Abstract
Cumulus oophorus cells play an essential role in oocyte development. They are also widely employed as donor cells for cloning by somatic cell nuclear transfer. Our previous studies revealed that Cbx4 mRNA was overexpressed in cloned two-cell embryos. These data indicated that CBX4 may regulate normal cumulus cell differentiation and that its overexpression in clones could contribute to aberrant gene regulation. We used siRNA-mediated knockdown of Cbx4 to assess its role in determining cumulus cell phenotype and compared the effects of this knockdown to published data for aberrant gene regulation in cloned embryos. We observed widespread effects on the expression of genes related to diverse processes in cultured cumulus cells, including cell assembly/proliferation and DNA replication/repair, endocrine function, carbohydrate and lipid metabolism, inflammation, and cell morphology, with apparent effects of CBX4 in promoting cumulus cell proliferation and survival and inhibiting differentiation. Overall, the data implicate CBX4 as a key component in the pathway integrating endocrine signals, intraovarian paracrine factors, and oocyte-derived factors in the control of cumulus cell functions. We also observed altered expression of 25 cumulus cell markers of oocyte quality, indicating an important role of CBX4 in production of high quality oocytes. Finally, we found that about one-quarter of the genes showing aberrant transcription in cloned embryos are sensitive to Cbx4 knockdown in cumulus cells, consistent with a role for aberrant Cbx4 regulation in elaborating abnormal cloned embryo characteristics.
Keywords: polycomb, differentiation, chromatin, nuclear reprogramming, ovary, follicle, cloning, somatic cell nuclear transfer
the cumulus oophorus cell is an essential companion cell that supports the growth and maturation of mammalian oocytes. The oocyte recruits cells from the follicular granulosa cell population to become cumulus cells, which proliferate and eventually surround the oocyte in multiple layers and remain in contact with the oocyte through cytoplasmic processes that penetrate the oocyte zona pellucida. The cumulus cells and the oocyte are connected via gap junctions. The cumulus cells provide metabolites to the oocyte to promote its development and play a key role in controlling meiotic progression ( 1, 19, 38, 51). Thus, while the oocyte orchestrates the overall pace of oogenesis, a complex dialog between cumulus cells and oocyte takes place, so that the phenotypic characteristics of both cell types evolve in coordination with each other during the overall process of oogenesis. Cumulus cells promote the formation of high-quality oocytes during in vitro maturation ( 12). The cumulus cells remain attached to the oocyte after ovulation and revert to a more granulosa cell-like state, in which they may replace intrafollicular paracrine signals that emanate from the granulosa cells to maintain oocyte quality ( 14). Interestingly, transcriptome profiles of cumulus cells appear more highly predictive of oocyte quality than the oocyte's own transcriptome ( 32, 33). Whereas transcriptome comparisons of high- and low-quality MII oocytes yielded just 59 genes with significant differences in expression, an equivalent comparison of transcriptomes of cumulus cells associated with those oocytes yielded 452 gene with significantly altered expression and a large number of additional genes displaying altered transitions from pre- to postmaturation stages. These observations indicate that oocyte quality is dramatically affected by aberrant gene regulation in the cumulus cell. The simplest explanation for this is that altered cumulus cell gene regulation controls the synthesis, stability, and accumulation of key macromolecular components of the oocyte that affects its quality.
The cumulus cells thus undergo a complex and dynamic history with respect to differentiated phenotype. This situation differs considerably from the more common conceptualization of cellular differentiation of many other cell types, in which cells transition to a single stable differentiated state and maintain that state until they are eliminated. The regulatory mechanisms that enable such a dynamic and flexible differentiated state in the cumulus cell are not well understood.
Chromatin regulators comprise a key class of gene regulatory factors that may contribute to establishment of a differentiated cell state that is dynamic and flexible. Such factors enable stable, heritable chromatin states to be programmed into cells during development but are also amenable to dynamic reprogramming by allowing the regulated reversal or modification of posttranslational changes in histones and changes in DNA methylation. Cloning by somatic cell nuclear transfer has offered a unique portal by which to view nuclear reprogramming and by which to assess the relative stability of chromatin states.
In an earlier analysis, we observed that the mRNA encoding the Polycomb group protein gene Chromobox 4 ( Cbx4) is overexpressed in two-cell stage cloned mouse embryos compared with fertilized embryos ( 57). CBX4 is enriched at sites of facultative heterochromatin ( 9). CBX family members are found in distinct complexes that regulate distinct sets of target genes, with distinct effects on phenotype ( 31, 43, 56). For example, CBX7 promotes pluripotency in embryonic stem cells and hematopoietic stem cell renewal ( 31, 43, 44) by suppressing other CBX family members ( 43). In contrast, CBX4 promotes lineage commitment and differentiation in embryonic stem cells and other cell types ( 43, 44). CBX4 also promotes cell proliferation in some cell types ( 35). Its loss is associated with some forms of cancer ( 50). CBX4 also inhibits cellular senescence in human epidermal stem cells ( 37). CBX4 is itself regulated by sumoylation ( 9) and in turn sumoylates DNA methyltransferase 3a and promote DNMT3A and DNMT3B recruitment to sites of gene repression ( 30, 34), SMAD-interacting protein, and other transcription factors to promote their binding to sites of repression ( 36, 45), and BMI1 to promote its recruitment to sites of DNA damage ( 28). CBX4 possesses SUMO E3 ligase activity and promotes the sumoylation of transcriptional co-repressors and other transcription and chromatin regulators ( 42), as well as DNA repair factors ( 28), and is required for p53/TP53 transcriptional activation. Hence, CBX4 exerts many genome-wide effects, some repressive and others activating.
Because cloned embryos manifest numerous somatic cell-like features ( 21, 22), overexpression of Cbx4 mRNA in clones raised the possibility that CBX4 may play an important role in elaborating the cumulus cell state and that its continued expression in clones could promote continued expression of cumulus cell characteristics. To learn the overall degree to which CBX4 contributes to the cumulus cell differentiated state, we undertook siRNA-mediated knockdown of CBX4 expression in cultured cumulus cells. We observed widespread impact on the mRNA expression profile, confirming that CBX4 plays a key role in cumulus cell differentiation. We also found that approximately one-quarter of the genes showing aberrant transcription regulation in cloned embryos are affected by Cbx4 siRNA in cumulus cells, consistent with the hypothesis that a small number of transcription regulators expressed aberrantly in clones due to incomplete reprogramming generates an expanded array of gene expression changes. Additionally, nearly half of the affected genes are likely regulated in a cell type-specific manner. These results provide new insight into the role of CBX4 in controlling cellular phenotype and the role of CBX4 in limiting successful cloning outcome.
MATERIALS AND METHODS
Cumulus cell culture and siRNA transfection.
Cumulus cells were harvested from BDF1 mice that had been superovulated by injection of 5 IU equine chorionic gonadotropin followed by 5 IU human chorionic gonadotropin 46–48 h later. Cumulus cells were isolated from ovulated MII stage cumulus-oocyte complexes, washed with M2 medium, resuspended in MEM-α containing 10% FCS, penicillin, and streptomycin, and then plated in six-well plates at a density of 2 × 10 4 cells/well. They were cultured overnight before transfection.
Double-stranded siRNAs (21-mer) targeting mouse CBX4 were purchased from Qiagen (Valencia, CA). The corresponding target mRNA sequences for the siRNAs were: Cbx4-6 (S102731834): CAGGAAGAGCGG-CAAGT ATTA, Cbx4-7 (S104446673): AAGGTCCGAAGTTGAGGGAAA. Scrambled siRNA was used as a negative control. Cumulus cells were transfected with siRNA (25 nM Cbx4-6 and 25 nM Cbx4-7) using lipofectamine RNAiMAX reagent (Invitrogen/Life Technologies, Grand Island, NY) according to the manufacturer protocol. After overnight culture, the medium was replaced and the cells were transfected by the forward transfection method. We diluted 30 pmol of siRNA in 50 μl Opti-MEM medium (Invitrogen) and mixed that with 1 μl of Lipofectamine RNAiMAX reagent (Invitrogen) diluted in 49 μl of Opti-MEM medium. After incubation for 20 min at room temperature, the mixture was added to wells containing the cumulus cells. Cumulus cells were harvested after 72 h of incubation at 37°C in a humidified 5% CO 2 atmosphere.
qRT-PCR assay.
The RNA interference (RNAi) results were evaluated with a Taqman real-time polymerase chain reaction assay, for which RNA was extracted 3 days after transfection by the Pico Pure RNA Isolation Kit (Arcturus/Life Technologies) and reverse transcribed with Superscript III First-Strand Synthesis System (Invitrogen). The TaqMan gene expression primers/probes were from ABI (Applied Biosystems/Life Technologies). We amplified 20 μl reactions through 40 cycles under universal cycling conditions at 95°C for 15 s; 60°C for 1 min on a StepOnePlus Real-Time PCR System. Quantification was normalized to the endogenous histone H2A [Mm-00501974_s1, (Hisst2ah2aa10)] within the log linear phase of the amplification curve by the comparative C t method, where C t is threshold cycle.
Western blot analysis.
