Abstract
In the current study, the role of cAMP in stimulating Na+ uptake in larval zebrafish was investigated. Treating larvae at 4 days postfertilization (dpf) with 10 μM forskolin or 1 μM 8-bromo cAMP significantly increased Na+ uptake by three-fold and twofold, respectively. The cAMP-dependent stimulation of Na+ uptake was probably unrelated to protein trafficking via microtubules because pretreatment with 200 μM colchicine or 30 μM nocodazole did not attenuate the magnitude of the response. Na+ uptake was stimulated markedly following acute (2 h) exposure to acidic water. The acid-induced increase in Na+ uptake was accompanied by a twofold elevation in whole body cAMP levels and attenuated by inhibiting PKA with 10 μM H-89. Knockdown of Na+-H+ exchanger 3b (NHE3b) attenuated, but did not abolish, the stimulation of Na+ uptake during forskolin treatment. In glial cell missing 2 morphants, in which the role of NHE3b in Na+ uptake is diminished and the Na+-Cl− cotransporter (NCC) becomes the predominant route of Na+ entry, forskolin treatment continued to increase Na+ uptake. These data suggest that at least NHE3b and NCC are targeted by cAMP in zebrafish larvae. Staining of larvae with fluorescent forskolin and propranolol revealed the presence of transmembrane adenylyl cyclase within multiple subtypes of ionocytes expressing β-adrenergic receptors. Taken together, results of the present study demonstrate that cAMP-mediated intracellular signaling may regulate multiple Na+ transporters and plays an important role in regulating Na+ uptake in zebrafish larvae during acute exposure to an acidic environment.
Keywords: β-adrenergic receptor, adenylyl cyclase, H+-ATPase-rich cell, low pH, protein kinase A
maintaining body fluid electrolyte balance is crucial for the survival of most vertebrates. Because fish living in freshwater (FW) suffer from the constant passive loss of ions by diffusion, they must actively absorb the major ions (Na+, Cl− and Ca2+) from the environment. The molecular mechanisms for the absorption of these ions have been intensely investigated (for reviews, see Refs. 12, 20–23, and 31). The results of these studies revealed significant roles for several hormones in regulating ionic uptake, including cortisol (11, 14, 27, 29, 33, 35), prolactin (5, 6, 47, 49), isotocin (10), stanniocalcin (63), vitamin D (34), ANG II (19) and endothelin (24), as well as neuroendocrine factors (e.g., catecholamines) (25, 39, 46, 64).
A series of recent studies (28–30, 32) demonstrated that in both larval and adult zebrafish, Na+ uptake is significantly elevated following exposure to extremely acidic (pH 4) water. In larvae, the stimulation of Na+ uptake in acidic water largely reflects activation of Na+/H+ exchange (NHE3b) (71) that is functionally linked to ammonia excretion via Rhcg1, an apically expressed ammonia channel (4, 30, 41, 51; for review, see Refs. 69 and 70). These effects are mediated, at least in part, by cortisol (acting via glucocorticoid receptors) (29) and catecholamines (acting via β-adrenergic receptors) (32). Although the mode of action of cortisol in stimulating Na+ uptake in acidic water was recently investigated (29), the specific downstream signaling pathways activated by catecholamines have not been identified.
Stimulation of β-adrenergic receptors, like other G protein-coupled receptors (GPCR) activates transmembrane adenylyl cyclase (tmAC), which initiates downstream signaling pathways mediated by 3′,5′-cyclic adenosine monophosphate (cAMP) (for review, see Ref. 48). Discovered almost 60 years ago (54), cAMP is known to act as an important second messenger in virtually all physiological systems (2). In mammalian tissues, cAMP inhibits Na+-H+ exchanger 3 activity through interaction with Na+-H+-exchanger regulatory factor 1 (NHERF1) (1, 17, 66, 67). cAMP can also be formed via activation of soluble adenylyl cyclase (sAC or AC10) (8). Unlike other isoforms of AC, which are transmembrane proteins, sAC is distributed in the cytosol and activated by a rise in [HCO3−] (8). The activation of sAC rapidly induces the apical accumulation of H+-ATPase in epididymis clear cells (42, 43) and A-type intercalated cells in renal collecting duct (44, 45). In dogfish (Squalus acanthias) gill ionocytes, sAC-derived cAMP also evokes the translocation of H+-ATPase, although in this case, the direction of movement is toward the basolateral membrane (60). Additionally, sAC contributes to the regulation of intestinal Na+ and Cl− reabsorption in the marine teleost, Opsanus beta (58). The results of these studies clearly implicate cAMP (derived either from tmAC or sAC) as an important intracellular signal promoting body fluid electrolyte homeostasis across vertebrate taxa. However, surprisingly little is known about the cAMP-mediated regulation of ion, especially Na+, uptake in FW fish.
On the basis of the role of β-adrenergic receptor activation in regulating Na+ uptake in acid-exposed zebrafish larvae and the extensive involvement of cAMP (a product of β-adrenergic receptor activation) as a regulator of transepithelial ion (and acid/base) transport, the present study was designed to assess the potential for cAMP to modulate Na+ uptake in zebrafish larvae. The specific objectives were to 1) directly demonstrate that cAMP is capable of stimulating Na+ uptake, 2) identify the Na+ transporters being regulated by cAMP, and 3) implicate cAMP in acutely inducing Na+ uptake during acid exposure.
MATERIALS AND METHODS
Experimental animals and water preparation.
Adult zebrafish (Danio rerio Hamilton-Buchanan 1822) were purchased from Big Al's Aquarium Services (Ottawa, ON, Canada) and kept in the University of Ottawa Aquatic Care Facility, where they were maintained in plastic tanks supplied with aerated, dechlorinated City of Ottawa tap water [the ionic composition of the water was (in mM) 0.78 Na+, 0.4 Cl−, 0.25 Ca2+, 0.025 K+, pH 7.0] at 28°C. Fish were subjected to a constant 14:10-h light-dark photoperiod and fed daily until satiation with no. 1 crumble-Zeigler (Aquatic Habitats, Apopka, FL). Embryos were collected and reared in 50-ml Petri dishes with dechlorinated City of Ottawa tap water (pH 7.3–7.5) supplemented with 0.05% methylene blue. The petri dishes were kept in incubators set at 28.5°C. Dead embryos were removed, and water was changed daily. The experiments were conducted in compliance with guidelines of the Canadian Council of Animal Care and after the approval of the University of Ottawa Animal Care Committee (protocol BL-226).
To determine the effect of cAMP on Na+ uptake in developing zebrafish, the following series of experiments were performed. Unless stated otherwise, all chemicals used for the experiments were purchased from Sigma (St. Louis, MO).
Series 1. Consequences of increasing cAMP levels on Na+ uptake.
cAMP levels in larval zebrafish were elevated by treatment with 1) forskolin, a pan-tmAC activator and 2) 8-bromo cAMP, a membrane-permeable, phosphodiestrase-resistant cAMP analog. Zebrafish larvae were raised in control water until 4 days postfertilization (dpf) and exposed to either forskolin at 0.1, 1, or 10 μM or 1 μM 8-bromo cAMP (Santa Cruz Biotechnology, Santa Cruz, CA). A forskolin stock solution was prepared in DMSO; the control group was treated with DMSO as a vehicle control. The final concentration of DMSO did not exceed 0.1%. Na+ uptake measurements began 5 min after the addition of forskolin or 8-bromo cAMP, in the continued presence of forskolin or cAMP. To measure Na+ uptake, 12 larvae were placed in a 2-ml microcentrifuge tube, and 0.25 μCi 22Na (in the form of NaCl; Perkin Elmer, Woodbridge, ON, Canada) was added to each tube to a final activity of 0.15 μCi/ml. Water samples (50 μl) were collected at 5 min and 2 h after the addition of isotope. At the end of the 2-h flux period, larvae were killed by overdose with ethyl 3-aminobenzoate methanesulfonate (MS-222) and briefly washed in isotope-free water containing high levels of Na+ (>200 mM) to displace residual isotope attached to the surface of the fish. The remaining water in the tube was stored separately for later measurement of total [Na+].
Series 2. Consequences of microtubule disruption on Na+ uptake during acute exposure to acidic water.
Previous studies demonstrated that activation of electrolyte transporters by cAMP may be associated with microtubule-dependent translocation of target proteins (59, 60). To test whether the increase in Na+ uptake triggered by forskolin treatment also might be associated with physical relocation of target transporters, zebrafish were pretreated with two microtubule disruptors, either colchicine (200 μM for 4 h; stock solution prepared in water) (62) or nocodazole [10–30 μM for 30 min; stock solution prepared in DMSO; Santa Cruz Biotechnology (72)] or nocodazole [10–30 μM for 30 min; stock solution prepared in DMSO; Santa Cruz Biotechnology (67)] before further treatment with 10 μM forskolin. Na+ uptake measurements began 5 min after the addition of these pharmacological agents, as described above.
