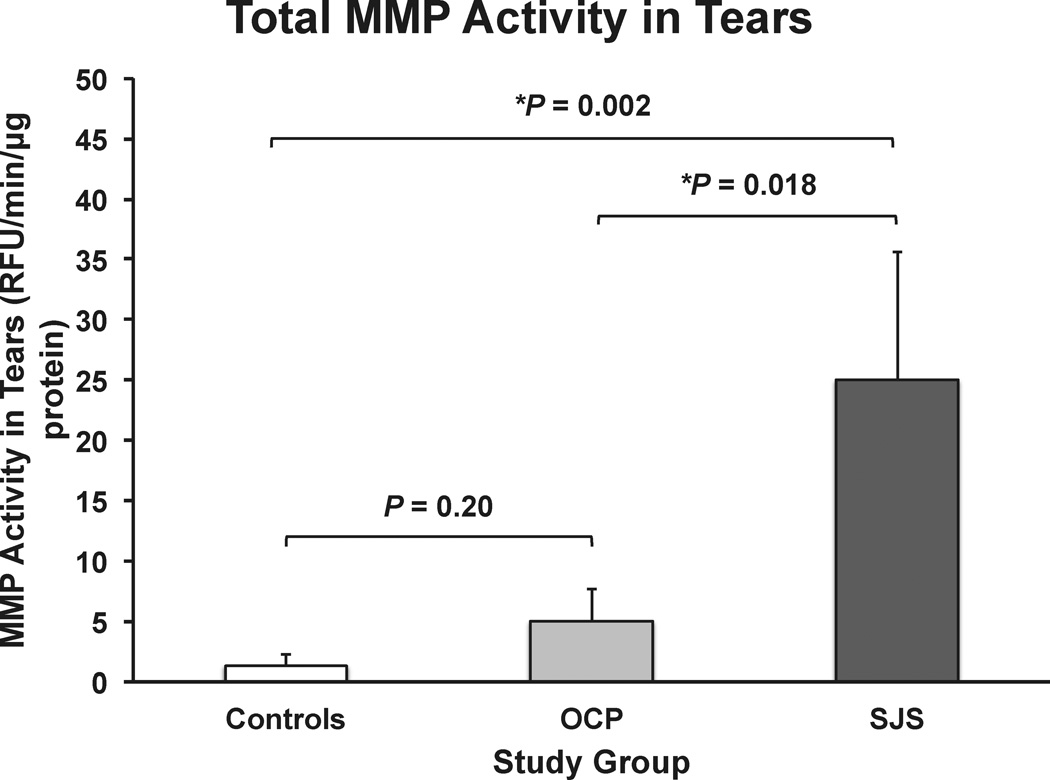
MMP fluorimetric activity assay demonstrated a 20-fold higher level of total MMP activity in tears of SJS patients and a 4-fold increase in OCP patients when compared with controls (in RFU/min/µg protein, SJS: 25.0 ± 10.6, OCP: 5.0 ± 2.7 and controls: 1.3 ± 0.9; Fig 2). Levels of activity in SJS patients were significantly higher when compared to both OCP patients and controls. However, total MMP activity was not significantly different between OCP and controls.
Figure 2.
Bar graph showing the mean total matrix metalloproteinase (MMP) activity in Stevens-Johnson syndrome (SJS) (n = 7 eyes), ocular cicatricial pemphigoid (OCP) (n = 27 eyes) and healthy control (controls) (n = 37 eyes) tears using an MMP fluorimetric activity assay. All values were standardized to µg of protein loaded (RFU/min/µg protein). The P values are shown on the graph (* statistically significant, Kruskal-Wallis test). The error bars indicate the standard errors of the mean.