We prepared whole protein extracts by lysing the cumulus cells 3 days after siRNA transfection in lysis buffer containing: 50 mM Tris·HCl (pH 7.4), 5 mM EDTA, 250 mM NaCl, 50 mM NaF, 0.1% Triton X-100, 0.1 mM Na 3VO 4, 2 mM PMSF, 10 μg/ml leupeptin, 4 μg/ml aprotinin, and 4 μg/ml pepstatin. Protein concentrations were quantified with the Bradford assay (Bio-Rad, Hercules, CA). Western blots were performed by resolving 10 mg of protein extracts on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to PVDF membranes in 10 mM CAPS (pH 11) containing 10% methanol, and probed with antibodies against CBX4 (rabbit polyclonal anti-CBX, #ARP30002-P050; Aviva Systems Biology, San Diego, CA) and bound antibody visualized using horseradish peroxidase-conjugated secondary antibodies (GE Healthcare Life Sciences, Pittsburgh, PA) and Western Lighting Plus ECL reagent (Perkin Elmer, Waltham, MA) and a FluorChemQ Imaging System (Protein Simple, Santa Clara, CA).
Microarray hybridization.
After 72 h of treatment with Cbx4 siRNAs, cumulus cells RNA was isolated as above and processed for microarray analysis. The transfection was done the same as described above except that cell number and reagent volumes were increased by fourfold. Up to 50 ng of total RNA from each sample were subjected to two rounds of cDNA synthesis using the Arcturus RiboAmp HS Plus kit (Invitrogen). Labeled cRNA was produced using the Affymetrix GeneChip Expression 3′ Amplification for IVT Labeling Kit (Affymetrix, Santa Clara, CA). The biotin-labeled cRNA samples were fragmented, and 10 μg were hybridized onto arrays. Posthybridization washing, staining, and scanning were performed as described in the Affymetrix GeneChip Expression Analysis Technical Manual. The amplified cRNA samples were fragmented, and 10 μg was hybridized to Affymetrix MOE 430 v2.0 arrays, and the arrays were washed, stained on fluidic stations, and scanned according to the manufacturer instructions.
Microarray data analysis.
Microarray data were preprocessed and analyzed with scripts written in R(48), utilizing routines from Bioconductor ( 23) and Significance Analysis of Microarrays (SAM) ( 54) packages. The quality of data from individual arrays was assessed by examining the standard indicators: minimum, maximum, and average background, percentage of present calls by the Affymetrix MAS5 algorithm, scaling factor, and ratios of expression between 3′ and 5′ probes for spike-in probe sets. The array quality control parameters for those samples accepted for further analysis were all within the acceptable ranges. Probe-set expression values were summarized and normalized by robust multiarray analysis ( 27). Control and treatment groups of microarrays were compared to identify differentially expressed genes using the SAM algorithm ( 54). Probe sets with all expression values below the threshold cutoff of 100 raw intensity units and the probe sets with all absent calls in both treatment groups were excluded. The parameters for SAM analysis were: false discovery rate threshold for q value 0.01, number of permutations 1,000. Array data were deposited with the Gene Expression Omnibus database (accession number GSE46565).
To reduce the potential impact of cross-hybridization to off-target mRNAs and to maximize the confidence and number of gene identities applied to probe sets, a custom set of probe-set definitions and annotations was used instead of those provided by Affymetrix. We performed alignment of probe sequences to RefSeq mRNA sequences and to genomic sequences for genes in GRC38 mouse genome assembly (downloaded on November, 14, 2012). Based on the alignment results we reduced some of the probe sets by removing probes that did not match their intended targets or matched two or more unrelated gene transcripts. We compiled custom annotations for probe sets. We annotated only those probe sets for which we found sufficient evidence of possible probe hybridization to published gene transcripts (probes mapped to published mRNA sequences) or to not-yet-reported gene transcripts (probes mapped to published genomic sequences). For some probe sets included on the chip we could not identify any complementary mouse transcripts or genomic sequences, and these probe sets were left unannotated. We note that the lack of annotation does not imply that these probe sets are irrelevant, as their annotation status could change with future improvements of genome assemblies and gene annotations.
Gene lists were analyzed with the Ingenuity Pathway Analysis (IPA) program (Ingenuity Systems, Rockwood, CA). This program provides the opportunity to evaluate statistically significant network associations and pathways that incorporate genes from the target list, as well as statistically significant effects on specific cellular process and biological functions. For biological functional analysis, the Fisher's exact test was performed with the P value threshold of 0.05 to identify molecular and cellular functional categories with statistical significance. Biofunctions were also ranked according to the absolute value of the activation z score, which is a statistic estimating the congruence of observed gene expression and previously reported activity for genes related to the particular biofunction and is used to predict biofunctions' activation state (decreased or increased). IPA also identifies potential upstream transcriptional regulators that may be responsible for the observed changes in expression. Similarly to analysis of biological functions, Fisher's exact test is used to predict which upstream regulators are involved and activation z score to predict whether they are activated or inhibited. It should be noted that the z-score can be used in conjunction with other measures of significance (e.g., P value, number of affected molecules, biofunctions). The z score may not fully account for inconsistent observations for gene product interactions or cell type-specific interactions that may arise and, due to undiscovered interactions being absent from the IPA knowledge database, may lead to false negatives. The z score, however, is a valuable aid in identifying affected networks and potential involvements of upstream regulators.
RESULTS
Experimental strategy for determining CBX4 function in cumulus cells.
Our objective was to evaluate the degree to which CBX4 contributes to the differentiated state of cumulus cells associated with matured oocytes by knocking down expression of CBX4 and determining how this affects the transcriptome. We chose a siRNA knockdown approach because this could be performed readily on mice of the same genetic background as those employed as oocyte donors for cloning (B6D2F1). Effective siRNA-mediated knockdown of expression of a target protein requires a period of time in order for existing supplies of that protein and its mRNA to decline. A change in CBX4 target gene expression in turn would require additional time to permit epigenetic changes pursuant to loss of CBX4 expression and an associated decline in target gene mRNA expression; a 72 h treatment was selected to meet these requirements.
Knockdown of CBX4 expression by siRNA transfection.
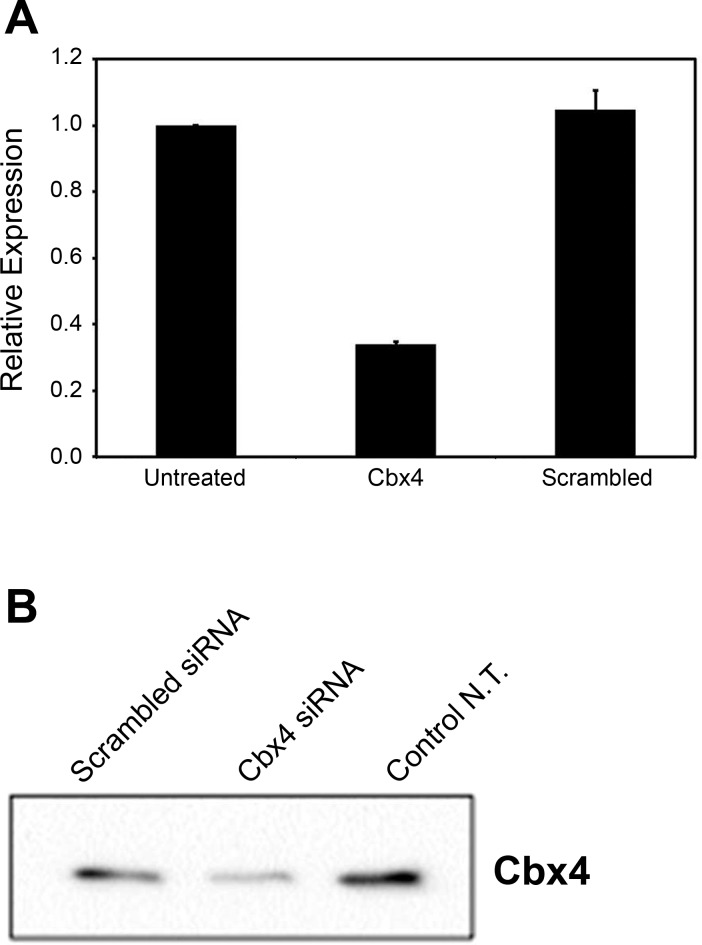
We found that lipofectamine-mediated siRNA delivery was more effective than adenoviral-mediated delivery and chose this approach for application in these studies. We verified the knockdown of Cbx4 gene expression 3 days posttransfection by quantitative RT-PCR and by Western blot analysis. At the mRNA level, Cbx4 expression was reduced to 33% of the control values (cells not transfected or cells transfected with scrambled siRNA) ( Fig. 1 A). Western blotting revealed 75 and 60% reductions in protein expression compared with nontransfected control cells and cells transfected with scrambled siRNA, respectively ( Fig. 1 B).
Fig. 1.
Confirmation of Cbx4 gene expression knockdown after siRNA treatment. Cells were lysed at 72 h of siRNA treatment and processed either for RNA or protein isolation. A: qRT-PCR analysis revealing reduced expression of Cbx4 mRNA. B: Western blot revealing reduction at the level of CBX4 protein.
Effects of Cbx4 siRNA knockdown on gene expression.
We obtained four high-quality arrays for Cbx4 siRNA transfected cells and three arrays for scrambled siRNA treated controls ( Table 1). The percent P calls and average backgrounds were all within acceptable ranges and similar between arrays. The scaling factors were close to each other, consistent with acceptable quality ( 40).
Table 1.