It was recently demonstrated that zebrafish larvae are able to increase Na+ uptake after only brief (2 h) exposure to acidic or ion-poor environments. To test whether microtubule-dependent transporter trafficking is involved in such rapid activation of Na+ uptake, larvae were pretreated with colchicine or nocodazole, and then transferred to acidic water [prepared by adding H2SO4 to Ottawa tapwater (pH ∼ 4.0)] for 2 h in the continuing presence of these inhibitors. After the 2-h exposure to acidic water, larvae were transferred to the control water, and Na+ uptake was measured as described above.
Series 3. Consequences of PKA inhibition during acute exposure to acidic water or forskolin.
To determine whether cAMP induces Na+ uptake through PKA, and whether such signaling is involved in the induction of Na+ uptake during acute challenge, the following experiments were performed. In one experiment, 4 dpf larvae were pretreated for 2 h with two commonly used PKA inhibitors, H-89 (10 μM, stock prepared in DMSO; LC Labs, Woburn, MA) or rp-cAMPs (10 μM, stock prepared in water; Santa Cruz Biochemicals). Larvae were then treated with 10 μM forskolin in the continued presence of either inhibitor. Na+ flux measurements began 5 min after the addition of forskolin. To test the involvement of PKA-dependent signaling during acute acid exposure, 4 dpf larvae were similarly pretreated with H-89 or rp-cAMPs before being exposed to acidic water for 2 h (in the continued presence of inhibitors). After the 2-h exposure, larvae were transferred to the control media (in the continued presence of inhibitors), and Na+ uptake was measured as described above.
Series 4. Identification of Na+ transporters regulated by cAMP.
To test whether NHE3b, a major route for Na+ uptake by zebrafish, is regulated by cAMP, we injected a previously validated NHE3b morpholino (30) and assessed the effect of forskolin treatment as described above. Larvae were injected with either control or NHE3b morpholino and raised until 4 dpf in control Ottawa tapwater. They were then treated with 10 μM forskolin and their Na+ uptake was measured as described in Series 1.
The transcription factor, glial cell missing 2 (gcm2) plays a critical role in the differentiation of H+-ATPase-rich cells (HRCs) and its knockdown prevents HRC formation (7). The loss of HRCs is accompanied by a compensatory increase in the numbers of Na+-Cl− cotransporter (NCC; zslc12a10.2)-expressing cells (7, 53, 65). To test whether NCC is regulated by cAMP, embryos were injected with a morpholino against gcm2 or a control morpholino (29). The larvae were raised in control water until 3 dpf, and Na+ uptake was measured in response to forskolin treatment as described above. In addition, sham- and morpholino-injected larvae were stained with Alexa Fluor 488-conjugated concanavalin A (conA), a lectin that has been used extensively as a marker for HRC in zebrafish larvae, following the previous protocol (29).
Series 5. Consequences of acute acid exposure on whole body cAMP levels.
To demonstrate the effect of acute acid exposure on cAMP metabolism, whole body cAMP content was analyzed using a commercial ELISA kit (Cayman Chemicals). 4 dpf larvae were exposed to acidic water for 3 h or kept in control water. After the treatment, larvae were killed (30 larvae were pooled for n = 1), homogenized in 100 μl 0.1 N HCl, and centrifuged at 6,000 g for 10 min at 4°C. Supernatant was collected and further diluted 10-fold using 0.1 N HCl. The diluted extract was stored at −80°C until analysis and directly used for the ELISA assay, according to the manufacturer's instructions.
Series 6. Vital staining of larvae using fluorescently tagged forskolin or propranolol.
To visualize forskolin-binding sites (presumed to be AC) in zebrafish, 4 dpf larvae were vitally stained with BODIPY-forskolin (Invitrogen) at 15 nM for 40 min. Larvae were stained simultaneously with Alexa Fluor 633-conjugated conA to visualize HRCs, as described in Series 4. Subsequently, larvae were killed with MS-222, briefly rinsed with PBS, and observed using a confocal microscope (Olympus FV1000 BX-61 with FluoView software).
It was previously shown that a subset of larval zebrafish ionocytes are enriched with β-adrenergic receptors based on their staining with fluorescently tagged propranolol (32). To demonstrate that these propranolol-positive ionocytes also express forskolin-binding AC, 4 dpf larvae were stained with BODIPY-propranolol and MitoTracker (a pan-ionocyte marker), as described by Kumai et al. (32), as well as BODIPY-forskolin, as described above. Furthermore, to test the specificity of BODIPY-forskolin, a group of larvae was pretreated with 10 μM nonfluorescent forskolin for 10 min before vital staining with propranolol, MitoTracker, and forskolin.
Analytical tools and calculations.
To determine Na+ uptake, all collected water samples were supplemented with 5 ml of scintillation cocktail (Biosafe-II, RPI, Mt. Prospect, IL), and their radioactivity was measured with a liquid scintillation counter (model LS-6500 Beckman Coulter, Mississauga ON, Canada). After being rinsed in an isotope-free medium, larvae were digested in a tissue solubilizer (Solvable, Perkin Elmer) for 4 h at 65°C. After complete digestion, samples were supplemented with 5 ml of the same scintillation cocktail. Samples were then neutralized by adding 500 μl of glacial acetic acid before measuring their radioactivity. The concentration of total Na+ in the water was measured using flame emission spectrophotometry (model AA260, Varian, Palo Alto, CA). For Na+ uptake experiments in Series 4.1, samples were counted directly using a gamma counter (Wallac 1470 Wizard Gamma Counter, PerkinElmer, Turku, Finland), without tissue digestion/scintillation cocktail. Owing to the limited volume of water, [Na+] was measured, and hence, external specific activity was determined, only at the end of the flux period. It was assumed that, given the typical [Na+] of the experimental water (700–1,000 μM), Na+ influx rate (less than 1 nmol·fish−1·h−1 or less) and even smaller expected net flux of Na+ (difference between influx and efflux), changes in total [Na+] during the flux period would be negligible. The rate of Na+ uptake (JinNa, pmol·fish−1·h−1) was calculated as follows:
where F is the total incorporated radioactivity (DPM, disintegration per minute), SA is the specific activity of the medium (DPM/pmol), n is the number of larvae (typically 1) and t is the duration of the incubation (h). DPM was calculated by the liquid scintillation counting program after taking quenching and counting efficiency into consideration.
Statistical analysis.
All statistical analyses were performed with SigmaPlot (v. 11, Systat, Chicago, IL). Student's t-test was used to analyze data from Series 1 (8-bromo cAMP treatment) and Series 5. One-way ANOVA followed by Tukey post hoc test was used to analyze data from Series 1 (forskolin treatment). Two-way ANOVA was used to analyze flux data from Series 2–4. When assumptions of normality or equal variance were violated, data were transformed using natural log- or square-root transformation. For all analyses, the level of statistical significance was set at P < 0.05.
RESULTS
Series 1. Consequences of increasing [cAMP] levels on Na+ uptake.