TIMP-1, MMP-8/TIMP-1 and MMP-9/ TIMP-1 Ratios in the Tears
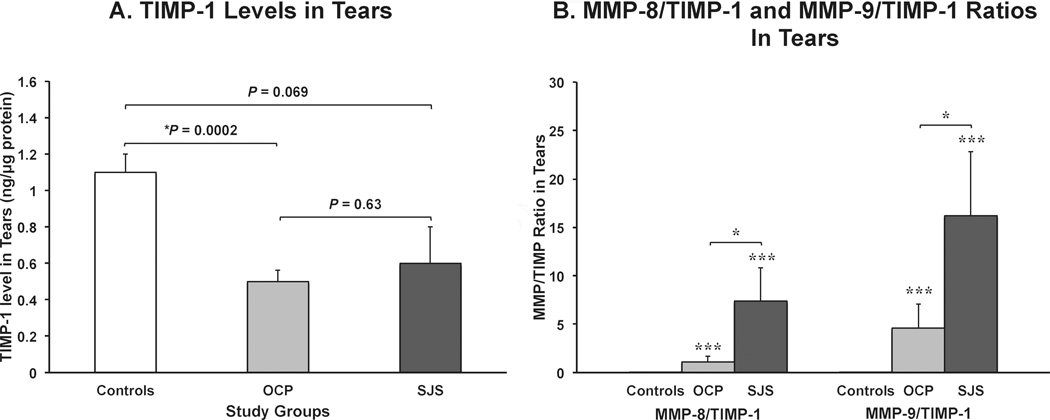
The levels of TIMP-1 in SJS tears were 1.7-fold lower and OCP tears were 2.2-fold lower when compared with controls (in ng/µg protein, SJS: 0.6 ± 0.2, OCP: 0.5 ± 0.06 and controls: 1.1 ± 0.1; Fig 3A). TIMP-1 levels in control tears was statistically higher than in the OCP tears, however, both OCP and controls levels were not significantly higher than in SJS tears. Interestingly, when the MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios were calculated, both ratios were markedly and statistically higher in patients with SJS and OCP (SJS > OCP) when compared to controls. MMP-8/TIMP-1 ratios were 7.4 ± 3.4 in SJS, 1.1 ± 0.6 in OCP and 0.01 ± 0.003 in controls (Fig 3B) and the MMP-9/TIMP-1 ratios were 16.2 ± 6.6 in SJS, 4.6 ± 2.5 in OCP and 0.05 ± 0.01 in controls (Fig 3B).
Figure 3.
Bar graphs showing (A) mean levels of tissue inhibitor of metalloproteinases-1 (TIMP-1) and (B) the ratios of the amounts of neutrophil collagenase (MMP-8) and gelatinase B (MMP-9) to TIMP-1 in tears of Stevens-Johnson syndrome (SJS), ocular cicatricial pemphigoid (OCP) and healthy control (controls) eyes. (A) SJS (7 eyes), OCP (24 eyes) and controls (33 eyes) tears were assayed using a fluorimetric bead-based enzyme-linked immunosorbent assay where all values were standardized to µg of protein loaded (ng/µg protein). The P values are shown on the graph (* statistically significant, Kruskal-Wallis test). (B) MMP/TIMP-1 ratios in SJS (7 eyes), OCP (23 eyes) and controls (32 eyes) tears were calculated and compared to their controls and between disease groups (*P<0.05, **P<0.01, ***P<0.001, Kruskal-Wallis test). The error bars indicate the standard errors of the mean.