Quality control parameters for arrays
| Assays, n | Present Calls, % | Probe sets With Present Calls, n | Scale Factor | Average Background | |
|---|---|---|---|---|---|
| Control scrambled siRNA | 3 | 42.05 (41.54–42.42) | 18,965 (18,736–19,131) | 0.39 (0.33–0.45) | 58.88 (55.70–64.92) |
| Cbx4 siRNA | 4 | 43.23 (42.55–44.36) | 19,496 (19,192–20,007) | 0.32 (0.28–0.41) | 67.97 (55.16–76.28) |
SAM analysis revealed extensive effects of Cbx4 knockdown on mRNA expression profiles ( Table 2 and Supplemental Tables S1 and S2). 1 We observed 3,014 probe sets significantly reduced and 2,502 probe sets with significantly increased signals in Cbx4 siRNA treated cells. Of these, 613 probe sets were decreased and 365 were increased by twofold (459 and 295 annotated genes, respectively), 64 and 56 by fourfold (total 93 annotated genes), and two and 15 by 10-fold or more.
Table 2.
Numbers of probe sets affected by Cbx4 siRNA treatment
| All | 1.5-fold | 2-fold | 4-fold | 10-fold | |
|---|---|---|---|---|---|
| Decreased | 3,014 | 1,475 | 613 | 64 | 2 |
| Increased | 2,502 | 1,150 | 365 | 56 | 15 |
Because CBX4 is a component of the PRC1 complex, we compared the lists of affected genes to published lists of PRC1 target genes identified using gene arrays in mouse ES cells and Drosophila cells ( 11, 53) and target genes identifiable through intermolecular association or other databases based on data from mammalian cells (list databases) (Supplemental Tables S1 and S2, Column I). This comparison revealed 99 probe-set IDs (63 genes) on the list of probe-set IDs with signals reduced by Cbx4 siRNA that were shared with the ES cell list and 174 probe-set IDs (121 genes) shared with the Drosophila list (250 probe sets, 174 genes total, 56 genes with twofold or greater effects). For probe-set IDs with signals increased by siRNA treatment, 52 (39 genes) and 150 (114 genes) were shared with the two lists (197 probe sets, 150 genes total, 39 genes with 2-fold or greater effects). The total number of genes affected by Cbx4 siRNA and previously described as PRC1 targets was 319, with 95 having expression affected by Cbx4 siRNA by twofold or more. This corresponds 12.6% of the 754 genes affected by twofold or more.
Ingenuity pathway and biofunction analysis of affected genes.
We submitted the list of annotated genes affected by Cbx4 siRNA at the level of twofold or greater, combining genes with increased and decreased expression (Supplemental Table S3). The list was analyzed with the biofunction, canonical pathway, network, and upstream regulator analysis modules. These tools search for biofunctions and canonical pathways for which lists of associated genes have significant overlap with the query gene list, construct networks (subsets of global molecular network in IPA knowledge base) that contain a high density of genes in the query list, and search for potential upstream regulators for which lists of downstream/regulated molecules have significant overlap with the query gene. For biofunction analysis, the most significant results based on z score were for multiple categories related to cell proliferation, cytokinesis, DNA replication, and chromatin assembly (decreased), and organismal/embryo death (increased) (Supplemental Table S3). Affected canonical pathways include those related to stress response [growth arrest and DNA damage inducible 45 (GADD45)], checkpoint regulation, ataxia telangiectasia mutant (ATM) signaling, estrogen regulation, ephrin signaling, and ovarian cancer (Supplemental Table S4).
Affected networks (Supplemental Table S5, Figs. 2– 5) include multiple networks related to cell cycle/assembly/DNA replication and repair ( networks 1, 4, 5, 6, 8, 9, 11, 13, 14, 15), lipid metabolism and endocrine function ( networks 2, 20, 22), inflammation ( networks 3, 19, 23), cell morphology and interactions ( network 7, 12, 18, 21, 24, 25), carbohydrate metabolism ( network 16), and protein synthesis ( network 17). Several of these networks involve follicle-stimulating hormone (FSH) or its receptor (FSHR), either affected by Cbx4 siRNA or as an upstream regulator (Networks 2, 10, 18, 22). Target genes known to interact with CBX4 are indicated for each figure ( Figs. 2– 5) and in Supplemental Table S5.
Fig. 2.
Ingenuity Pathway Analysis (IPA) network 1 (see Supplemental Table S6). Names of molecules that are affected by Cbx4 siRNA treatment are preceded by ↑ (increased) and ↓ (decreased by treatment), and their symbols filled gray; darker shades of gray correspond to higher fold-change. Upstream regulators are marked with a thicker border. IPA employs the following symbols in its networks: A, activation; CP, chemical-protein interaction; E, expression; I, inhibition; LO, localization; M, biochemical modification; MB, group/complex membership; nTRR, nontargeting RNA-RNA interaction; P, phosphorylation/dephosphorylation; PD, protein-DNA interaction; PP, protein-protein interaction; PR, Protein-RNA binding; RB, regulation of binding; RE, reaction; T, transcription; TR, translocation; UB, ubiquitination; line, binding only; line with filled arrowhead, downstream effect; line with bar and arrowhead, inhibition and downstream effect; line with outlined arrowhead, translocation; solid line, direct interaction; broken line, indirect interaction; vertical diamond, enzyme; horizontal diamond, peptidase; horizontal oval, transcription regulator; vertical oval, transmembrane receptor; trapezoid, transporter; down-pointing triangle, kinase; square, cytokine; vertical rectangle, G-protein coupled receptor, horizontal rectangle, ligand-dependent nuclear receptor; dashed square, growth factor; dashed rectangle, ion channel; double circle, complex/group; circle, other.
Fig. 5.
IPA network 18 (see Supplemental Table S6). Symbols are as described in Fig. 2.
Fig. 3.
IPA network 2 (see Supplemental Table S6). Symbols are as described in Fig. 2.
The IPA upstream regulator analysis tool can reveal key molecules that regulate the affected networks and hence are strong candidates for molecules that are mechanistically related to the observed effects. Some of these directly regulate the networks and may themselves be affected by Cbx4 siRNA treatment, while others may indirectly regulate the affected networks and may be unaffected by Cbx4 siRNA treatment. Some key upstream regulators appeared repeatedly among the affected networks ( Table 3 and Supplemental Table S5). Some of these are decreased in expression by Cbx4 siRNA treatment (BMPR2, BRCA1, CCND1&2, CDC20, CKS2, CSF1R, E2F1&6&8, ETS1, GREM1, IGF1, INHA, KRAS, LEPR, MKI67, MYC, NCAM1, NR3C1, NR4A1, NR5A2, NUPR1, PRDX2, RUNX2, S100A6, SEC16B, TIMP1, ZBTB20, ZEB1&2). Other upstream regulators are increased by Cbx4 siRNA treatment (CCDC80, CDH1, CDK4, CDKN1A, CDKN2A, COL4A3, DDX58, DNMT3B, EGFR, EGR1, ERBB3, FOXO1, FSHR, KRT18, LIF, LRP6, PRKCI, PTGS2, PTK2, TXNIP, ZFPM2) (see Supplemental Tables S1 and S2 for gene symbol definitions).
Table 3.
Overview of the most prominent upstream regulators in IPA networks