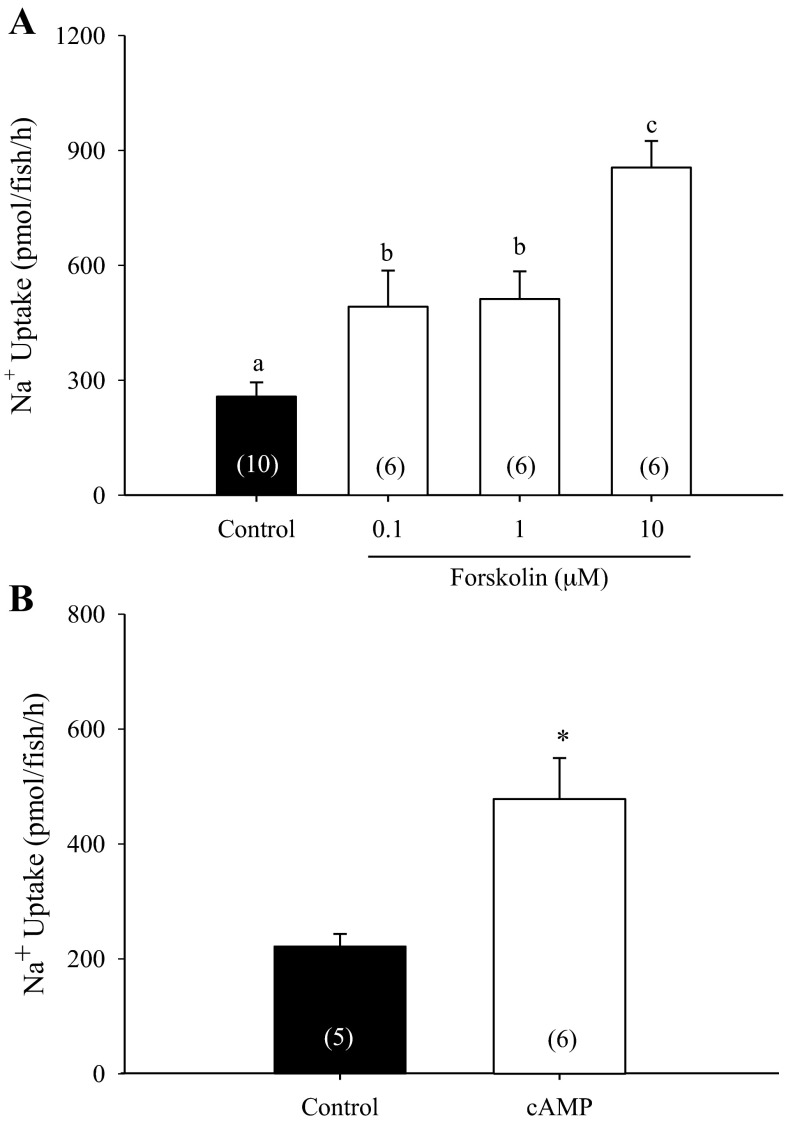
Acute treatment with forskolin between 0.1 and 10 μM significantly elevated Na+ uptake in 4 dpf zebrafish larvae maintained in control water (Fig. 1A; n = 6–10; one way ANOVA). Similarly, treatment with 1 μM 8-bromo cAMP led to a significant increase in Na+ uptake (Fig. 1B; n = 5 or 6; Student's t-test).
Fig. 1.
Effect of forskolin treatment on Na+ uptake. Treatment with 0.1–10 μM forskolin increased Na+ uptake in zebrafish larvae in a dose-dependent manner (A; n = 6–10; one-way ANOVA). Treatment with 8-bromo cAMP also increased the uptake of Na+ significantly (B; n = 5 or 6; Student's t-test). a,b,cDifferent letters denote significant differences among the treatment groups. *Significant difference between control and cAMP-treated groups. A number in parentheses indicates sample size for each group. Data are presented as means ± SE.
Series 2. Consequences of microtubule disruption on Na+ uptake during acute exposure to acidic water and activation of cAMP signaling.
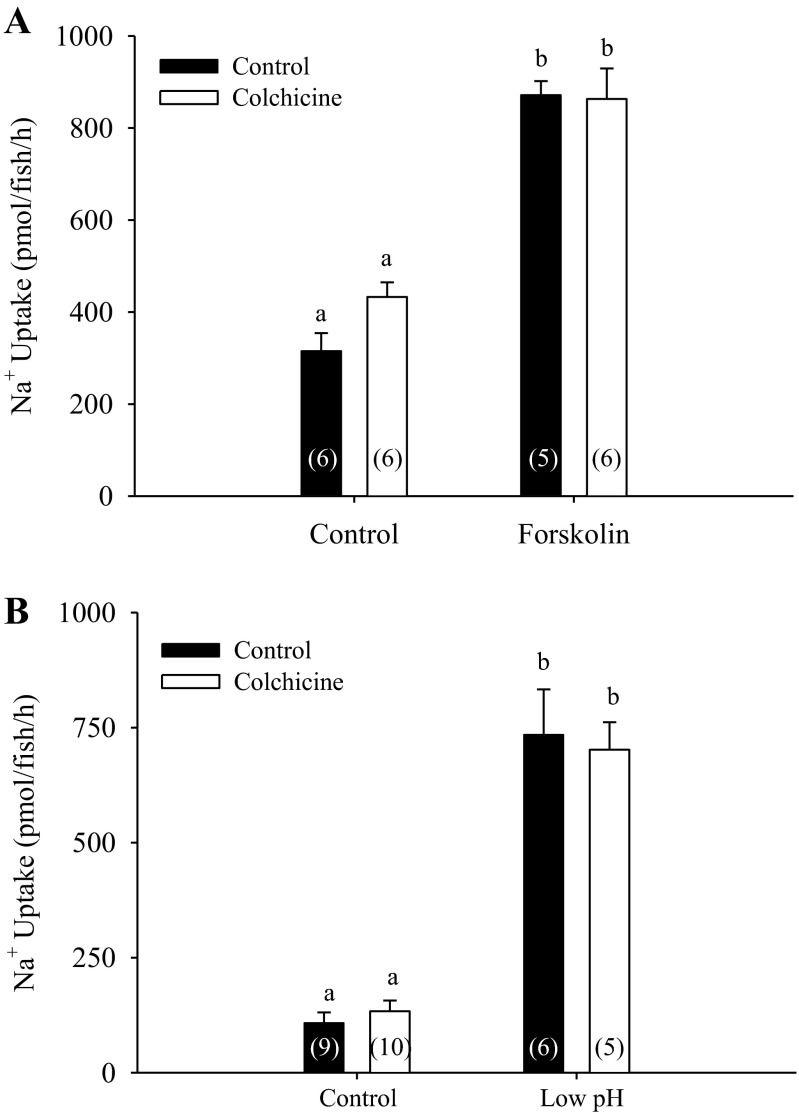
Pretreatment with 200 μM colchicine did not affect the stimulation of Na+ uptake caused by forskolin treatment (Fig. 2A; two-way ANOVA, n = 5 or 6). The pretreatment with colchicine did not affect stimulation of Na+ uptake following acute exposure to acidic water (Fig. 2B; two-way ANOVA, n = 5–10). Similarly, treatment with nocodazole also did not modify Na+ uptake in response to forskolin or acidic water (data not shown).
Fig. 2.
Effect of colchicine pretreatment on Na+ uptake after forskolin treatment and acid exposure. Pretreating larvae with 200 μM colchicine for 4 h did not inhibit stimulation of Na+ uptake following their treatment with forskolin (A; n = 5 or 6; two way ANOVA). Similarly, pretreating larvae with 200 μM colchicine did not attenuate the stimulation of Na+ uptake following acute-acid exposure (B; n = 5–10; two-way ANOVA) a,bDifferent letters denote significant difference between control and treated (forskolin or acid) groups. A number in parentheses indicates sample size for each group. Data are presented as means ± SE.
Series 3. Consequences of PKA inhibition on Na+ uptake.
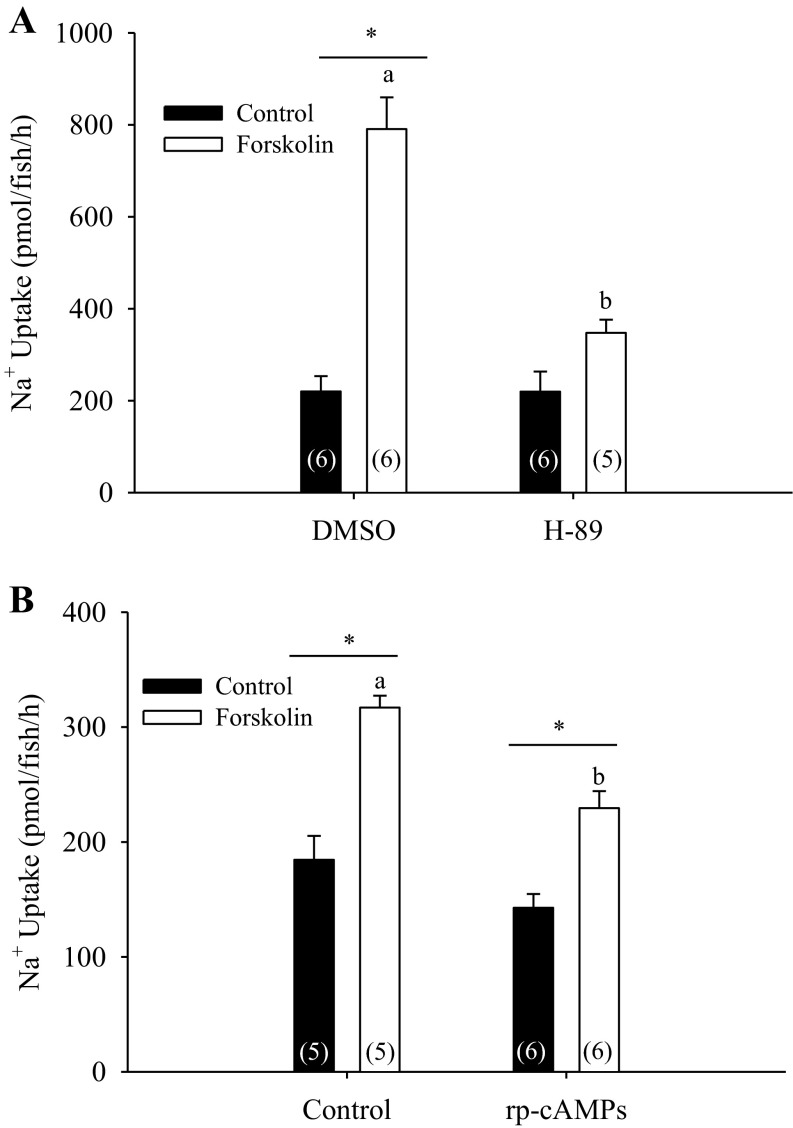
Both H-89 and rp-cAMPs treatment significantly attenuated the forskolin-mediated stimulation of Na+ uptake, although the inhibition seen with H-89 treatment was more severe (Fig. 3, A and B; two way ANOVA, n = 5 or 6).