Correlations Between MMP-8, MMP-9 and MPO in the Tears
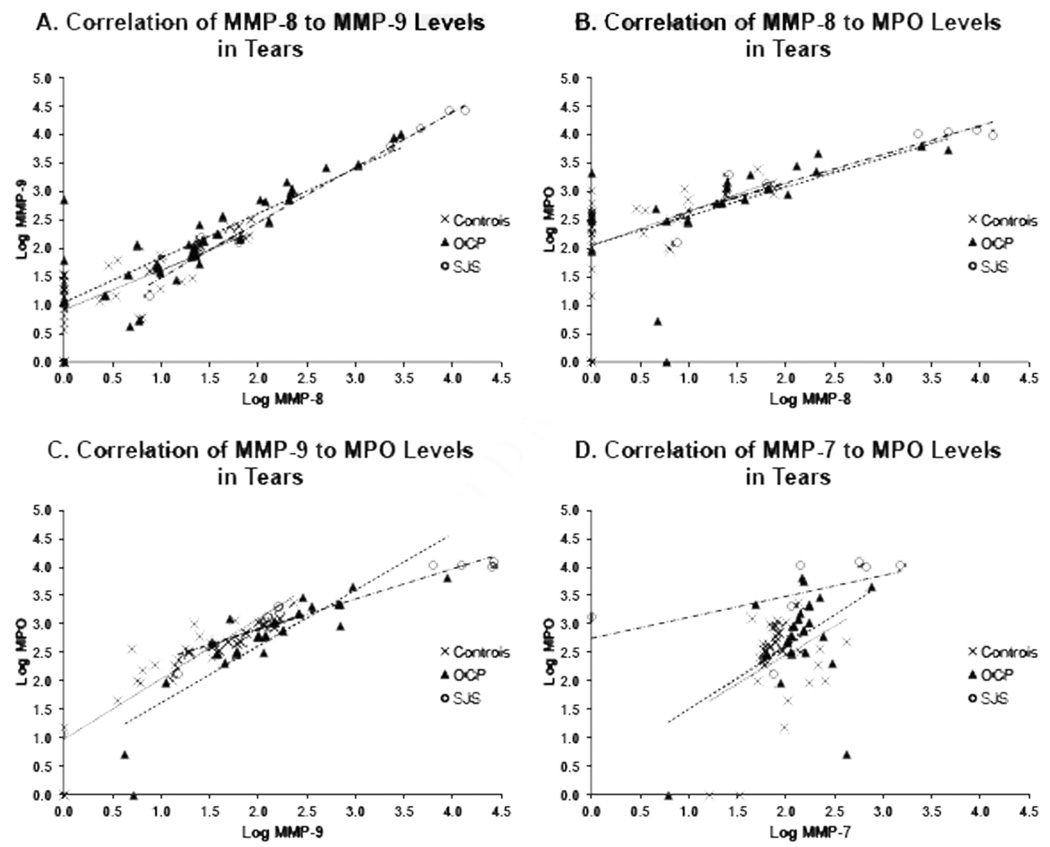
Using the data reported above, we evaluated correlations between levels of MMP-8, MMP-9 and MPO in tears since MMP-8 and MPO are known products of inflammatory cells, primarily neutrophils and MMP-9 can be derived from both inflammatory cells and ocular surface tissue (Figures 4A–C).
Figure 4.
Scatter graphs illustrating the correlations between the levels (in logarithmic values) of (A) neutrophil collagenase (MMP-8) and gelatinase B (MMP-9), (B) MMP-8 and myeloperoxidase (MPO), (C) MMP-9 and MPO and (D) matrilysin (MMP-7) and MPO in Stevens-Johnson syndrome (SJS), ocular cicatricial pemphigoid (OCP) and healthy control (controls) tears using Spearman rank correlation tests. The best fit lines: SJS (-.-), OCP (---) and controls (…). (A) In SJS (7 eyes), OCP (36 eyes) and controls (38 eyes) tears, significant correlation was found in all study groups: SJS (r = 0.96, P<0.01), OCP (r = 0.87, P<0.0001) and controls (r = 0.78, P<0.0001). (B) In SJS (7 eyes), OCP (22 eyes) and controls (32 eyes) tears, correlation was found in all study groups. Significance was achieved in OCP (r = 0.84, P<0.0001) and controls (r = 0.68, P<0.0001) but not in SJS (r = 0.75, P = 0.067). (C) In SJS (7 eyes), OCP (21 eyes) and controls (32 eyes) tears, significant correlation was found in all study groups: SJS (r = 0.85, P<0.05), OCP (r = 0.93, P<0.0001) and controls (r = 0.91, P<0.0001). (D) In SJS (7 eyes), OCP (22 eyes) and controls (30 eyes) tears, no significant correlation was demonstrated in all study groups: SJS (r = 0.68, P = 0.11), OCP (0.26, P = 0.24) and controls (r = 0.089, P = 0.64).