| Upstream Regulator | Log-ratio | Activation z Score | Overlap P Value | Networks, n | Networks (molecules regulated, n) | Upstream regulator | Log-ratio | Activation z Score | Overlap P Value | Networks, n | Networks (molecules regulated, n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓MYC[2] | −0.885 | −3.676 | 5.98 × 10 −5 | 4 | Net4(6), Net13(10), Net15(4), Net18(5) | ↓NR3C1 | −0.514 | −0.795 | 1.36 × 10 −3 | 1 | Net25(2) |
| ↑CDKN1A | 0.69 | 2.839 | 5.19 × 10 −20 | 3 | Net4(4), Net5(4), Net13(9) | TGFB1 | −1.739 | 5.45 × 10 −16 | 16 | Net1(12), Net2(6), Net3(5), Net4(6), Net5(5), Net6(6), Net7(9), Net8(6), Net10(4), Net11(8), Net12(8), Net15(4), Net17(5), Net18(5), Net19(4), Net21(8) | |
| ↓↓CCND1[2] | −2.787 | −3.478 | 3.51 × 10 −16 | 2 | Net1(8), Net8(M/10) | TP53 | 3.629 | 3.59 × 10 −13 | 13 | Net1(8), Net3(4), Net4(6), Net5(5), Net7(4), Net8(6), Net10(6), Net13(12), Net14(6), Net15(5), Net18(4), Net21(5) Net25(2) | |
| ↑CDK4 | 0.45 | 8.60 × 10 −12 | 2 | Net1(6), Net8(7) | TNF | 1.34 | 4.29 × 10 −6 | 13 | Net2(6), Net3(4), Net8(6), Net9(7), Net11(5), Net12(6), Net13(8), Net14(6), Net17(5), Net18(4), Net19(6), Net21(8), Net22(2) | ||
| ↓E2F1[1] | −0.403 | −2.339 | 4.76 × 10 −11 | 2 | Net4(4), Net6(7) | HNF4A | −0.11 | 6.96 × 10 −3 | 11 | Net3(3), Net4(8), Net5(6), Net6(6), Net7(4), Net10(9), Net19(6), Net20(3), Net21(5), Net24(4), Net25(2) | |
| ↓IGF1 | −0.488 | −2.067 | 3.47 × 10 −7 | 2 | Net12(4), Net17(4) | ERBB2 | −1.147 | 2.19 × 10 −23 | 10 | Net1(9), Net5(4), Net8(6), Net9(5), Net10(6), Net13(9), Net15(4), Net18(6), Net19(3), Net20(3) | |
| ↓NUPR1 | −0.783 | 4.562 | 4.12 × 10 −4 | 2 | Net24(2), Net25(2) | HGF | −2.973 | 3.09 × 10 −16 | 6 | Net1(5), Net3(4), Net6(11), Net9(5), Net13(8), Net21(5) | |
| ↓↓E2F8 | −1.136 | 5.65 × 10 −10 | 1 | Net1(M/1) | FSH | −2.042 | 2.62 × 10 −5 | 4 | Net2(6), Net10(M/3), Net18(4), Net22(2) | ||
| ↑FOXO1 | 0.32 | −2.643 | 8.35 × 10 −5 | 1 | Net1(5) | FOS | 0.314 | 9.14 × 10 −5 | 4 | Net3(3), Net9(4), Net10(5), Net20(3) | |
| ↓↓S100A6 | −1.107 | −2.646 | 7.47 × 10 −5 | 1 | Net1(M/3) | IL1B | 0.555 | 2.04 × 10 −2 | 4 | Net11(5), Net19(3), Net21(5), Net22(2) | |
| ↓↓E2F6[2] | −1.059 | 0 | 3.57 × 10 −4 | 1 | Net2(M/5) | E2F4 | 4.28 × 10 −20 | 3 | Net1(6), Net4(4), Net6(6) | ||
| ↑↑FSHR | 1.22 | −1.634 | 5.89 × 10 −4 | 1 | Net2(M/5) | Cg | −0.811 | 6.44 × 10 −12 | 3 | Net2(M/12), Net9(4), Net11(4) | |
| ↓↓NR5A2 | −1.138 | −1.953 | 3.82 × 10 −1 | 1 | Net2(M/3) | CTNNB1 | −1.534 | 3.50 × 10 −5 | 3 | Net7(5), Net11(4), Net21(6) | |
| ↓↓ZBTB20 | −1.92 | 0.447 | 2.46 × 10 −2 | 1 | Net3(M/2) | IL2 | −1.286 | 8.48 × 10 −5 | 3 | Net10(4), Net12(4), Net14(4) | |
| ↓↓BRCA1 | −1.502 | −0.1 | 1.68 × 10 −2 | 1 | Net4(M/1) | OSM | 0.674 | 9.55 × 10 −4 | 3 | Net10(4), Net19(5), Net20(4) | |
| ↑↑CCDC80 | 1.008 | 1.54 × 10 −3 | 1 | Net7(M/1) | Lh | −1.822 | 2.07 × 10 −3 | 3 | Net10(M/2), Net18(4), Net22(2) | ||
| ↓↓GREM1 | −1.112 | 2.60 × 10 −3 | 1 | Net7(M/2) | IL6 | −0.62 | 3.64 × 10 −9 | 3 | Net12(4), Net13(9), Net22(2) | ||
| ↓INHA | −0.789 | −0.172 | 1.07 × 10 −4 | 1 | Net7(4) | CSF2 | −4.66 | 2.45 × 10 −14 | 2 | Net1(8), Net13(10) | |
| ↑↑KRT18 | 1.024 | 4.40 × 10 −2 | 1 | Net7(M/1) | EGF | −4.214 | 1.08 × 10 −7 | 2 | Net2(6), Net3(3) | ||
| ↓↓ZEB2 | −1.522 | −0.872 | 1.96 × 10 −2 | 1 | Net7(M/1) | IFNB1 | 1.949 | 4.50 × 10 −2 | 2 | Net3(3), Net19(4) | |
| ↑↑ZFPM2[2] | 1.347 | 2.60 × 10 −2 | 1 | Net7(M/2) | RB1 | 3.971 | 5.99 × 10 −11 | 2 | Net6(6), Net8(8) | ||
| ↑↑CDKN2A | 1.87 | 1.737 | 5.23 × 10 −6 | 1 | Net8(M/6) | PI3K (complex) | −1.578 | 1.45 × 10 −4 | 2 | Net7(M/1), Net19(3) | |
| ↑↑PTGS2 | 1.49 | −0.997 | 4.00 × 10 −4 | 1 | Net8(M/2) | CEBPA | −1.279 | 3.79 × 10 −4 | 2 | Net9(4), Net12(5) | |
| ↓↓BMPR2 | −1.013 | 6.49 × 10 −4 | 1 | Net9(M/3) | CEBPB | −0.498 | 6.45 × 10 −5 | 2 | Net9(4), Net12(5) | ||
| ↓↓ZEB1 | −1.027 | −1.973 | 4.16 × 10 −3 | 1 | Net9(M/1) | IL4 | −2.072 | 2.39 × 10 −1 | 2 | Net10(4), Net22(3) | |
| ↑↑ERBB3 | 1.672 | 0.92 | 4.93 × 10 −6 | 2 | Net10(M/1), Net18(4) | TBX2 | −4.219 | 6.00 × 10 −13 | 1 | Net1(5) | |
| ↑↑PTK2 | 1.232 | −1.011 | 3.03 × 10 −5 | 1 | Net11(M/4) | YY1 | −0.662 | 5.24 × 10 −16 | 1 | Net2(6) | |
| ↓↓TIMP1 | −1.416 | 0 | 1.03 × 10 −2 | 1 | Net11(M/1) | RNA polymerase II | 1.90 × 10 −4 | 1 | Net4(M/1) | ||
| ↓↓CSF1R | −1.186 | −0.218 | 4.16 × 10 −3 | 1 | Net12(M/1) | let-7 | 4.571 | 1.31 × 10 −11 | 1 | Net6(6) | |
| ↑LIF | 0.439 | 0.417 | 6.99 × 10 −3 | 1 | Net12(5) | LDL | −0.578 | 3.13 × 10 −2 | 1 | Net8(M/5) | |
| ↓↓LEPR[1,2] | −1.648 | 4.51 × 10 −2 | 1 | Net12(M/3) | HRAS | −1.043 | 1.15 × 10 −5 | 1 | Net11(4) | ||
| ↓↓NCAM1 | −1.311 | 2.15 × 10 −2 | 1 | Net12(M/2) | JUN | −1.095 | 1.91 × 10 −7 | 1 | Net12(4) | ||
| ↓↓CDC20[1] | −1.92 | 1.94 × 10 −3 | 1 | Net13(M/2) | NFkB (complex) | 0.095 | 2.20 × 10 −2 | 1 | Net13(M/7) | ||
| ↓↓CKS2 | −1.311 | 8.50 × 10 −5 | 1 | Net13(M/1) | Ins1 | −2.021 | 1.89 × 10 −2 | 1 | Net15(4) | ||
| ↓↓SEC16B | −1.283 | 4.40 × 10 −2 | 1 | Net14(M/1) | Pdgf (complex) | −1.768 | 1.77 × 10 −3 | 1 | Net15(M/1) | ||
| ↑↑PRKCI[2] | 1.092 | 2.60 × 10 −2 | 1 | Net15(M/1) | PDGF BB | −2.277 | 1.51 × 10 −7 | 1 | Net15(M/5) | ||
| ↓↓CCND2[1,2] | −2.065 | 2.58 × 10 −2 | 1 | Net17(M/1) | TCR | −2.132 | 4.27 × 10 −1 | 1 | Net15(M/4) | ||
| ↑↑COL4A3 | 3.856 | 1.69 × 10 −2 | 1 | Net18(M/2) | IgG | 0.102 | 4.70 × 10 −2 | 1 | Net17(M/4) | ||
| ↑EGFR[1] | 0.368 | −0.405 | 1.06 × 10 −8 | 1 | Net18(4) | Immunoglobulin | 0.905 | 1.34 × 10 −2 | 1 | Net17(M/4) | |
| ↑↑DDX58 | 1.437 | 0.421 | 2.46 × 10 −2 | 1 | Net19(M/2) | CTNNβ-TCF/LEF | −1.534 | 3.50 × 10 −5 | 1 | Net19(M/1) | |
| ↓↓PRDX2 | −1.56 | 2.58 × 10 −2 | 1 | Net19(M/1) | TLR3 | −0.217 | 4.93 × 10 −2 | 1 | Net19(3) | ||
| ↑↑TXNIP | 1.118 | 2.177 | 1.39 × 10 −2 | 1 | Net19(M/1) | AHR | −0.947 | 1.03 × 10 −4 | 1 | Net20(2) | |
| ↓ETS1[1] | −0.767 | −0.504 | 1.76 × 10 −2 | 1 | Net20(M/3) | SIRT1 | −1.351 | 2.54 × 10 −2 | 1 | Net20(M/2) | |
| ↑↑CDH1 | 2.653 | 0.317 | 2.51 × 10 −3 | 1 | Net21(M/2) | Ap1 | 0.239 | 1.58 × 10 −2 | 1 | Net21(M/1) | |
| ↓↓MKI67[1] | −1.766 | 4.40 × 10 −2 | 1 | Net21(M/1) | ESR2 | 1.326 | 1.18 × 10 −3 | 1 | Net21(6) | ||
| ↓↓RUNX2 | −1.082 | −1.238 | 3.43 × 10 −3 | 1 | Net21(M/4) | Fibrin | −2 | 8.51 × 10 −3 | 1 | Net21(M/1) | |
| ↑EGR1 | 0.837 | −0.19 | 6.08 × 10 −4 | 1 | Net22(2) | MTOR | −2.219 | 3.92 × 10 −1 | 1 | Net22(M/1) | |
| ↓NR4A1 | −0.618 | −1.42 | 2.27 × 10 −4 | 1 | Net22(M/3) | SPP1 | −0.97 | 2.56 × 10 −2 | 1 | Net22(M/1) | |
| ↑DNMT3B | 0.65 | 0.333 | 3.96 × 10 −4 | 1 | Net24(M/1) | NFYC | 1.57 × 10 −2 | 1 | Net24(M/1) | ||
| ↑LRP6 | 0.287 | 4.87 × 10 −2 | 1 | Net24(M/1) | SMARCA4 | 1.422 | 4.87 × 10 −6 | 1 | Net24(2) | ||
| ↓KRAS | −0.737 | 1.409 | 5.51 × 10 −5 | 1 | Net25(2) | TREM1 | −0.243 | 1.06 × 10 −3 | 1 | Net24(M/1) |
PRC1 targets are labeled with [. . .] in the first column, based on data from [1] Boyer et al. (11) and [2] Tolhuis et al. (53). Symbols for genes with increased and decreased expression and fold-change ≥2 are preceded with ↑↑ and ↓↓, respectively. Symbols for genes with increased and decreased expression and fold-change <2 are preceded with ↑ and ↓. Number (. . .) next to a network ID in the 6th column signifies how many molecules in that network the regulator is affecting. Label (M/. . .) signifies that the regulator is also a member of that network.