Fig. 3.
Effect of PKA inhibition on cAMP-dependent Na+ uptake stimulation. Pretreatment with two commonly used PKA inhibitors, H-89 (A; n = 5 or 6; two-way ANOVA) and rp-cAMPs (B; n = 5 or 6; two-way ANOVA) both attenuated the rapid stimulation of Na+ uptake following treatment with 10 μM forskolin. *Significant difference between control and forskolin-treated groups, whereas different letters denote significant differences between control and PKA-inhibitor pretreated groups. A number in parentheses indicates sample size for each group. Data are presented as means ± SE.
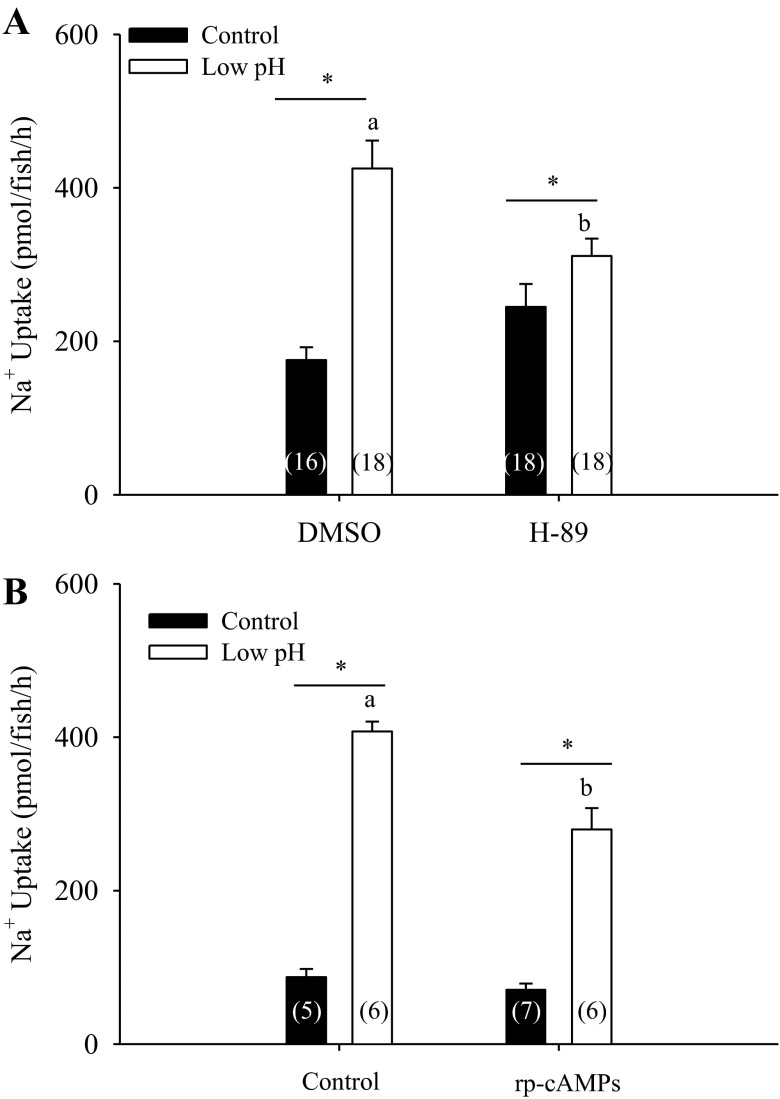
Furthermore, both H-89 (Fig. 4A; two-way ANOVA, n = 16–18) and rp-cAMPs (Fig. 4B; two-way ANOVA, n = 5–7) treatments significantly attenuated the stimulation of Na+ uptake following exposure to acidic water, although Na+ uptake was still significantly elevated following acid exposure, even in the presence of inhibitors.
Fig. 4.
Effect of PKA inhibition on stimulation of Na+ uptake following acid exposure. Pretreatment with two commonly used PKA inhibitors, H-89 (A; n = 16–18; two-way ANOVA) and rp-cAMPs (B; n = 5–7; two-way ANOVA) both attenuated the stimulation of Na+ uptake following acute exposure to acidic water, although the larvae still managed to elevate their Na+ uptake significantly. *Significant difference between control and acid-exposed groups. a,bDifferent letters denote significant differences between control and PKA inhibitor-pretreated groups. A number in parentheses indicates sample size for each group. Data are presented as means ± SE.
Series 4. Identification of Na+ transporters regulated by cAMP.
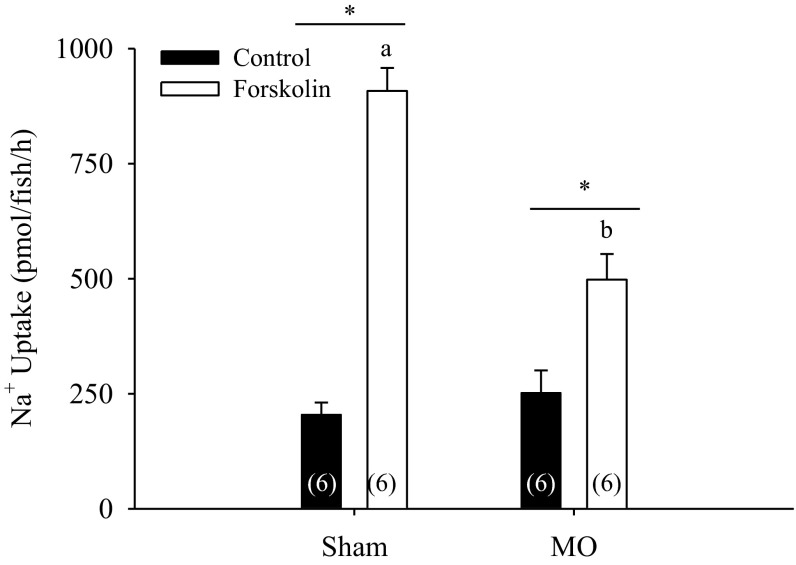
Knockdown of NHE3b did not affect Na+ uptake in larvae raised in control water (Fig. 5). However, in the forskolin-treated fish, Na+ uptake was significantly lower in the NHE3b morphants [although forskolin still induced Na+ uptake (Fig. 5; two-way ANOVA, n = 6)].
Fig. 5.
Effect of forskolin treatment in NHE3b morphants. Knockdown of NHE3b did not affect Na+ uptake in 4 days postfertilization (dpf) zebrafish larvae raised in control water. However, Na+ uptake, while stimulated, was significantly lower in NHE3b morphants following forskolin treatment (n = 6; two-way ANOVA). a,bDifferent letters denote significant difference between sham and morphants. *Significant difference between DMSO (vehicle) and forskolin treated groups.