The levels of MMP-8 correlated with those of MMP-9 in tears of all patients (Spearman rank correlation tests, SJS: r = 0.96 (P<0.01), OCP: r = 0.87 (P<0.0001) and controls: r = 0.78 (P<0.0001); Fig 4A). The levels of MMP-8 correlated with the levels of MPO in OCP and control groups but the correlation was not statistically significant in the SJS group (SJS: r = 0.75 (P = 0.067), OCP: r = 0.84 (P<0.0001) and controls: r = 0.68 (P<0.0001); Fig 4B). On the other hand, the levels of MMP-9 were strongly and statistically correlated to MPO levels in tears of all patients (SJS: 0.85 (P<0.05), OCP: 0.93 (P<0.0001) and controls: 0.91 (P<0.0001); Fig 4C).
The correlation studies of the remaining MMPs (MMP-2, -3, -7 and -12) to MPO varied widely. A significant correlation between MMP-2 and MPO was found in SJS and OCP patients (SJS: r = 0.82 (P<0.05) and OCP: r = 0.48 (P<0.05); no figure) but not in controls. Conversely, MMP-3 correlated with MPO in controls only (r = 0.55 (P<0.01); no figure). There were no correlations observed between MMP-7 to MPO (Fig 4D), and MMP-12 to MPO (data not shown).
Discussion
This study is the first, to our knowledge, to report markedly elevated MMP-8, MMP-9, MPO and MMP activity levels in tears of patients with chronic SJS and OCP when compared to healthy controls. We also demonstrate strong correlations between the tear levels of MMP-8 and MMP-9 and between those enzymes and the specific inflammatory marker MPO. These relationships by comparison to lack of correlations between levels of MMP-7 and MPO suggest that inflammatory cells, primarily neutrophils, are the common source of elevated MMP-8 and MMP-9 on the ocular surface of these two severe inflammatory diseases.
The MMP findings in SJS and OCP agree with previous reports that demonstrated elevated tear levels of MMPs in a variety of inflammatory ocular surface diseases11–17. Membrane array analysis of OCP tears in active disease revealed markedly elevated MMP-9 thought to be released by activated neutrophils26. MPO is a well-established marker of neutrophil infiltration whose activity has been used to quantitate the density of polymorphonuclear cells in corneal and conjunctival tissues27,28. In tears, elevated MPO has been reported in patients with allergy22 and, in one study, increased MPO levels were demonstrated in homogenized dry eye tears29. Congruent changes in MPO and MMP levels have been shown in conditions such as chronic periodontitis30. Although several studies evaluated both MMP-8 and MMP-9 tear levels on the ocular surface12,16, to our knowledge, this study is the first to assess these enzymes with respect to MPO tear levels in any ocular surface disease.
Tissue inhibitors of metalloproteinases (TIMPs) inhibit MMPs in a 1:1 stoichiometric ratio of TIMP to MMP by interacting with the N-terminal domain of the TIMP to the active site of the MMP31. Therefore, the elevated MMP-8/TIMP-1 and MMP-9/TIMP-1 ratios in the SJS and OCP suggest an MMP imbalance on the ocular surface since TIMPs are the primary regulators of MMP activity31. A limitation of the assay was its inability to discriminate between active and inactive MMPs or between free TIMPs and TIMPs complexed with MMPs. Therefore; the results are only suggestive of an MMP homeostatic imbalance. However, since the hallmark of MMP dysfunction is MMP overactivation, the total MMP activity observed in the tears of SJS and OCP were not only higher than in control but they were congruent with higher MMP/TIMP ratios. These results point again to a potential imbalance in the MMP/TIMP dynamic on the ocular surface milieu in favor of excessive MMP activity. MMP overactivation may impart its potential deleterious effects on the underlying ocular surface tissue such as disturbing the corneal epithelial barrier function, increasing corneal epithelial desquamation and corneal surface irregularity32. The contribution of each MMP studied to the overall activity observed remains unknown.