The upstream regulator analysis tool yielded many regulators predicted to be affected, but which displayed either no expression or no change in expression with Cbx4 siRNA (Supplemental Table S5). These observations may be explained in several ways. First, the array probe sets may not have been adequate to reveal changes in mRNA expression for some regulators following treatment. Alternatively, the activities of some of the regulators may be affected secondarily at a translational or posttranslational level, effects that would not be measured by array analysis but could nevertheless be arising following treatment. A third explanation could be that some z scores may reflect inconsistent or cell type-specific effects in the IPA knowledge database. The upstream regulators that were affected by Cbx4 siRNA were found in narrow subsets of the affected networks compared with the other regulators that appeared repeatedly in multiple networks but were not affected by Cbx4 siRNA. Cbx4 siRNA thus affects the expression of a narrow array of upstream regulators that reside in larger pathways and networks that may be modulated by other upstream regulators with widespread effects. Additionally, downstream genes affected by Cbx4 siRNA treatment that are potential targets of regulators that are not expressed or not affected may indicate an interesting potential for exogenous regulatory factors to partially phenocopy Cbx4 siRNA effects, implicating CBX4 as a possible part of cumulus cell responses to such factors.
We also note that in some instances, the Cbx4 siRNA elicited effects on some upstream regulators that were at odds with the directionality of predicted effects reflected in the z scores for networks (e.g., FOXO1, NUPR1). This suggests that some of the effects of CBX4 in cumulus cells differ from effects in the knowledge database and thus may be cell type specific.
The most notable among regulators affected by Cbx4 siRNA treatment are MYC (regulates networks 4, 13, 15, and 18), cyclin D1 (CCND1; regulates network 1 and 8, member of network 8), and E2F transcription factor 1 (E2F1, regulates networks 4 and 6), which are all regulated by PRC1. Other notable regulators affected by Cbx4 siRNA treatment are cyclin-dependent kinase inhibitor 1A (CDKN1A, regulates 3 networks), and cyclin-dependent kinase 4 (CDK4), insulin-like growth factor 1 (IGF1), and nuclear protein transcription regulator (NUPR1) (these regulate 2 networks).
Among regulators not affected by Cbx4 siRNA treatment, the most notable is transforming growth factor beta 1 (TGFB1), which appears as a prominent regulator of 16 of the networks (11 of the top 12) and regulates between four and 12 member genes in those networks; it also regulates one gene in six other networks. TGFB1 was neither a member of any network nor a Cbx4 siRNA affected factor, however, indicating its effects on these networks may be indirect. Transforming related protein 53 (TP53, aka TRP53) appears as a prominent regulator of 13 of the networks (7 of the top 10), regulates up to 12 members of the affected networks and is a member of network 12. Hepatic nuclear factor 4A (HNF4A) regulates 11 of the affected networks (6 of the top 10) and regulates as many as nine members of affected networks; however, it is not a member of any of these networks. V-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (ERBB2) regulates 10 of the affected networks related to DNA replication and cell proliferation and is a member of network 3. FSH is a regulator of four of the networks.
The most prominent network in terms of statistical significance and number of affected molecules is network 1, which is associated with the key functions of cell cycle, cellular assembly and organization, and DNA replication, recombination, and repair. This network contains a rich array of nuclear factors, many of which are affected by Cbx4 siRNA treatment and other genes that are targets of PRC1. Network 1 is notable for its inclusion of CBX4 itself (Supplemental Table S5, Fig. 2).
Several other networks stand out as particularly relevant to cumulus cell biology. Chief among these is network 8 (functionally annotated as cell cycle and connective tissue development), which contains two affected upstream regulators, cyclin-dependent kinase inhibitor 2A (CDKN2A) and prostaglandin endoperoxidase synthase 2 (PTGS2), both upregulated by Cbx4 siRNA treatment. PTGS2 plays a vital role ovulation, being induced by oocyte-derived growth and differentiation factor 9 (GDF9), and subsequently required for cumulus cell expansion ( 15, 17). Network 8 also encompasses many of the most prominent upstream regulators listed above, including TGFB1, TP53, tumor necrosis factor (TNF), CCND1, ERBB2, CDK4, retinoblastoma 1 (RB1), and low-density lipoprotein (LDL). The presence of these regulators also links this network to many of the other affected networks. Network 8 also incorporates the key cellular regulator peroxisome proliferator activated receptor gamma (PPARG), which is also affected by Cbx4 siRNA treatment. PTGS2 impinges directly on a number of these other key molecules, including TGFB1, TNF, ERBB2, and PPARG.
The FSH-related networks are networks 2, 10, 18, 22. Network 2 (endocrine system development and lipid metabolism) contains FSHR, which is upregulated by Cbx4 siRNA treatment and in turn affects other key regulators [TGFB1, TNF, ying-yang 1 (YY1), nuclear receptor family 5 A2 (NR5A2), and E2F transcription factor 6 (E2F6)]. Nearly all of the molecules in this network are affected by Cbx4 siRNA. Network 10 (cellular maintenance) elaborates connections with an even larger array of upstream regulators, including prominent regulators such as TGFB1, TP53, and ERBB2, as well as HNF4A, FBJ Osteosarcoma oncogene (FOS), interleukins, and the key endocrine regulator luteinizing hormone (LH). Network 18 (connective tissue disorders and immunological disease) encompasses two additional regulators that are affected by Cbx4 siRNA but not contained in network 8, collagen type 4 alpha 3 (COL4A3) and ERBB3 (also in network 10). Network 18 is notable for the inclusion of extracellular matrix components such as laminins and collagen. Network 22 (drug metabolism and carbohydrate metabolism) shares key regulators with the other networks (TNF, FSH, interleukins, LH) and also has unique ones [early growth response (EGR), mechanistic target of rapamycin (MTOR), NR4A1, and secreted phosphoprotein (SPP1)].
In considering the endocrine regulation of follicle cell function, we examined the lists of affected genes for components related to activin and inhibin signaling and responses. We observed reduced expression of mRNAs encoding Inhibin alpha (INHA), cytochrome P450 family member 11A1 (CYP11A1), hydroxy-delta-5-steroid dehydrogenase, 3 beta (HSD3B), steroidogenic acute regulatory protein (STAR), and follistatin like (FSTL1) (Supplemental Table S1), some of which are regulated by activin (in conjunction with estrogen stimulation) in granulosa or corpus luteum cells. We also observed increased expression of mRNAs encoding FSHR (positively regulated by activin), ACVR1, and SMADs 3 and 4 (mediators of activin responses) and BMP and activin membrane-bound inhibitor (BAMBI, a negative regulator of activin signaling) (Supplemental Table S2). Further evidence of a link between cumulus cell expressed CBX4 and oocyte quality is the increased expression of PTGS2 (a cumulus cell expressed gene associated with high quality oocytes) and its association with an affected network (no. 8, Fig. 4).
Fig. 4.
IPA network 8 (see Supplemental Table S6). Symbols are as described in Fig. 2.
We next submitted for IPA analysis a list of 95 genes that were affected by at least twofold by Cbx4 siRNA and also appeared on the published lists of PRC1 target genes. Biofunction analysis yielded significant associations with cell growth and proliferation, cell death and survival, cell cycle, cellular organization, cellular assembly, and cellular function and maintenance (Supplemental Table S6). An analysis of canonical pathways associated with the PRC1 target genes yielded highly significant results for many of the same categories, including those mentioned above with direct physiological relevance to folliculogenesis, such as estrogen signaling, aryl hydrocarbon signaling, and signaling through several hormones and growth factors (Supplemental Table S7). The results with the known PRC1 targets therefore reinforce the results obtained with the entire gene lists.
Effects of Cbx4 siRNA on cumulus markers of oocyte and embryo quality.
Many studies in recent years have examined genes expressed in cumulus cells at levels that differ according to whether the oocyte is deemed of high or low quality or subsequently judged to be of higher or lower developmental competence. Effects of Cbx4 siRNA on these genes would indicate possible effects of CBX4 on crucial differentiative parameters of cumulus cells and their appropriate specialization to support production of high-quality oocytes. We inspected our lists of affected genes to determine whether CBX4 might positively or negatively regulate cumulus cell phenotype in a way that could affect oocyte quality. We observed 12 alterations with Cbx4 siRNA that would correlate with increased oocyte quality and 13 that would correlate with lower oocyte quality ( Table 4).
Table 4.