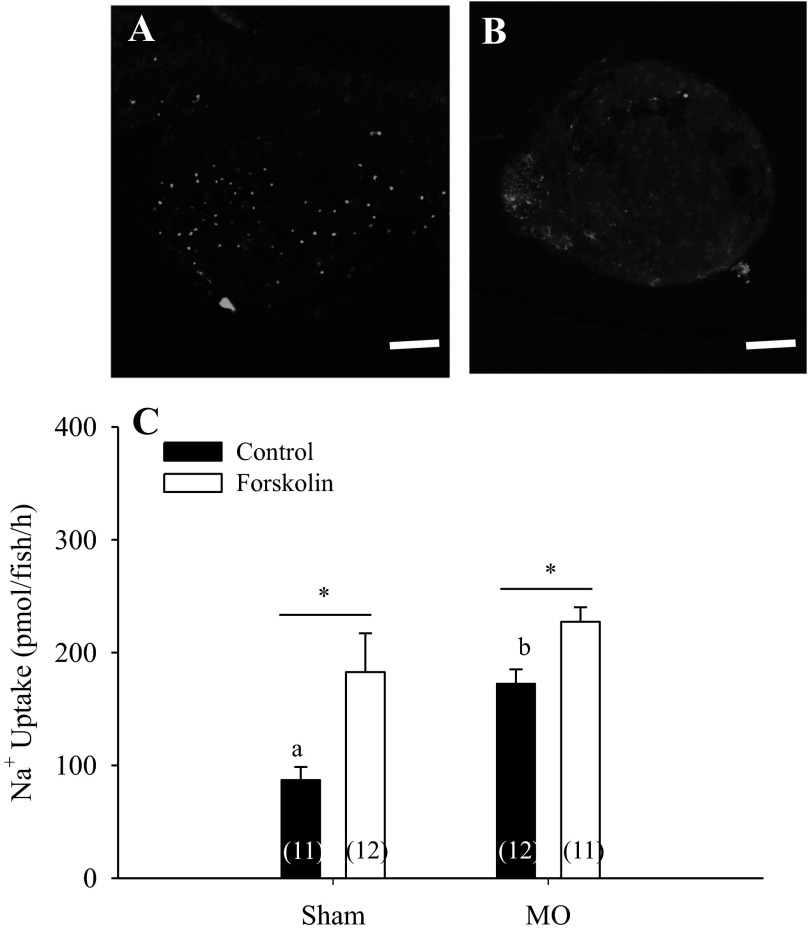
As reported previously (29), knockdown of gcm2 led to a significant increase in Na+ uptake (Fig. 6, A and B). Treatment of morphants with 10 μM forskolin further increased the uptake of Na+, suggesting that Na+ uptake via NCC is being stimulated by forskolin (Fig. 6C; two-way ANOVA; n = 11 or 12).
Fig. 6.
Effect of forskolin treatment on gcm2 morphants. Knockdown of gcm2 led to almost complete loss of conA-positive cells in zebrafish larvae (A and B). Exposure to 10 μM forskolin significantly increased Na+ uptake in both sham and gcm2 morphants (C; n = 11 or 12; two-way ANOVA). a,bDifferent letters denote significant difference between sham and morphants. *Significant difference between DMSO (vehicle) and forskolin-treated groups. Scale bars in A and B = 100 μm. A number in parentheses indicates sample size for each group. Data are presented as means ± SE.
Series 5. Consequences of acute acid exposure on whole body cAMP levels.
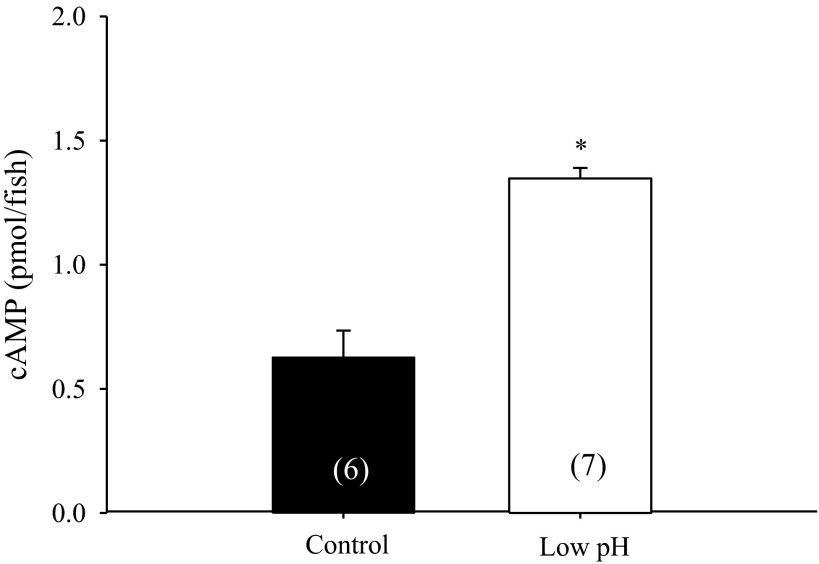
Whole body cAMP content was significantly increased following 3-h exposure to acidic water (Fig. 7; n = 6 or 7; Student's t-test).
Fig. 7.
Effect of acid exposure on whole body cAMP content. A brief (3 h) exposure to acidic water significantly elevated whole body cAMP content in 4 dpf zebrafish larvae (n = 6 or 7; Student's t-test). *Significant difference between groups. A number in parentheses indicates sample size for each group. Data are presented as means ± SE.
Series 6. Vital staining of larvae using fluorescently tagged forskolin and propranolol.
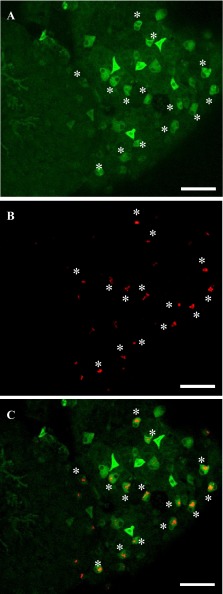
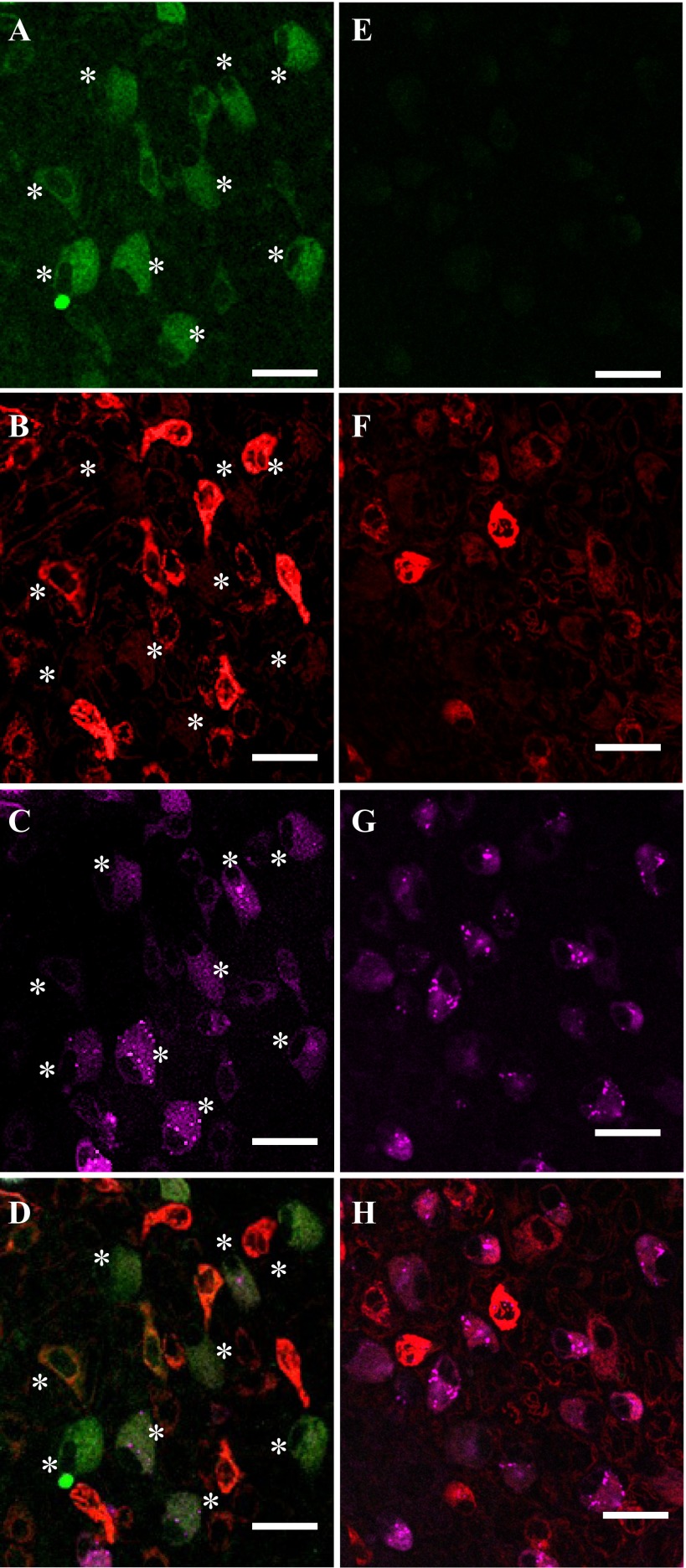
The conA-positive HRCs were also forskolin-positive, whereas forskolin also stained additional con-A-negative cells (Fig. 8, A–C). As expected, most of the forskolin-positive cells also expressed high levels of β-adrenergic receptors as visualized using fluorescent propranolol (Fig. 9, A–D). As previously reported by Kumai et al. (32) and also in agreement with Fig. 8, most of the forskolin/propranolol-positive cells also stained with MitoTracker (Fig. 8). The specificity of forskolin was confirmed as pretreatment with “cold” forskolin greatly reduced staining intensity with BODIPY-forskolin (Fig. 9, E–H).
