Abstract
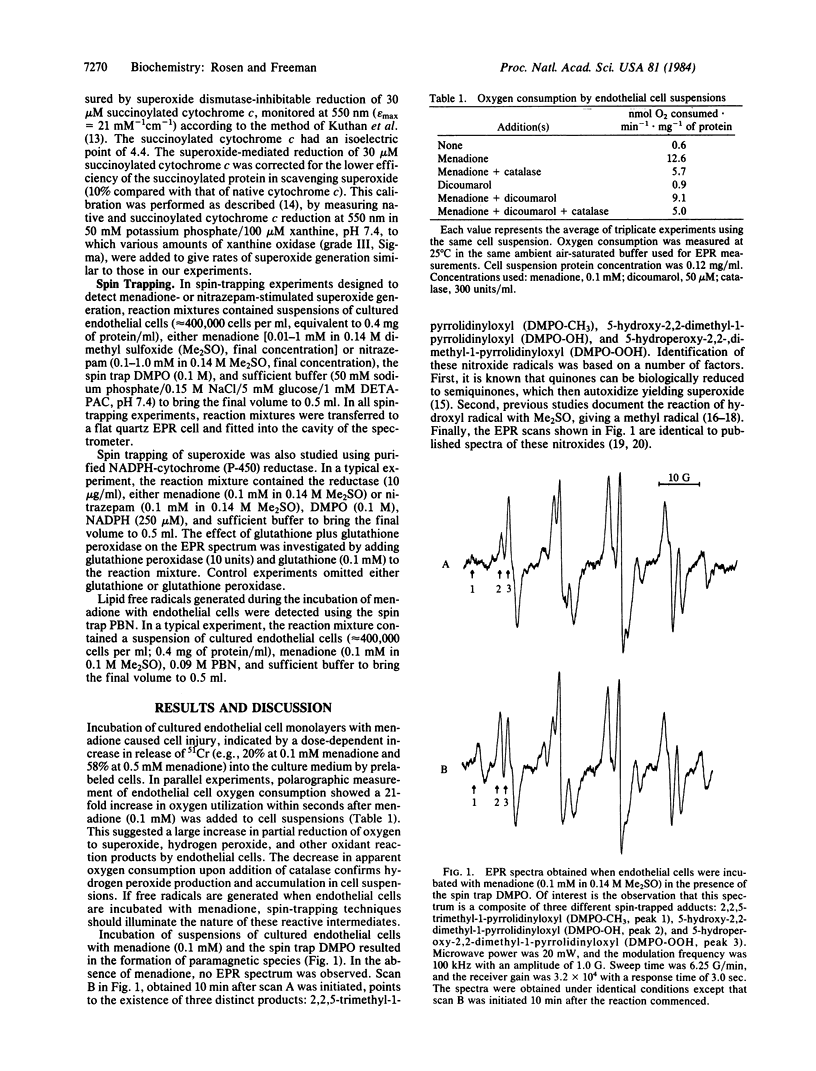
Superoxide and lipid free-radical generation in cultured endothelial cells treated with menadione or nitrazepam were measured using electron paramagnetic resonance spectroscopy. Superoxide was detected both intracellularly and extracellularly. Extracellular generation of superoxide and hydrogen peroxide was also measured, either by spectrophotometric measurement of succinoylated cytochrome c reduction or by polarography. Extracellular superoxide was generated due to reduced menadione diffusing across the plasma membrane and reacting with oxygen to generate superoxide in the medium. Increased intracellular oxygen tension favored intracellular oxidation of reduced menadione, thus decreasing diffusion of reduced menadione from the cells and, hence, decreasing extracellular superoxide production. The nitro anion free radical of reduced nitrazepam, which cannot cross the plasma membrane, did not generate detectable extracellular superoxide. Our results show that intracellular superoxide can be spin-trapped using 5,5-dimethyl-1-pyrroline-1-oxide and that secondary free-radical injury to membrane lipids, due to excess production of partially reduced species of oxygen by intact cells, can be detected by spin-trapping lipid free radicals with phenyl N-tert-butylnitrone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwood-Smith M. J. Current concepts concerning radioprotective and cryoprotective properties of dimethyl sulfoxide in cellular systems. Ann N Y Acad Sci. 1975 Jan 27;243:246–256. doi: 10.1111/j.1749-6632.1975.tb25363.x. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W., Leuthauser S. W. The effect of iron on the distribution of superoxide and hydroxyl radicals as seen by spin trapping and on the superoxide dismutase assay. Photochem Photobiol. 1978 Oct-Nov;28(4-5):693–695. doi: 10.1111/j.1751-1097.1978.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Cohen G., Cederbaum A. I. Chemical evidence for production of hydroxyl radicals during microsomal electron transfer. Science. 1979 Apr 6;204(4388):66–68. doi: 10.1126/science.432627. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J., Paxton J. Spin trapping of superoxide. Mol Pharmacol. 1979 Sep;16(2):676–685. [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol Pharmacol. 1982 Mar;21(2):262–265. [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Young S. L., Crapo J. D. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983 Oct 25;258(20):12534–12542. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Kuthan H., Ullrich V., Estabrook R. W. A quantitative test for superoxide radicals produced in biological systems. Biochem J. 1982 Jun 1;203(3):551–558. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. K., McCay P. B., Noguchi T., Fong K. L. In vivo spin-trapping of trichloromethyl radicals formed from CCl4. Biochem Pharmacol. 1979 Jul 15;28(14):2231–2235. doi: 10.1016/0006-2952(79)90212-0. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- Poyer J. L., Floyd R. A., McCay P. B., Janzen E. G., Davis E. R. Spin-trapping of the trichloromethyl radical produced during enzymic NADPH oxidation in the presence of carbon tetrachloride or bromotrichloromethane. Biochim Biophys Acta. 1978 Mar 20;539(3):402–409. doi: 10.1016/0304-4165(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. IV. Dependence on Fe3+. J Biol Chem. 1971 Jan 10;246(1):263–269. [PubMed] [Google Scholar]
- Rosen G. M., Demos H. A., Rauckman E. J. Not all aromatic nitro compounds form free radicals. Toxicol Lett. 1984 Aug;22(2):145–152. doi: 10.1016/0378-4274(84)90058-4. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Finkelstein E., Rauckman E. J. A method for the detection of superoxide in biological systems. Arch Biochem Biophys. 1982 May;215(2):367–378. doi: 10.1016/0003-9861(82)90097-2. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]
- Yusa T., Crapo J. D., Freeman B. A. Hyperoxia enhances lung and liver nuclear superoxide generation. Biochim Biophys Acta. 1984 Apr 10;798(2):167–174. doi: 10.1016/0304-4165(84)90299-x. [DOI] [PubMed] [Google Scholar]