Interestingly, MPO has been found to indirectly disrupt the physiologic balance of the proteolytic activity of MMPs and the function of endogenous MMP inhibitors in inflammation33. When activated, neutrophils release MPO from their azurophilic granules to generate antimicrobial antioxidants and other reactive species to kill bacteria and other pathogens20,21. These reactive species have also been shown to modify host proteins by oxidizing amino acid side chains, N-terminal amino groups and disulfides34. In controlled studies, one of these reactive species, hypochlorous acid (HOCl), was shown to activate MMP-833,35 and suggested to activate MMP-933,36 and, conversely, inactivate TIMP-1 (can no longer form complexes with MMPs)37. To test this hypothesis within our samples, attempts were made to compare the specific activity of MMP-8 in the tears of the three study groups. However, due to the relatively low protein recovered from single tear collections from all patients and the absence of available sensitive MMP-8 activity assays and MMP-8 specific fluorimetric substrates, MMP-8 activity in tears could not be reliably measured. Future studies determining MMP-8 and MMP-9 activities and the levels of MPO-generated reactive species in tears of patients with ocular surface disease may be of value in ascertaining the role of MPO in MMP activation. Perhaps pooling tear washes daily from each eye over days or, preferably, hourly over a single day would improve protein yield to facilitate such studies.
The elevated MMPs and MPO tear levels seen in SJS and OCP patients diagnosed to be in clinical remission is intriguing. Ours is the first study, to our knowledge, to suggest the presence of activated neutrophils on the ocular surface in the chronic stages of inflammation in these diseases. Histologic studies of conjunctival tissues in cicatrizing diseases have implicated mononuclear cells, including macrophages, lymphocytes and plasma cells as the principal effectors of chronic inflammation and the major causes of conjunctival fibrosis38. A study that examined conjunctivalized corneas from chronic SJS patients showed extensive subepithelial mononuclear infiltration with no polymorphonuclear cells except within vascular lumen39. Conjunctival biopsies from patients with episodic conjunctival inflammation following SJS have shown a preponderance of T lymphocytes, macrophages and Langerhans’ cells and infrequent neutrophils40. The presence of neutrophils in SJS tears was only demonstrated in the acute stage from serial tear analysis of a single SJS patient followed for weeks after the acute onset of the disease41.
With respect to OCP, immunohistological study of conjunctival specimens from patients at various stages of the disease has shown a marked neutrophilic infiltration in the acute stage that substantially dropped in the chronic stage. In the acute stage, macrophages were as elevated as neutrophils that mildly declined yet remained relatively elevated in the subacute and chronic stages42. Lymphocytes were the major inflammatory cells in the chronic stage. To our knowledge, there have been no OCP tear studies on neutrophil quantification. Despite the fact that most studies on cellular inflammation in SJS and OCP were limited to the conjunctiva, the common presence of macrophages in the chronic stages of both diseases and the fact that these cells are also known to synthesize MPO and MMPs raises the possibility that macrophages may be a source of these enzymes in SJS and OCP tears. However, macrophages are not known to synthesize MMP-8, one of two MMPs that correlated with MPO levels in the diseased tears in our study. Additionally, our study showed negligible levels of MMP-12 in SJS and OCP tears; a macrophage-derived elastinolytic protease in inflammation. While these data may not completely negate a possible contribution of macrophages to the influx of MMPs and MPO in the tears, it strongly suggests that they may not be the dominant inflammatory source in the diseased tears in chronic stages.