Fig. 8.
Distribution of forskolin-binding sites (asterisks) in developing zebrafish larvae. Incubation of 4 dpf zebrafish larvae with BODIPY-conjugated forskolin revealed a subset of epithelial cells enriched with forskolin binding sites (presumed to be tmAC; A). A portion of tmAC-rich cells were con-A-positive HRCs (B and C). Scale bars: 50 μm.
Fig. 9.
Colocalization of forskolin-binding sites (asterisks) with β-adrenergic receptors. Incubation of 4 dpf zebrafish larvae with BODIPY-conjugated forskolin (A and E), MitoTracker (B and F), and BODIPY-conjugated propranolol (C and G) demonstrated that forskolin-biding tmAC-enriched cells also express high level of β-adrenergic receptors, and that those cells are MitoTracker-positive ionocytes, although the expression level of MitoTracker was relatively weaker than some other, forskolin-negative, MitoTracker-positive cells. Pretreatment with nonfluorescent forskolin greatly reduced staining intensity of fluorescent forskolin (E), while staining from MitoTracker and propranolol (F and G) was not affected. Scale bars: 20 μm.
DISCUSSION
The present study demonstrates that the rapid increase in Na+ uptake following exposure of larval zebrafish to acidic water is caused, at least in part, by cAMP-mediated intracellular signaling. These data expand on the previous observation that in zebrafish, stimulation of Na+ uptake is linked to activation of β-adrenergic receptors. The current study further reveals that both NHE3b and NCC are a potential regulatory target of cAMP signaling.
cAMP stimulates Na+ uptake.
While the results of previous studies demonstrated a role for cAMP-mediated signaling in stimulating Cl− secretion in isolated opercular epithelium of SW-acclimated killifish, Fundulus heteroclitus (38), and decreasing Na+-K+-ATPase activity in gill cells isolated from brown trout, Salmo trutta (55), surprisingly little is known about the role of this universal second messenger in regulating ion fluxes in FW fish in vivo. On the basis of the stimulatory effects of forskolin and 8-bromo cAMP on Na+ uptake, the present study clearly demonstrated the potential for cAMP to rapidly regulate Na+ balance in zebrafish larvae. Because the activation of Na+ uptake during exposure of zebrafish larvae to acidic water is linked to β-adrenergic stimulation uptake (32), these data are consistent with the previously reported stimulatory role of β-adrenergic receptor activation (which promotes downstream signaling mediated by cAMP) on Na+ uptake.
cAMP is synthesized either by tmAC or AC1–9 or by sAC or AC 10. Because sAC is activated by a rise in [HCO3−], its potential role in body fluid pH and electrolyte regulation has received considerable research interest in many animal and tissue models (16, 42–44, 58, 60). Surprisingly, despite the widespread presence of sAC across phyla, phylogenetic analysis of vertebrate AC isoforms has not yet identified sAC (or a sAC-like isoform) in the zebrafish genome. In addition, treatment of zebrafish larvae with two frequently used sAC inhibitors, KH-7 and 4-catechol estradiol (4-CE) (60), was without effect on Na+ uptake (Y. Kumai and S. F. Perry, unpublished results), further supporting the idea that there is no functional AC10 ortholog in zebrafish. However, it cannot be excluded that other AC isoforms have acquired sensitivity to [HCO3−], and potentially assume functions similar to sAC as a HCO3− sensor in zebrafish. Measurement of cAMP levels in isolated adult gill tissue during exposure to elevated [HCO3−] might provide insight on this issue.
Because the stimulation of Na+ uptake after forskolin treatment was apparent within 2 h, it was likely related to, at least initially, activation of existing proteins rather than to increased transcription and protein synthesis. Indeed, mRNA levels of NHE3b, previously implicated in the stimulation of Na+ uptake by zebrafish larvae in acidic water (30) did not change after 2-h exposure to acidic water (data not shown). Microtubule disruption using colchicine previously used in larval tilapia Oreochromis mossambicus (61, 62) was employed to determine whether the stimulation of Na+ uptake was caused by trafficking of internal proteins toward the apical membrane (59, 60) or the activation of existing apical membrane proteins. Because pretreatment with colchicine did not impede the stimulation of Na+ uptake associated with forskolin treatment (Fig. 2A) or acid exposure (Fig. 2B), it seems unlikely that protein trafficking was involved. The same results (data not shown) were obtained using an alternate MT inhibitor, nocodazole, at a dose almost 5 times higher than previously used to inhibit MT-dependent cell migration in zebrafish larvae (72). Thus, it is suggested that acute treatment with cAMP or acidic water increases Na+ uptake by activating Na+ transporters (see below) already expressed on the apical membrane. On the other hand, it was recently reported that knockdown of miR-8 results in significant disruption of intracellular trafficking within HRCs (as demonstrated by conA-staining pattern). The same morphants exhibited significantly lower accumulation of Na+ green in HRCs following 2-day acid exposure. Although no causal relationship between disruption of conA stain and intensity of Na+ green staining was directly demonstrated, these observations indicate potential role of MT in osmoregulation during chronic acid exposure (13).
cAMP acts through PKA to activate Na+ uptake.
Although PKA initially was recognized as the sole mediator of downstream cAMP signaling, it was recently demonstrated that a second protein, “exchange protein directly activated by cAMP” (Epac), can initiate PKA-independent downstream signaling (9, 15). Previous studies showed that Epac1 is expressed in the mouse proximal tubule and that its activation inhibited NHE3 activity similarly to PKA activation (17) by interacting with NHERF1 (40). Interestingly, even for a common physiological endpoint, the mechanisms of cAMP signaling may be tissue-(and likely species-) dependent. For example, Murtazina et al. (40) showed that in ileum, the inhibitory effect of cAMP on NHE3 activity was abolished by pretreating the tissue with H-89, a commonly used PKA inhibitor, despite the presence of Epac1 in this tissue. Very recently, BioLog introduced three new Epac inhibitors. However, our preliminary experiments with these inhibitors caused high mortality in larvae, especially during their exposure to acidic water. Because of the lack of alternative, more suitable tools to study the functional significance of Epac, the present study focused on the role of PKA.
Treatment with two PKA inhibitors, H-89, and rp-cAMPs, demonstrated a significant role of PKA in mediating cAMP-dependent stimulation on Na+ uptake by zebrafish larvae (Fig. 3). That two PKA inhibitors with different modes of action (37) caused similar physiological response (although H-89 exhibited greater inhibition) strengthens the conclusion that PKA plays an important role in mediating cAMP-dependent signaling to stimulate Na+ uptake. However, further experiments with more specific tools for PKA inhibition, such as a recently introduced new class of photo-activatable morpholinos (50) targeting PKA, would provide better evidence. In addition, because of the lack of suitable inhibitors for Epac, whether cAMP acts solely though PKA (or also acts though Epac) in inducing Na+ uptake awaits future research.
When zebrafish larvae were exposed to acidic water in the presence of PKA inhibitors, the stimulation of Na+ uptake was attenuated (Fig. 4), suggesting that at least in part, the increase in Na+ uptake during acute exposure to acidic water is controlled by a cAMP-PKA signaling pathway. In agreement with this idea, whole body cAMP content significantly increased in larvae exposed for 3 h to acidic water (Fig. 7). It is important to note, however, that the observed rise in whole body cAMP content likely reflects the rapid induction of multiple physiological signaling pathways in response to acid exposure, many of which would be occurring outside of ionocytes and thus not be directly related to ionic regulation. To better define the role of cAMP-mediated signaling in Na+ uptake, live imaging of cAMP at the level of specific ionocytes, using techniques such as fluorescence resonance energy transfer-based cAMP measurement (3, 68) would be helpful.
cAMP may regulate multiple Na+ transporters.
Na+ uptake by zebrafish larvae could occur at either HR or NCC cells (12, 21). The observed colocalization between conA (a vital dye for HRCs) and BODIPY-forskolin (Fig. 8) suggests that one mechanism of Na+ uptake being regulated by cAMP is occurring at HRCs. HRCs are known to express NHE3b and H+-ATPase, both of which have been proposed to contribute to Na+ uptake (18, 30). On the basis of the knockdown of NHE3b, the present study demonstrates that Na+ uptake via NHE3b is likely regulated by cAMP. This situation is in contrast to the effect of cAMP on NHE3b in mammalian tissues, where cAMP was shown to inhibit NHE3 activity (for review, see Ref. 1). Although the difference in the potential effects of cAMP on NHE3b between mammalian and piscine systems is interesting, an important difference is that in freshwater fish, because of thermodynamic constraints, Na+ uptake via NHE3b depends on other transporters and enzymes, most notably, an apical ammonia conducting channel Rhcg1 and carbonic anhydrase (26, 30, 36, 52). Thus, the predicted increase in Na+ uptake via NHE3b in zebrafish larvae following forskolin treatment might be driven primarily by the stimulation of those other proteins required for NHE3b to function. Expressing zebrafish NHE3b in oocytes and measuring its activity in response to forskolin treatment could provide important insights as to whether cAMP could directly stimulate NHE3b in zebrafish.
