Abstract
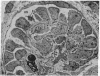
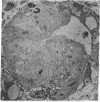
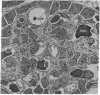
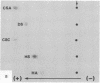
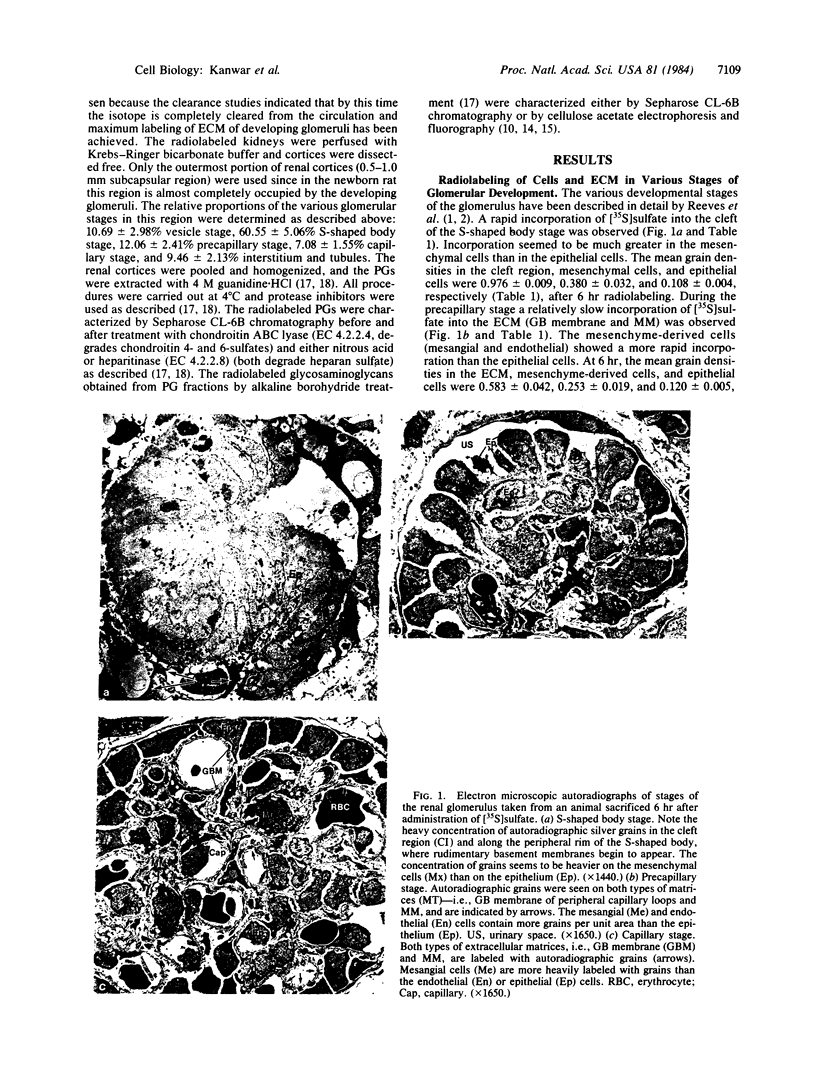
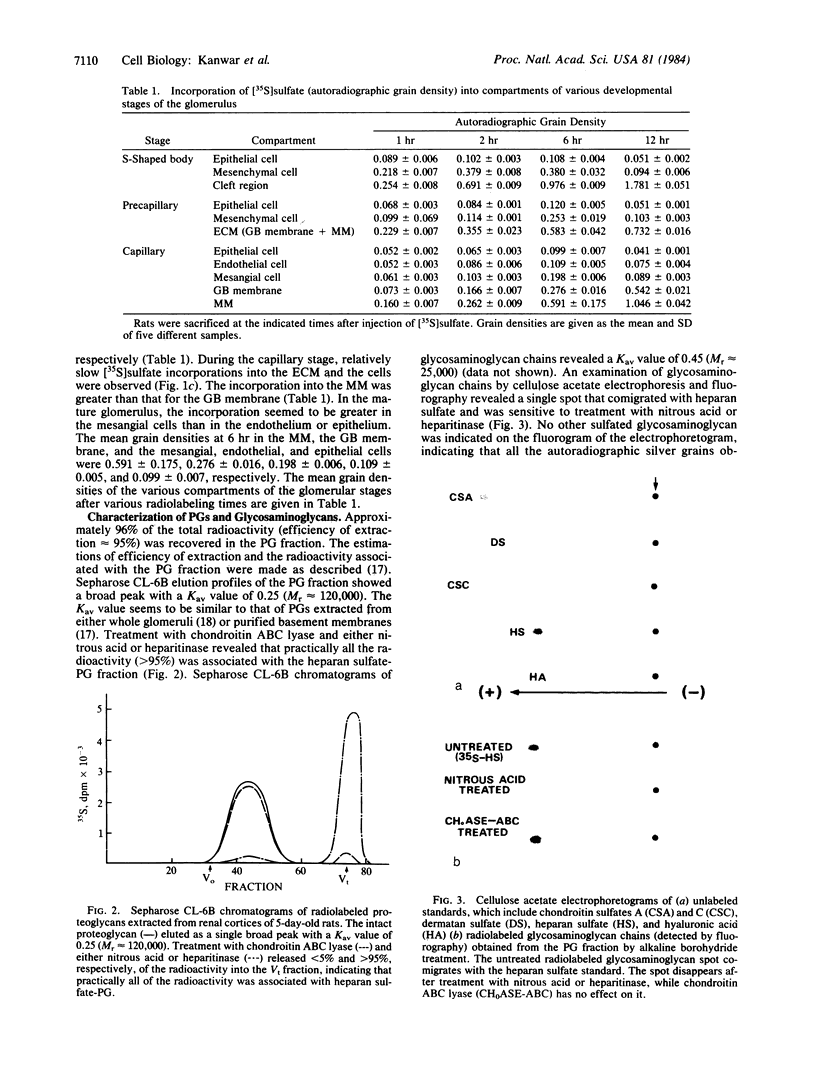
The site of cellular synthesis of glomerular proteoglycans was investigated in developing glomeruli of 4- to 5-day-old rats. [35S]Sulfate was administered intravenously and animals were sacrificed 15 min to 12 hr later. The outermost layers of the kidney cortices were utilized for characterization of proteoglycans and electron microscopic autoradiography. Sepharose CL-6B chromatography and cellulose acetate electrophoresis revealed that most (approximately equal to 96%) of the radioactivity was associated with heparan sulfate-proteoglycan synthesized during maturation of glomerular capillaries. Tissue autoradiography revealed the following: (i) during the S-shaped body stage, there is rapid incorporation of [35S]sulfate by mesenchymal cells into the cleft region (site for development of future glomerular extracellular matrices); (ii) during the precapillary stage, mesenchyme-derived cells showed higher incorporation of radioisotope than did epithelial cells; and (iii) during the mature capillary stage, all glomerular cell types (mesangial, endothelial, and epithelial) incorporated [35S]sulfate, incorporation by mesangial cells being the greatest. Radiolabeling was also higher in the mesangial matrix than in the glomerular basement membrane of peripheral capillary loops. Synthesis of a single major species of sulfated glycosaminoglycan by cells of different embryologic origin may be unique to glomerular capillaries.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S. D., Cohn R. H., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977 May;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M., Banerjee S. D. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev Biol. 1982 Apr;90(2):291–305. doi: 10.1016/0012-1606(82)90378-5. [DOI] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976 May;73(5):1646–1650. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P. Formation of basement membranes in the embryonic kidney: an immunohistological study. J Cell Biol. 1981 Oct;91(1):1–10. doi: 10.1083/jcb.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. R., Caulin-Glaser T., Lamm M. E. Charge of circulating immune complexes as a factor in glomerular basement membrane localization in mice. J Clin Invest. 1981 May;67(5):1305–1313. doi: 10.1172/JCI110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4493–4497. doi: 10.1073/pnas.76.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Hascall V. C., Farquhar M. G. Partial characterization of newly synthesized proteoglycans isolated from the glomerular basement membrane. J Cell Biol. 1981 Aug;90(2):527–532. doi: 10.1083/jcb.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Jakubowski M. L., Rosenzweig L. J. Distribution of sulfated glycosaminoglycans in the glomerular basement membrane and mesangial matrix. Eur J Cell Biol. 1983 Sep;31(2):290–295. [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J., Jakubowski M. L. Distribution of de novo synthesized sulfated glycosaminoglycans in the glomerular basement membrane and mesangial matrix. Lab Invest. 