MPO and MMP-8 are derived from inflammatory cells and correlated strongly to MMP-9 levels in this study to suggest an inflammatory source for all three enzymes. However, since both inflammatory cells and ocular surface epithelial tissue can produce MMP-9, we cannot rule out epithelial cells as a secondary source of MMP-9. The control group had 14 eyes (37%) that showed the presence of MMP-9 and the absence of MMP-8 in their tears compared to 4 eyes (11%) in the OCP group and no eyes (0%) in the SJS group. This interesting finding infers, at least with respect to MMP-9, that inflammatory cells recruited into the tears may be the dominant source of MMP-9 in SJS and OCP. Further tear studies with specific markers for a wide range of inflammatory cells including macrophages and T lymphocytes will help determine if the inflammatory profile in tears mimics that in conjunctival tissue.
The high levels of MMP-8, MMP-9 and MPO enzymes in patients in clinical remission may indicate exceptionally high levels in tears of patients in acute stages of the disease or in patients whose ocular inflammation has not been adequately controlled. It may also point to the presence of low-grade conjunctival inflammation that remains undiagnosed by slit lamp examination. It is difficult to discern whether the levels found in this study indicate successful management or not. Multicenter studies involving larger study populations looking into the enzyme levels at different stages of the disease and in healthy age-matched gender-matched controls may provide a grading system to stratify disease severity on the basis of inflammatory markers in tears.
Grading of conjunctival injection (0–4) by slit lamp exam is the common method used to better evaluate inflammation on the ocular surface. Despite its advantages, its subjective nature inspired studies to look for other methods to better evaluate inflammation on the ocular surface43. The results of this study show that MPO is a sensitive and specific marker for potentially detecting and quantitating active cellular inflammation on the ocular surface in the presence or absence of clinically visible inflammation. A study by Kawasaki et al surprisingly found substantial inflammatory cell infiltration in the conjunctiva of SJS patients with little or no clinically obvious inflammation39. Large prospective studies are needed to test the efficacy of using an MPO assay, alone or as adjunct to other available modalities, to evaluate ocular surface inflammation. Perhaps such studies will open opportunities, as seen with assay of MMP-9 in dry eye44, to develop easy-to-use, fast sensitive devices that can measure tear MPO levels in tears of patients by the bedside.
Acknowledgments
Financial Support The Boston Keratoprosthesis Research Fund (MEEI), Boston, Massachusetts and the National Institutes of Health, National Eye Institute, Bethesda, Maryland (NEI 03306 to IKG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicting relationship exists for any author.
Financial Disclosure(s)
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Gregory DG. The ophthalmologic management of acute Stevens-Johnson syndrome. Ocul Surf. 2008;6:87–95. doi: 10.1016/s1542-0124(12)70273-2. [DOI] [PubMed] [Google Scholar]
- 2.Power WJ, Ghoraishi M, Merayo-Lloves J, et al. Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology. 1995;102:1669–1676. doi: 10.1016/s0161-6420(95)30811-1. [DOI] [PubMed] [Google Scholar]
- 3.Kirzhner M, Jakobiec FA. Ocular cicatricial pemphigoid: a review of clinical features, immunopathology, differential diagnosis, and current management. Semin Ophthalmol. 2011;26:270–277. doi: 10.3109/08820538.2011.588660. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed M, Zein G, Khawaja F, Foster CS. Ocular cicatricial pemphigoid: pathogenesis, diagnosis and treatment. Prog Retin Eye Res. 2004;23:579–592. doi: 10.1016/j.preteyeres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Hsu M, Jayaram A, Verner R, et al. Indications and outcomes of amniotic membrane transplantation in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea. 2012;31:1394–1402. doi: 10.1097/ICO.0b013e31823d02a8. [DOI] [PubMed] [Google Scholar]