Even after NHE3b knockdown, Na+ uptake was elevated in response to forskolin treatment (Fig. 5). This observation suggests there is another Na+ uptake pathway controlled by cAMP. In gcm2 morphants, which lack HRCs (Fig. 6, A and B), forskolin treatment stimulated Na+ uptake (Fig. 6C). These observations suggest that NCC (zslc12a10.2) might also be induced by cAMP. Although we observed that BODIPY-forskolin also stained a MitoTracker-positive population of cells in addition to HRCs (Figs. 8 and 9), we could not directly demonstrate whether these cells are NCC cells because of our lack of suitable antibodies or vital dyes for this cell type. Identification of specific AC isoform(s) expressed on NCC cells, using double in situ hybridization against NCC and AC isoforms, and knockdown of such isoform(s) would strengthen the proposed regulation of NCC via cAMP.
On the basis of the available data, it is feasible that both NCC and NHE3b contribute to the stimulation of Na+ uptake during acute exposure to acidic water. The predicted role of NCC is of interest because we did not detect any inhibition of Na+ uptake by metolazone (an NCC inhibitor) treatment (30). The potential difference in the proposed role of NCC in Na+ uptake during acid exposure is likely due to the difference in experimental protocol, as acid exposure (30) was >24 h as opposed to 2 h in the present study. Thus, potential involvement of transporters involved with Na+ uptake, including NCC, NHE3b, and Rhcg1 in Na+ uptake stimulation during acute acid-exposure would benefit from further investigation.
Although these results provide exciting new insights into the regulation of Na+ transport by cAMP, it is important to recognize two experimental limitations. First, because whole larvae were given waterborne treatment of forskolin, for the moment it is difficult to determine whether the observed effects reflect the direct action of forskolin on ionocytes (and activation of intracellular signaling), especially for NCC. Furthermore, as discussed previously (56, 57), increases in tissue cAMP levels could reach supraphysiological levels. Indeed, following 10 μM forskolin treatment, whole body cAMP content in zebrafish larvae rose by almost 30-fold (M. Tresguerres, Y. Kumai, and S. F. Perry, unpublished data), and an increase of similar magnitude was reported in a previous study (55).
Perspectives and Significance
Despite its prominent role as a universal second messenger, the role of cAMP-dependent signaling on osmoregulation in FW fish is not well established. While the present study provides new findings implicating the presence of cAMP-mediated signaling mechanism(s) capable of activating several Na+ transporters, it also raises a number of issues that need to be addressed such as 1) the relative roles of PKA and Epac in mediating cAMP-signaling, 2) whether specific isoforms of tmAC are involved in mediating cAMP-dependent uptake of Na+, 3) whether the uptake of other ions (e.g., Ca2+ and Cl−) is controlled by cAMP, and 4) whether there is one or more functional analogs of sAC in zebrafish (or in other FW fish) playing a role similar to that in dogfish gill and mammalian kidney.
GRANTS
This study was funded by Natural Sciences and Engineering Research Council of Canada Discovery and Research Tools and Innovation grants to S. F. Perry. Y. Kumai was supported by an Ontario Graduate Scholarship during the tenure of this study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.K. conception and design of research; Y.K. and R.W.K. performed experiments; Y.K., R.W.K., and S.F.P. analyzed data; Y.K., R.W.K., and S.F.P. interpreted results of experiments; Y.K. and S.F.P. prepared figures; Y.K. drafted manuscript; Y.K. and S.F.P. edited and revised manuscript; Y.K., R.W.K., and S.F.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Vishal Saxena and Bill Fletcher at University of Ottawa for their excellent animal care. We also appreciate insightful tips from Dr. Martin Tresguerres (University of California, San Diego) for measurement of whole body cAMP.
Present address for Y. Kumai: Department of Physiology and Biophysics, Case Western Reserve University School of Medicine, 10900 Euclid Ave. Cleveland, OH 44106, USA.
REFERENCES
- 1.Alexander RT, Grinstein S. Tethering, recycling and activation of the epithelial sodium proton exchanger, NHE3. J Exp Biol 212: 1630–1637, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Beavo JA, Brunton LL. Cyclic nucleotide research- still expanding after half a century. Nat Rev Mol Cell Biol 3: 710–718, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Borner S, Schwede F, Schlipp A, Berisha F, Calebiro D, Lohse MJ, Nikolaev VO. FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nat Protoc 6: 427–438, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Braun MH, Steele SL, Ekker M, Perry SF. Nitrogen excretion in developing zebrafish (Danio rerio): a role for Rh proteins and urea transporters. Am J Physiol Renal Physiol 296: F994–F1005, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Breves JP, Serizier SB, Goffin V, McCormick SD, Karlstrom RO. Prolactin regulates transcription of the ion uptake Na+/Cl− cotransporter (ncc) gene in zebrafish gill. Mol Cell Endocrinol 369: 98–106, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breves JP, Watanabe S, Kaneko T, Hirano T, Grau EG. Prolactin restores branchial mitochondrion-rich cells expressing Na+/Cl− cotransporter in hypophysectomized Mozambique tilapia. Am J Physiol Regul Integr Comp Physiol 299: R702–R710, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Chang WJ, Horng JL, Yan JJ, Hsiao CD, Hwang PP. The transcription factor, glial cell missing 2, is involved in differentiation and functional regulation of H+-ATPase-rich cells in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 296: R1192–R1201, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cclase as an eolutionarily cnserved bcarbonate snsor. Science 289: 625–628, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin 40: 651–662, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou MY, Hung JC, Wu LC, Hwang SP, Hwang PP. Isotocin controls ion regulation through regulating ionocyte progenitor differentiation and proliferation. Cell Mol Life Sci 68: 2797–2809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz SA, Chao PL, Hwang PP. Cortisol promotes differentiation of epidermal ionocytes through Foxi3 transcription factors in zebrafish (Danio rerio). Comp Biochem Physiol Part A 164: 249–257, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Dymowska AK, Hwang PP, Goss GG. Structure and function of ionocytes in the freshwater fish gill. Respir Physiol Neurobiol 184: 282–292, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Flynt AS, Thatcher EJ, Burkewitz K, Li N, Liu Y, Patton JG. miR-8 microRNAs regulate the response to osmotic stress in zebrafish embryos. J Cell Biol 185: 115–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmour K, Collier C, Dey C, Perry S. Roles of cortisol and carbonic anhydrase in acid-base compensation in rainbow trout, Oncorhynchus mykiss. J Comp Physiol B 181: 501–515, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Ann Rev Pharmacol Toxicol 50: 355–375, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem 284: 5774–5783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honegger KJ, Capuano P, Winter C, Bacic D, Stange G, Wagner CA, Biber J, Murer H, Hernando N. Regulation of sodium-proton exchanger isoform 3 (NHE3) by PKA and exchange protein directly activated by cAMP (EPAC). Proc Natl Acad Sci USA 103: 803–808, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horng JL, Lin LY, Huang CJ, Katoh F, Kaneko T, Hwang PP. Knockdown of V-ATPase subunit A (atp6v1a) impairs acid secretion and ion balance in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 292: R2068–R2076, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hoshijima K, Hirose S. Expression of endocrine genes in zebrafish larvae in response to environmental salinity. J Endocrinol 193: 481–491, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hwang PP. Ion uptake and acid secretion in zebrafish (Danio rerio). J Exp Biol 212: 1745–1752, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Hwang PP, Chou MY. Zebrafish as an animal model to study ion homeostasis. Pflügers Arch 465: 1233–1247, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang PP, Perry SF. Ionic and acid-base regulation. In: Zebrafish. Series in Fish Physiology, vol 29, edited by Perry SF, Ekker M, Farrell AP, Brauner CJ. San Diego, CA: Academic, 2010, p. 311–344 [Google Scholar]