Effects of Cbx4 siRNA on cumulus cell markers of oocyte quality
| Decreased | Increased | Predicted Cbx4 siRNA effect on Oocyte Quality | Predicted Cbx4 effect on Oocyte Quality | Reference | |
|---|---|---|---|---|---|
| High-Quality Markers | |||||
| ACPP | Lee et al., 2011 | ||||
| ADFP | Assidi et al., 2010 | ||||
| AKAP7 | √ | + | − | Assidi et al., 2010 | |
| ALCAM | Adriaenssens et al., 2010 | ||||
| ATP6V1C1 | Assidi et al., 2010 | ||||
| BCL2L11 | Assou et al., 2010 | ||||
| BCL2L11 | Assou et al., 2008, 2010 | ||||
| BTC | Assidi et al., 2008 | ||||
| CD44 | Assidi et al., 2008 | ||||
| CDC126 | Lee et al., 2011 | ||||
| CDC42 | √ | − | + | Hamel et al., 2008 | |
| CKB | √ | − | + | Lee et al., 2008 | |
| CYP19A1 | Hamel et al., 2008 | ||||
| EGFR | √ | + | − | Assidi et al., 2008 | |
| EGR3 | Lee et al., 2011 | ||||
| EREG | Assidi et al., 2010 | ||||
| FDX1 | √ | − | + | Hamel et al., 2008 | |
| FOSL2 | Lee et al., 2011 | ||||
| FSHR | √ | + | − | Caixeta et al., 2009 | |
| GHR | √ | + | − | Caixeta et al., 2009 | |
| GREM1 | √ | − | + | Adriaenssens et al., 2010; Assidi et al., 2008; McKenzie et al., 2004 | |
| GSTT1 | Ito et al., 2008 (granulosa) | ||||
| H2A | √ | + | − | Caixeta et al., 2009 | |
| HAS2 | Assidi et al., 2008, 2010; McKenzie et al., 2004 | ||||
| HIGD1A | √ | + | − | Assidi et al., 2010 | |
| HSD3B1 | √ | − | + | Hamel et al., 2008 | |
| HSPA5 | Assidi et al., 2010 | ||||
| HSPA8 | √ | − | + | Assidi et al., 2010 | |
| IGF1 | √ | − | + | Lee et al., 2011 | |
| INHBA | Assidi et al., 2008, 2010 | ||||
| IRS1 | √ | + | − | Lee et al., 2011 | |
| KLF6 | Lee et al., 2011 | ||||
| LEPR | √ | − | + | van Tol et al., 2010 | |
| PCK1 | Assou et al., 2010 | ||||
| PCK1 | Assou et al., 2010 | ||||
| PRDX2 | √ | − | + | Lee et al., 2011 | |
| PTGS2 | √ | + | − | Assidi et al., 2008,2010; McKenzie et al., 2004 | |
| PTX3 | Zhang et al., 2005 | ||||
| RPS6K2 (RPS6KA2) | √ | − | + | Adriaenssens et al., 2010 | |
| SDC4 | √ | − | + | Adriaenssens et al., 2010 | |
| SERPINE2 | Hamel et al., 2008 | ||||
| SLC18A2 | Assidi et al., 2010 | ||||
| SLC39A10 | √ | − | + | Assidi et al., 2010 | |
| SPROUTY4 | Adriaenssens et al., 2010 | ||||
| THBS1 | √ | + | − | Assidi et al., 2010 | |
| TNFAIP6 | √ | − | + | Assidi et al., 2010 | |
| UTMP | Assidi et al., 2010 | ||||
| VCAN | √ | + | − | Adriaenssens et al., 2010 | |
| Low-Quality Markers | |||||
| AQP11 | Lee et al., 2011 | ||||
| CLU | Lee et al., 2011 | ||||
| CTSB | √ | + | − | Bettegowda et al., 2008; Balboula et al., 2010 | |
| CTSS | Bettegowda et al., 2008 | ||||
| CTSZ | Bettegowda et al., 2008 | ||||
| FN1 | Lee et al., 2011 | ||||
| GMNN | √ | + | − | Lee et al., 2011 | |
| HRAS | Lee et al., 2011 | ||||
| HSD11B2 | Lee et al., 2011 | ||||
| HSD17B1 | Lee et al., 2011 | ||||
| HSD3B2 | Lee et al., 2011 | ||||
| HSDL1 | Lee et al., 2011 | ||||
| IGFBP4 | Lee et al., 2011 | ||||
| IGFBP5 | Lee et al., 2011 | ||||
| KCNK3 | Lee et al., 2011 | ||||
| NEK6 | Lee et al., 2011 | ||||
| NF1B | Assou et al., 2010 | ||||
| PR | Hasegawa et al., 2005 | ||||
| SMAD7 | Lee et al., 2011 | ||||
| STC1 | Lee et al., 2011 | ||||
Relationship of Cbx4 siRNA effects to cloned embryo gene expression defects.
Because cloned embryos overexpress the Cbx4 mRNA and display numerous somatic cell-like characteristics, we evaluated the degree to which CBX4 might contribute to the aberrant cloned embryo phenotype. We compared the lists of genes affected by Cbx4 siRNA in cumulus cells to the lists of genes showing altered transcription-dependent expression in our previous study of cloned embryos compared with normal embryos ( sets 2J and 2M) ( 57).
Reducing Cbx4 mRNA expression with siRNA should mediate an effect opposite to the effect of elevated Cbx4 mRNA expression in clones. We first compared the list of embryonically transcribed genes with decreased expression in two-cell cloned embryos ( set 2M) ( 57) to those with increased expression arising from Cbx4 siRNA treatment of clones and divided these between known PRC1 targets and other genes as indicated in Supplemental Tables S1 and S2. These would be genes that would be repressed by CBX4 in both cumulus cells and clones. We observed three and 28 probe sets shared between the lists of underexpressed genes in clones and the lists of Cbx4 siRNA-enhanced PRC1 targets and other probe sets, respectively, corresponding to 1.0 and 9.3% of the 302 set 2M ( 57) probe sets (Supplemental Table S8).
Increased expression of genes in clones due to higher CBX4 expression could arise by the alleviation of indirect gene repression mediated by direct CBX4 targets, and these genes would be expressed at reduced levels in Cbx4 siRNA treated cumulus cells. We found 12 probe sets (11 genes) that were shared between the list of embryonically transcribed, overexpressed mRNAs in clones ( set 2J) ( 57) and the list of siRNA-diminished known PRC1 targets in cumulus cells, and 116 probe sets shared with the list of other siRNA-diminished probe sets, corresponding to 1.36 and 13.2%, respectively, of the 880 probe sets for genes overexpressed and embryonically transcribed in cloned two-cell stage embryos (Supplemental Table S8). Combining these two sets of genes regulated oppositely in clones vs. siRNA-treated cumulus cells yielded 150 out of 1,182 (12.7%) of the affected transcribed genes in cloned two-cell stage embryos.
We also observed genes that were regulated in the same direction in clones compared with normal embryos as by Cbx4 siRNA treatment of cumulus cells. We observed 57 probe sets (54 annotated genes) that were repressed in clones and also reduced with Cbx4 siRNA treatment of cumulus cells, only two of which were reported PRC1 targets (Supplemental Table S8). We also observed 86 probe sets (84 annotated genes) that were elevated in clones and also elevated by Cbx4 siRNA treatment of cumulus cells, two of which were reported PRC1 targets (Supplemental Table S8).
Combining the results of the inversely regulated gene sets and the genes that were affected in the same directions yields a total of 302 probe sets for genes sensitive to Cbx4 knockdown in cumulus cells genes and having altered embryonic transcription-dependent expression in clones (25.6% of the affected 1,182 genes), nearly half of which are potentially regulated differently between the two cell types.
DISCUSSION
The polycomb repressor complex 1 (PRC1) regulates gene expression and cellular differentiation in diverse cell types. The data here provide, for the first time, evidence indicative of a role for the PRC1 component CBX4 in cumulus cell development and differentiation. We observed widespread effects of Cbx4 siRNA treatment on the expression of genes related to diverse processes in cultured mouse cumulus cells, implicating CBX4 as a key component in the overall pathway integrating endocrine signals, intraovarian paracrine factors, and oocyte-derived factors in the control of cumulus cell function. Because cumulus cell differentiation and oocyte quality are intrinsically linked ( 2– 7, 10, 13, 33), these observations indicate an important role in CBX4 in controlling reproductive function in female mammals. Moreover, finding that 25 of the genes proposed to be cumulus cell-expressed markers of oocyte quality are affected by Cbx4 knockdown in a manner that could either increase or decrease oocyte quality indicates that fine regulation of CBX4 expression is likely important for generating high-quality oocytes. We also confirmed that CBX4 regulates genes that are aberrantly expressed in cloned embryos.
Our results provide novel information about the functions of CBX4. First, our results reveal for the first time functions of CBX4 in cumulus cells. Second, our results reveal effects of CBX4 on a variety of other regulatory factors in cells, some direct effects, and some indirect effects. Some of the observed effects were not previously in the IPA knowledge database, indicating newly discovered functions. An overview of the CBX4 functions in cumulus cells can be inferred from the effects of Cbx4 siRNA treatment, connections to members of affected networks, and the affected biological functions associated with the affected networks (Supplemental Table S9 and Fig. 6). The results indicate that CBX4 directly increases or decreases the activities of multiple regulators, which in turn act via a variety of networks to affect a range of biological processes. Multiple interactions of CBX4 with members of the affected networks indicate that CBX4 plays a role in the elaboration of a range of biological functions ( Fig. 6 and Supplemental Table S5, column F).
Fig. 6.
Summary of connections between CBX4 expression and cumulus cell characteristics. CBX4 activates or inhibits the expression of a variety of upstream regulators, which in turn regulate downstream gene networks that control the indicated cellular processes. *The observed effects of Cbx4 siRNA on FOXO1 and NUPR1 are opposite to the effects predicted by IPA on the basis of changes in the expression of downstream network members.