- 6.Di Pascuale MA, Espana EM, Liu DT, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112:904–912. doi: 10.1016/j.ophtha.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Tugal-Tutkun I, Akova YA, Foster CS. Penetrating keratoplasty in cicatrizing conjunctival diseases. Ophthalmology. 1995;102:576–585. doi: 10.1016/s0161-6420(95)30980-3. [DOI] [PubMed] [Google Scholar]
- 8.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon GM, Austin JS, Sklar AL, et al. Comprehensive gene expression profiling and functional analysis of matrix metalloproteinases and TIMPs, and identification of ADAM-10 gene expression, in a corneal model of epithelial resurfacing. J Cell Physiol. 2011;226:1461–1470. doi: 10.1002/jcp.22306. [DOI] [PubMed] [Google Scholar]
- 10.Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;290(suppl):S12–S23. doi: 10.1007/pl00007449. [DOI] [PubMed] [Google Scholar]
- 11.Chotikavanich S, de Paiva CS, Li D, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohini G, Murugeswari P, Prajna NV, et al. Matrix metalloproteinases (MMP-8, MMP-9) and the tissue inhibitors of metalloproteinases (TIMP-1, TIMP-2) in patients with fungal keratitis. Cornea. 2007;26:207–211. doi: 10.1097/01.ico.0000248384.16896.7d. [DOI] [PubMed] [Google Scholar]
- 13.Smith VA, Hoh HB, Easty DL. Role of ocular matrix metalloproteinases in peripheral ulcerative keratitis. Br J Ophthalmol. 1999;83:1376–1383. doi: 10.1136/bjo.83.12.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maatta M, Kari O, Tervahartiala T, et al. Elevated expression and activation of matrix metalloproteinase 8 in tear fluid in atopic blepharoconjunctivitis. Cornea. 2008;27:297–301. doi: 10.1097/ICO.0b013e31815c18d6. [DOI] [PubMed] [Google Scholar]
- 15.Maatta M, Kari O, Tervahartiala T, et al. Tear fluid levels of MMP-8 are elevated in ocular rosacea--treatment effect of oral doxycycline. Graefes Arch Clin Exp Ophthalmol. 2006;244:957–962. doi: 10.1007/s00417-005-0212-3. [DOI] [PubMed] [Google Scholar]
- 16.Leonardi A, Sathe S, Bortolotti M, et al. Cytokines, matrix metalloproteases, angiogenic and growth factors in tears of normal subjects and vernal keratoconjunctivitis patients. Allergy. 2009;64:710–717. doi: 10.1111/j.1398-9995.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 17.Kari O, Maatta M, Tervahartiala T, et al. Tear fluid concentration of MMP-8 is elevated in non-allergic eosinophilic conjunctivitis and correlates with conjunctival inflammatory cell infiltration. Graefes Arch Clin Exp Ophthalmol. 2009;247:681–686. doi: 10.1007/s00417-009-1042-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001;85:147–153. doi: 10.1136/bjo.85.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonardi A, Brun P, Abatangelo G, et al. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44:3052–3058. doi: 10.1167/iovs.02-0766. [DOI] [PubMed] [Google Scholar]
- 20.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 21.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 22.Monteseirin J, Fernandez-Pineda I, Chacon P, et al. Myeloperoxidase release after allergen-specific conjunctival challenge. J Asthma. 2004;41:639–643. doi: 10.1081/jas-200026407. [DOI] [PubMed] [Google Scholar]
- 23.Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc. 1986;84:527–663. [PMC free article] [PubMed] [Google Scholar]
- 24.Gipson IK, Spurr-Michaud SJ, Senchyna M, et al. Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye. Cornea. 2011;30:1346–1352. doi: 10.1097/ICO.0b013e31820d852a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sack RA, Sathe S, Beaton AR, et al. Changes in the diurnal pattern of the distribution of gelatinases and associated proteins in normal and pathological tear fluids: evidence that the PMN cell is a major source of MMP activity in tear fluid. Adv Exp Med Biol. 2002;506:539–545. doi: 10.1007/978-1-4615-0717-8_76. [DOI] [PubMed] [Google Scholar]