1983 Aug;49(2):216–225. [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J., Linker A., Jakubowski M. L. Decreased de novo synthesis of glomerular proteoglycans in diabetes: biochemical and autoradiographic evidence. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2272–2275. doi: 10.1073/pnas.80.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Veis A., Kimura J. H., Jakubowski M. L. Characterization of heparan sulfate-proteoglycan of glomerular basement membranes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):762–766. doi: 10.1073/pnas.81.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L., Maunsbach A. B. The ultrastructural development of the glomerular filtration barrier in the rat kidney: a morphometric analysis. J Ultrastruct Res. 1980 Sep;72(3):392–406. doi: 10.1016/s0022-5320(80)90074-x. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P., Kanwar Y. S., Farquhar M. G. Characterization of heparan sulfate isolated from drug glomerular basement membranes. Lab Invest. 1981 Jun;44(6):560–565. [PubMed] [Google Scholar]
- Michael A. F., Keane W. F., Raij L., Vernier R. L., Mauer S. M. The glomerular mesangium. Kidney Int. 1980 Feb;17(2):141–154. doi: 10.1038/ki.1980.18. [DOI] [PubMed] [Google Scholar]
- Reeves W. H., Kanwar Y. S., Farquhar M. G. Assembly of the glomerular filtration surface. Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J Cell Biol. 1980 Jun;85(3):735–753. doi: 10.1083/jcb.85.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W., Caulfield J. P., Farquhar M. G. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978 Aug;39(2):90–100. [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennke H. G., Patel Y., Venkatachalam M. A. Glomerular filtration of proteins: clearance of anionic, neutral, and cationic horseradish peroxidase in the rat. Kidney Int. 1978 Apr;13(4):278–288. doi: 10.1038/ki.1978.41. [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Daniel C. W. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol. 1982 Mar;90(1):215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Preferential distribution of anionic sites on the basement membrane and the abluminal aspect of the endothelium in fenestrated capillaries. J Cell Biol. 1982 Nov;95(2 Pt 1):425–434. doi: 10.1083/jcb.95.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. A., Spooner B. S. Proteoglycan and glycosaminoglycan synthesis in embryonic mouse salivary glands: effects of beta-D-xyloside, an inhibitor of branching morphogenesis. J Cell Biol. 1983 May;96(5):1443–1450. doi: 10.1083/jcb.96.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Toole B. P. Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol. 1974 Sep;62(3):815–830. doi: 10.1083/jcb.62.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro C. A., Brody J. S. Structural features of alveolar wall basement membrane in the adult rat lung. J Cell Biol. 1981 Nov;91(2 Pt 1):427–437. doi: 10.1083/jcb.91.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]