- 23.Hwang PP, Lee TH, Lin LY. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301: R28–R47, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Hyndman KA, Evans DH. Effects of environmental salinity on gill endothelin receptor expression in the killifish, Fundulus heteroclitus. Comp Biochem Physiol Part A 152: 58–65, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Isaia J, Maetz J, Haywood GP. Effects of epinephrine on branchial non-electrolyte permeability in rainbow trout. J Exp Biol 74: 227–237, 1978 [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Kobayashi S, Nakamura N, Esaki M, Miyagi H, Hoshijima K, Hirose S. Close association of carbonic anhydrase (CA2a & CA15a), Na+/H+ exchanger (Nhe3b), and ammonia transporter Rhcg1 in zebrafish ionocytes responsible for Na+ uptake. Front Physiol 4: 59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanis G, Esbaugh AJ, Perry SF. Branchial expression and localization of SLC9A2 and SLC9A3 sodium/hydrogen exchangers and their possible role in acid-base regulation in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 211: 2467–2477, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kumai Y, Bahubeshi A, Steele S, Perry SF. Strategies for maintaining Na+ balance in zebrafish (Danio rerio) during prolonged exposure to acidic water. Comp Biochem Physiol 160: 52–62, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Kumai Y, Nesan D, Vijayan MM, Perry SF. Cortisol regulates Na+ uptake in zebrafish, Danio rerio, larvae via the glucocorticoid receptor. Mol Cell Endocrinol 364: 113–125, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Kumai Y, Perry SF. Ammonia excretion via Rhcg1 facilitates Na+ uptake in larval zebrafish, Danio rerio, in acidic water. Am J Physiol Regul Integr Comp Physiol 301: R1517–R1528, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Kumai Y, Perry SF. Mechanisms and regulation of Na+ uptake by freshwater fish. Respir Physiol Neurobiol 184: 249–256, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Kumai Y, Ward M, Perry SF. Beta-adrenergic regulation of Na+ uptake by larval zebrafish, Danio rerio, in acidic and ion-poor environments. Am J Physiol Regul Integr Comp Physiol 303: R1031–R1041, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Kwong RWM, Perry SF. Cortisol regulates epithelial permeability and sodium losses in zebrafish exposed to acidic water. J Endocrinol 217: 253–264, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Lin CH, Su CH, Tseng DY, Ding FC, Hwang PP. Action of vitamin D and the receptor, VDRa, in calcium handling in zebrafish (Danio rerio). PLoS One 7: e45650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CH, Tsai IL, Su CH, Tseng DY, Hwang PP. Reverse effect of mammalian hypocalcemic cortisol in fish: cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism. PLoS One 6: e23689, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin TY, Liao BK, Horng JL, Yan JJ, Hsiao CD, Hwang PP. Carbonic anhydrase 2-like a and 15a are involved in acid-base regulation and Na+ uptake in zebrafish H+-ATPase-rich cells. Am J Physiol Cell Physiol 294: C1250–C1260, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev 24: 261–274, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Marshall WS, Bryson SE, Midelfart A, Hamilton WF. Low-conductance anion channel activated by cAMP in teleost Cl−-secreting cells. Am J Physiol Regul Integr Comp Physiol 268: R963–R969, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Morgan IJ, Potts WTW. The effects of the adrenoreceptor agonists phenylephrine and isoproterenol on the intracellular ion concentrations of branchial epithelial cells of brown trout (Salmo trutta L.). J Comp Physiol B 165: 458–463, 1995 [Google Scholar]
- 40.Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seidler U, Hoque KM, Tse CM, De Jonge HR, Weinman EJ, Donowitz M. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na+/H+ exchanger regulatory factor 1 (NHERF1) null mice: cAMP inhibition is diffrentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem 282: 25141–25151, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Nakada T, Hoshijima K, Esaki M, Nagayoshi S, Kawakami K, Hirose S. Localization of ammonia transporter Rhcg1 in mitochondrion-rich cells of yolk sac, gill, and kidney of zebrafish and its ionic strength-dependent expression. Am J Physiol Regul Integr Comp Physiol 293: R1743–R1753, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 278: 49523–49529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Păunescu TG, Da Silva N, Russo LM, McKee M, Lu HAJ, Breton S, Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Pǎunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry SF, Payan P, Girard JP. Adrenergic control of branchial chloride transport in the isolated perfused head of the freshwater trout (Salmo gairdneri). J Comp Physiol B 154: 269–274, 1984 [Google Scholar]
- 47.Pickford GE, Phillips JG. Prolactin, a factor in promoting survival of hypophysectomized killifish in fresh water. Science 130: 454–455, 1959 [DOI] [PubMed] [Google Scholar]
- 48.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Pisam M, Auperin B, Prunet P, Rentier-Delrue F, Martial J, Rambourg A. Effects of prolactin on α and β chloride cells in the gill epithelium of the saltwater adapted tilapia “Oreochromis niloticus”. Anat Rec 235: 275–284, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Shestopalov IA, Sinha S, Chen JK. Light-controlled gene silencing in zebrafish embryos. Nat Chem Biol 3: 650–651, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Shih TH, Horng JL, Hwang PP, Lin LY. Ammonia excretion by the skin of zebrafish (Danio rerio) larvae. Am J Physiol Cell Physiol 295: C1625–C1632, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Shih TH, Horng JL, Liu ST, Hwang PP, Lin LY. Rhcg1 and NHE3b are involved in ammonium-dependent sodium uptake by zebrafish larvae acclimated to low-sodium water. Am J Physiol Regul Integr Comp Physiol 302: R84–R93, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Shono T, Kurokawa D, Miyake T, Okabe M. Acquisition of glial cells missing 2 enhancers contributes to a diversity of ionocytes in zebrafish. PLoS One 6: e23746, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem 232: 1077–1091, 1958 [PubMed] [Google Scholar]
- 55.Tipsmark CK, Madsen SS. Rapid modulation of Na+/K+-ATPase activity in osmoregulatory tissues of a salmonid fish. J Exp Biol 204: 701–709, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Tresguerres M, Buck J, Levin L. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflügers Arch 460: 953–964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int 79: 1277–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tresguerres M, Levin LR, Buck J, Grosell M. Modulation of NaCl absorption by [HCO3−] in the marine teleost intestine is mediated by soluble adenylyl cyclase. Am J Physiol Regul Integr Comp Physiol 299: R62–R71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tresguerres M, Parks SK, Katoh F, Goss GG. Microtubule-dependent relocation of branchial V-H+-ATPase to the basolateral membrane in the Pacific spiny dogfish (Squalus acanthias): a role in base secretion. J Exp Biol 209: 599–609, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Tresguerres M, Parks SK, Salazar E, Levin LR, Goss GG, Buck J. Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc Natl Acad Sci USA 107: 442–447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai JC, Hwang PP. Effects of wheat germ agglutinin and colchicine on microtubules of the mitochondria-rich cells and Ca2+ uptake in tilapia (Oreochromis mossambicus) larvae. J Exp Biol 201: 2263–2271, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Tsai JC, Hwang PP. The wheat germ agglutinin binding sites and development of the mitochondria-rich cells in gills of tilapia (Oreochromis mossambicus). Fish Physiol Biochem 19: 95–102, 1998 [Google Scholar]
- 63.Tseng DY, Chou MY, Tseng YC, Hsiao CD, Huang CJ, Kaneko T, Hwang PP. Effects of stanniocalcin 1 on calcium uptake in zebrafish (Danio rerio) embryo. Am J Physiol Regul Integr Comp Physiol 296: R549–R557, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Vermette MG, Perry SF. The effects of prolonged epinephrine infusion on the physiology of the rainbow trout, Salmo gairdneri. II Branchial solute fluxes. J Exp Biol 128: 255–267, 1987 [DOI] [PubMed] [Google Scholar]
- 65.Wang YF, Tseng YC, Yan JJ, Hiroi J, Hwang PP. Role of SLC12A10.2, a Na-Cl cotransporter-like protein, in a Cl− uptake mechanism in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 296: R1650–R1660, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Weinman EJ, Minkoff C, Shenolikar S. Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA. Am J Physiol Renal Physiol 279: F393–F399, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Weinman EJ, Shenolikar S, Kahn AM. cAMP-associated inhibition of Na+-H+ exchanger in rabbit kidney brush-border membranes. Am J Physiol Renal Fluid Electrolyte Physiol 252: F19–F25, 1987 [DOI] [PubMed] [Google Scholar]
- 68.Willoughby D, Cooper DMF. Live-cell imaging of cAMP dynamics. Nat Methods 5: 29–36, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Wright PA, Wood CM. 7 Things fish know about ammonia and we don't. Respir Physiol Neurobiol 184: 231–240, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Wright PA, Wood CM. A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins. J Exp Biol 212: 2303–2312, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Yan JJ, Chou MY, Kaneko T, Hwang PP. Gene expression of Na+/H+ exchanger in zebrafish H+-ATPase-rich cells during acclimation to low-Na+ and acidic environments. Am J Physiol Cell Physiol 293: C1814–C1823, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Yoo SK, Lam Py Eichelberg MR, Zasadil L, Bement WM, Huttenlocher A. Role of microtubules in neutrophil polarity and migration in live zebrafish. J Cell Sci 125: 5702–5710, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]