- 26.Chan MF, Sack R, Quigley DA, et al. Membrane array analysis of tear proteins in ocular cicatricial pemphigoid. Optom Vis Sci. 2011;88:1005–1009. doi: 10.1097/OPX.0b013e31821ddc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang A, Balzli CL, Caballero AR, et al. Staphylococcus aureus infection of the rabbit cornea following topical administration. Curr Eye Res. 2012;37:1075–1083. doi: 10.3109/02713683.2012.716485. [DOI] [PubMed] [Google Scholar]
- 28.McCormick CC, Caballero AR, Balzli CL, et al. Diverse virulence of Staphylococcus aureus strains for the conjunctiva. Curr Eye Res. 2011;36:14–20. doi: 10.3109/02713683.2010.523194. [DOI] [PubMed] [Google Scholar]
- 29.Augustin AJ, Spitznas M, Kaviani N, et al. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol. 1995;233:694–698. doi: 10.1007/BF00164671. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez M, Gamonal J, Tervahartiala T, et al. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: a longitudinal study. J Periodontol. 2010;81:1644–1652. doi: 10.1902/jop.2010.100196. [DOI] [PubMed] [Google Scholar]
- 31.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pflugfelder SC, Farley W, Luo L, et al. Matrixmetalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mainnemare A, Megarbane B, Soueidan A, et al. Hypochlorous acid and taurine-N-monochloramine in periodontal diseases. J Dent Res. 2004;83:823–831. doi: 10.1177/154405910408301101. [DOI] [PubMed] [Google Scholar]
- 34.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Saari H, Suomalainen K, Lindy O, et al. Activation of latent human neutrophil collagenase by reactive oxygen species and serine proteases. Biochem Biophys Res Commun. 1990;171:979–987. doi: 10.1016/0006-291x(90)90780-q. [DOI] [PubMed] [Google Scholar]
- 36.Meli DN, Christen S, Leib SL. Matrix metalloproteinase-9 in pneumococcal meningitis: activation via an oxidative pathway. J Infect Dis. 2003;187:1411–1415. doi: 10.1086/374644. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Rosen H, Madtes DK, et al. Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue: an oxidative mechanism for regulating proteolysis during inflammation. J Biol Chem. 2007;282:31826–31834. doi: 10.1074/jbc.M704894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faraj HG, Hoang-Xuan T. Chronic cicatrizing conjunctivitis. Curr Opin Ophthalmol. 2001;12:250–257. doi: 10.1097/00055735-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Kawasaki S, Nishida K, Sotozono C, et al. Conjunctival inflammation in the chronic phase of Stevens-Johnson syndrome. Br J Ophthalmol. 2000;84:1191–1193. doi: 10.1136/bjo.84.10.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster CS, Fong LP, Azar D, Kenyon KR. Episodic conjunctival inflammation after Stevens-Johnson syndrome. Ophthalmology. 1988;95:453–462. doi: 10.1016/s0161-6420(88)33165-9. [DOI] [PubMed] [Google Scholar]
- 41.Yagi T, Sotozono C, Tanaka M, et al. Cytokine storm arising on the ocular surface in a patient with Stevens-Johnson syndrome [letter] Br J Ophthalmol. 2011;95:1030–1031. doi: 10.1136/bjo.2010.196295. [DOI] [PubMed] [Google Scholar]
- 42.Bernauer W, Wright P, Dart JK, et al. The conjunctiva in acute and chronic mucous membrane pemphigoid. An immunohistochemical analysis. Ophthalmology. 1993;100:339–346. doi: 10.1016/s0161-6420(93)31644-1. [DOI] [PubMed] [Google Scholar]
- 43.Wakamatsu TH, Okada N, Kojima T, et al. Evaluation of conjunctival inflammatory status by confocal scanning laser microscopy and conjunctival brush cytology in patients with atopic keratoconjunctivitis (AKC) [Accessed June 18, 2013];Mol Vis. 2009 15:1611–1619. [serial online] Available at: http://www.molvis.org/molvis/v15/a172/. [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman HE. The practical detection of MMP-9 diagnoses ocular surface disease and may help prevent its complications. Cornea. 2013;32:211–216. doi: 10.1097/ICO.0b013e3182541e9a. [DOI] [PubMed] [Google Scholar]