The IPA indicates that in cumulus cells CBX4 regulates genes that are associated with cellular proliferation and cellular differentiation, as it does in other cell types. The IPA analysis also revealed effects on genes that are associated with the specific developmental transitions in these cells related to folliculogenesis, ovulation, and other processes in the ovary. CBX4 may modulate cumulus cell functions during folliculogenesis by affecting transcriptional responses to diverse paracrine and endocrine stimuli including FSH, activin, growth hormone, and insulin, as well as oocyte-derived factors such as the TGFβ family members, GDF9 and bone morphogenetic protein 15 (BMP15).
Our results indicate that part of CBX4 effects may be mediated via genes in the activin response pathway. We observed reduced expression of several genes that are negatively regulated by activin in granulosa cells ( Inha, Cyp11a1, Hsd3b, Star) and increased expression of the Fshr gene, which is positively regulated by activin in granulosa cells. These effects are not completely in concordance with reported effects of activins in granulosa cells but indicate that some of the genes that are regulated by activin and inhibin in granulosa cells are sensitive to CBX4 modulation in cumulus cells. The array of affected genes in cumulus cells and the manner in which they are regulated may be distinct from activin and inhibin effects in granulosa cells and other cell types. The responses of cumulus cells to endocrine stimuli could differ markedly from granulosa cells, and so the effects of CBX4 could likewise differ. The responses of cumulus cells to such stimuli have not been studied in detail. CBX4 may thus contribute to the responses of cumulus cells to activin and inhibin.
Treatment with the Cbx4 siRNA increased array expression values for mRNAs encoding FSHR and PTGS2, two key regulators of ovarian biology, key for promoting cumulus cell differentiation and expansion. PTGS2 provides an essential link in the pathway by which the cellular activities of the cumulus cell are integrated with the needs of the developing oocyte, culminating in successful ovulation of a high-quality oocyte for fertilization. PTGS2 is essential for cumulus cell function, cumulus cell expansion, and ovulation. Its expression is regulated by oocyte-derived GDF9, and this in turn regulates numerous downstream pathways ( 15, 18). FSH stimulation is key for granulosa cell differentiation and folliculogenesis ( 25) and stimulates cumulus cell expansion ( 20). Oocyte-derived BMP15 inhibits FSHR expression and thereby coordinates granulosa cell proliferation and differentiation ( 46). GDF9 promotes granulosa cell proliferation and inhibits FSH-induced granulosa cell differentiation ( 39) but enables FSH-induced cumulus cell expansion ( 16). Mouse and human GDF9:BMP15 heterodimers potently stimulate cumulus cell expansion ( 47). Gene expression in cumulus cells is sensitive to the amount of FSH stimulation ( 49).
Our data indicate that CBX4 may normally discourage cumulus cell differentiation and/or expansion. This may be in part by modulating expression of FSHR and PTGS2 in cumulus cells. Prominent affected networks include those related to cell proliferation and DNA replication/repair, cell assembly and morphology, and endocrine system development and function. Accounting for directionality of effects on biofunctions ( z scores, Supplemental Table S3), the broader effects of Cbx4 siRNA were to inhibit cell proliferation and promote cell death, indicating a possible role for CBX4 in promoting cell proliferation and survival in these cells. These effects may involve a combination of upstream regulators ( Table 3 and Supplemental S5) acting through multiple signaling pathways. It is possible that CBX4 expression itself responds to exogenous factors such as FSH, BMP15, and GDF9. The impact of Cbx4 siRNA on the expression of FSHR and PTGS2 indicates that CBX4 may modulate changes in gene expression brought about by exogenous factors, such as FSH and estrogen, and oocyte-derived factors. However, additional effects of Cbx4 siRNA on receptors that function as upstream regulators, such as epidermal growth factor receptor (EGFR), leptin receptor (LEPR, a PRC2 target), bone morphogenetic protein receptor type 2 (BMPR2), and colony stimulating factor 1 receptor (CSF1R), suggest that CBX4 expression regulates cumulus cell responses to a range of exogenous ligands. And effects of Cbx4 siRNA on a range of nuclear factors ( Table 3) suggest an even broader role for CBX4 in controlling cumulus cell differentiation. Interactions between TGFB1, FSH, and forkhead box 1 (FOXO1) in the regulated networks emphasize the opportunities for such effects. For example, CBX4 effects on FOXO1 could underlie the CBX4 support of cell survival. Collectively, these observations implicate CBX4 as a key regulator that may help integrate a range of stimuli into a definitive change in cell state leading to establishment of a developmentally competent oocyte. This ability of CBX4 to fine-tune cumulus cell state may account for its control in cumulus cells of genes for which expression correlates either positively or negatively with oocyte quality.
The dynamic nature of the cumulus cell differentiated state likely adds to the complexity of CBX4 effects. Our recent studies in the rhesus monkey indicate that cumulus cells, which manifest a phenotype distinct from mural granulosa cells prior to ovulation, transition to a more granulosa-like state during ovulation ( 14). Here, Cbx4 siRNA was applied to cumulus cells that were obtained from ovulated MII stage oocytes and then cultured in the absence of oocytes. A more granulosa-like state achieved during ovulation could be sustained during culture, providing a valuable opportunity for its study. The results described here may thus reflect how CBX4 contributes to the establishment of this unique cell state. Additionally, increases in expression of matrix metalloproteinase mRNAs raises the interesting possibility that cumulus cells may undergo a degenerative process sharing some features with luteolysis, which normally may work with apoptotic processes to facilitate the release of the fertilized oocyte from its associated cumulus cells after fertilization. CBX4 may modulate this transition as well.
The results presented here also support our previous conclusion that continued expression of donor cell transcription regulators contributes to the aberrant regulation of genes in early-stage cloned embryos. Approximately one-quarter of the aberrantly expressed genes in clones appear to be responsive to the level of CBX4 expression in cumulus cells. About half of these genes may affect the differentiative abilities of cells in the early-stage clones. Because CBX4 promotes lineage commitment and differentiation in stem cells, its apparent disruption of downstream genes in clones could alter cell lineage allocation during preimplantation development. We note that one of the genes affected in common between cumulus cells and clones is Kruppel-like factor 4 ( Klf4), which has a widely known role in regulating cellular pluripotency ( 52). CBX4 effects on DNA methyltransferases could also contribute to epigenetic abnormalities in clones.
The finding that nearly half of the transcribed genes that showed altered expression in clones and were sensitive to Cbx4 siRNA treatment of cumulus cells may respond to CBX4 expression differently between the two cell types indicates that, as a result of nuclear reprogramming, the transcription response to CBX4 has been altered by the late two-cell stage but that this reprogramming remains incomplete by the time gene transcription initiates in the cloned embryo. This is consistent with our earlier conclusion that reprogramming occurs over a prolonged period of time, and, as a consequence, clones elaborate a phenotype that is intermediate between somatic cell and normal embryo. This result also indicates that downstream effects of CBX4 are cell context dependent. The further study of CBX4 effects on gene regulation through the comparison of normal and cloned embryos could provide new insight into how the role of CBX4 and other PRC1 components changes as early development progresses. Additionally, the manipulation of other transcription factors in somatic cells combined with the analysis of their effects in normal and cloned embryos could provide a novel approach for understanding the stage-specific effects of other transcription factors during early development, as well as the degree to which the activities of such factors are amenable to the reprogramming process.
In summary, the results presented here for effects of Cbx4 siRNA on cumulus cells indicate a complex role of the PRC1 complex in controlling cumulus cell gene expression and may advance our understanding of infertility. Cbx4 siRNA exerted many effects in cumulus cells, including changes in the expression of a wide array of receptors and transcriptional regulators. This suggests that CBX4 exerts a broad range of effects in cumulus cells mediated by numerous downstream pathways and networks that contain these key regulators as key nodes of interactions. Additionally, these effects intersect in networks that are responsive to exogenous factors such as TGFB, interleukins, and endocrine factors. This suggests that CBX4 may play a key role during folliculogenesis by modulating cellular responses to these factors and highlights the likely sensitivity of cumulus-oocyte complexes to the maternal endocrine milieu. These results also indicate that modifying the availability of such factors could provide one approach to modifying cumulus cell function and thereby modifying oocyte quality. The effects of Cbx4 knockdown on previously reported markers of oocyte quality are consistent with a need for fine regulation of CBX4 expression during the production of high-quality oocytes. Cumulus cells undergo a continuous evolution in cell phenotype so that they can meet the changing needs of the developing oocyte before and after ovulation. Further studies examining the roles of other PRC1 and PRC2 components should help to clarify how the complex and dynamic pattern of gene expression is orchestrated to support cumulus cell development, differentiation, and degeneration and how this contributes to production of high-quality oocytes. Additionally, understanding the potential role of genetic, epigenetic, and environmentally induced variation of CBX4 expression may help to explain and avoid incidences of reduced oocyte quality.
GRANTS
This work was support in part by National Institutes of Health Grants RO1HD043092 and R24OD012221.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.H. and J.G. performed experiments; L.H., U.M., J.G., and K.E.L. prepared figures; L.H., U.M., J.G., and K.E.L. edited and revised manuscript; L.H., U.M., J.G., and K.E.L. approved final version of manuscript; U.M., J.G., and K.E.L. analyzed data; U.M., J.G., and K.E.L. interpreted results of experiments; U.M. and K.E.L. drafted manuscript; K.E.L. conception and design of research.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank George Smith for critical comments on the manuscript.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Ackert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 233: 258–270, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Adriaenssens T, Wathlet S, Segers I, Verheyen G, De Vos A, Van der Elst J, Coucke W, Devroey P, Smitz J. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod 25: 1259–1270, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Assidi M, Dieleman SJ, Sirard MA. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction 140: 835–852, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Assidi M, Dufort I, Ali A, Hamel M, Algriany O, Dielemann S, Sirard MA. Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol Reprod 79: 209–222, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Assidi M, Montag M, Van der Ven K, Sirard MA. Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: a preliminary study. J Assist Reprod Genet 28: 173–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Assou S, Haouzi D, De Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol Hum Reprod 16: 531–538, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Assou S, Haouzi D, Mahmoud K, Aouacheria A, Guillemin Y, Pantesco V, Reme T, Dechaud H, De Vos J, Hamamah S. A non-invasive test for assessing embryo potential by gene expression profiles of human cumulus cells: a proof of concept study. Mol Hum Reprod 14: 711–719, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Balboula AZ, Yamanaka K, Sakatani M, Hegab AO, Zaabel SM, Takahashi M. Cathepsin B activity is related to the quality of bovine cumulus oocyte complexes and its inhibition can improve their developmental competence. Mol Reprod Dev 77: 439–448, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Bernstein E, Duncan EM, Masui O, Gil J, Heard E, Allis CD. Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol Cell Biol 26: 2560–2569, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bettegowda A, Patel OV, Lee KB, Park KE, Salem M, Yao J, Ireland JJ, Smith GW. Identification of novel bovine cumulus cell molecular markers predictive of oocyte competence: functional and diagnostic implications. Biol Reprod 79: 301–309, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441: 349–353, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Buccione R, Schroeder AC, Eppig JJ. Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod 43: 543–547, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Caixeta ES, Ripamonte P, Franco MM, Junior JB, Dode MA. Effect of follicle size on mRNA expression in cumulus cells and oocytes of Bos indicus: an approach to identify marker genes for developmental competence. Reprod Fertil Dev 21: 655–664, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Chaffin CL, Lee YS, Vandevoort CA, Patel BG, Latham KE. Rhesus monkey cumulus cells revert to a mural granulosa cell state after an ovulatory stimulus. Endocrinology 153: 5535–5545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1beta. Endocrinology 140: 2685–2695, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, Gilchrist RB. Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology 146: 2798–2806, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 13: 1035–1048, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Elvin JA, Yan C, Matzuk MM. Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc Natl Acad Sci USA 97: 10288–10293, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 122: 829–838, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Eppig JJ. Role of serum in FSH stimulated cumulus expansion by mouse oocyte-cumulus cell complexes in vitro. Biol Reprod 22: 629–633, 1980 [DOI] [PubMed] [Google Scholar]
- 21. Gao S, Chung YG, Williams JW, Riley J, Moley K, Latham KE. Somatic cell-like features of cloned mouse embryos prepared with cultured myoblast nuclei. Biol Reprod 69: 48–56, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Gao S, Latham KE. Maternal and environmental factors in early cloned embryo development. Cytogenet Genome Res 105: 279–284, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamel M, Dufort I, Robert C, Gravel C, Leveille MC, Leader A, Sirard MA. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod 23: 1118–1127, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Harlow CR, Davidson L, Burns KH, Yan C, Matzuk MM, Hillier SG. FSH and TGF-beta superfamily members regulate granulosa cell connective tissue growth factor gene expression in vitro and in vivo. Endocrinology 143: 3316–3325, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hasegawa J, Yanaihara A, Iwasaki S, Otsuka Y, Negishi M, Akahane T, Okai T. Reduction of progesterone receptor expression in human cumulus cells at the time of oocyte collection during IVF is associated with good embryo quality. Hum Reprod 20: 2194–2200, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ismail IH, Gagne JP, Caron MC, McDonald D, Xu Z, Masson JY, Poirier GG, Hendzel MJ. CBX4-mediated SUMO modification regulates BMI1 recruitment at sites of DNA damage. Nucleic Acids Res 40: 5497–5510, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito M, Muraki M, Takahashi Y, Imai M, Tsukui T, Yamakawa N, Nakagawa K, Ohgi S, Horikawa T, Iwasaki W, Iida A, Nishi Y, Yanase T, Nawata H, Miyado K, Kono T, Hosoi Y, Saito H. Glutathione S-transferase theta 1 expressed in granulosa cells as a biomarker for oocyte quality in age-related infertility. Fertil Steril 90: 1026–1035, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Kim SH, Park J, Choi MC, Park JH, Kim HP, Lee JH, Oh DY, Im SA, Bang YJ, Kim TY. DNA methyltransferase 3B acts as a co-repressor of the human polycomb protein hPc2 to repress fibroblast growth factor receptor 3 transcription. Int J Biochem Cell Biol 40: 2462–2471, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Klauke K, Radulovic V, Broekhuis M, Weersing E, Zwart E, Olthof S, Ritsema M, Bruggeman S, Wu X, Helin K, Bystrykh L, de Haan G. Polycomb Cbx family members mediate the balance between haematopoietic stem cell self-renewal and differentiation. Nat Cell Biol 15: 353–362, 2013 [DOI] [PubMed] [Google Scholar]
- 32. Lee YS, Latham KE, Vandevoort CA. Effects of in vitro maturation on gene expression in rhesus monkey oocytes. Physiol Genomics 35: 145–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee YS, Vandevoort CA, Gaughan JP, Midic U, Obradovic Z, Latham KE. Extensive effects of in vitro oocyte maturation on rhesus monkey cumulus cell transcriptome. Am J Physiol Endocrinol Metab 301: E196–E209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li B, Zhou J, Liu P, Hu J, Jin H, Shimono Y, Takahashi M, Xu G. Polycomb protein Cbx4 promotes SUMO modification of de novo DNA methyltransferase Dnmt3a. Biochem J 405: 369–378, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu B, Liu YF, Du YR, Mardaryev AN, Yang W, Chen H, Xu ZM, Xu CQ, Zhang XR, Botchkarev VA, Zhang Y, Xu GL. Cbx4 regulates the proliferation of thymic epithelial cells and thymus function. Development 140: 780–788, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Long J, Zuo D, Park M. Pc2-mediated sumoylation of Smad-interacting protein 1 attenuates transcriptional repression of E-cadherin. J Biol Chem 280: 35477–35489, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Luis NM, Morey L, Mejetta S, Pascual G, Janich P, Kuebler B, Cozutto L, Roma G, Nascimento E, Frye M, Di Croce L, Benitah SA. Regulation of human epidermal stem cell proliferation and senescence requires polycomb-dependent and -independent functions of Cbx4. Cell Stem Cell 9: 233–246, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296: 2178–2180, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Mazerbourg S, Hsueh AJ. Growth differentiation factor-9 signaling in the ovary. Mol Cell Endocrinol 202: 31–36, 2003 [DOI] [PubMed] [Google Scholar]
- 40. McCall MN, Murakami PN, Lukk M, Huber W, Irizarry RA. Assessing affymetrix GeneChip microarray quality. BMC Bioinformatics 12: 137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, Amato P, Matzuk MM. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod 19: 2869–2874, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Merrill JC, Melhuish TA, Kagey MH, Yang SH, Sharrocks AD, Wotton D. A role for non-covalent SUMO interaction motifs in Pc2/CBX4 E3 activity. PLoS One 5: e8794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morey L, Pascual G, Cozzuto L, Roma G, Wutz A, Benitah SA, Di Croce L. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem Cell 10: 47–62, 2012 [DOI] [PubMed] [Google Scholar]
- 44. O'Loghlen A, Munoz-Cabello AM, Gaspar-Maia A, Wu HA, Banito A, Kunowska N, Racek T, Pemberton HN, Beolchi P, Lavial F, Masui O, Vermeulen M, Carroll T, Graumann J, Heard E, Dillon N, Azuara V, Snijders AP, Peters G, Bernstein E, Gil J. MicroRNA regulation of Cbx7 mediates a switch of Polycomb orthologs during ESC differentiation. Cell Stem Cell 10: 33–46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oh Y, Chung KC. Small ubiquitin-like modifier (SUMO) modification of zinc finger protein 131 potentiates its negative effect on estrogen signaling. J Biol Chem 287: 17517–17529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem 276: 11387–11392, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA 110: E776–E785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. R Development Core Team R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, p. 409, 2009 [Google Scholar]
- 49. Sanchez F, Adriaenssens T, Romero S, Smitz J. Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice. Biol Reprod 83: 514–524, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Satijn DP, Olson DJ, van der Vlag J, Hamer KM, Lambrechts C, Masselink H, Gunster MJ, Sewalt RG, van Driel R, Otte AP. Interference with the expression of a novel human polycomb protein, hPc2, results in cellular transformation and apoptosis. Mol Cell Biol 17: 6076–6086, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135: 111–121, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Tolhuis B, de Wit E, Muijrers I, Teunissen H, Talhout W, van Steensel B, van Lohuizen M. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat Genet 38: 694–699, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Tol HT, Vernooij JC, Colenbrander B, Gutknecht D, Macklon NS, Roelen BA. Expression of leptin receptor mRNA in cumulus cells is correlated with expression of PTX3. Reprod Biomed Online 20: 741–750, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Vandamme J, Völkel P, Rosnoblet C, Le Faou P, Angrand PO. Interaction proteomics analysis of polycomb proteins defines distinct PRC1 complexes in mammalian cells. Mol Cell Proteom 10: M110.002642, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vassena R, Han Z, Gao S, Baldwin DA, Schultz RM, Latham KE. Tough beginnings: alterations in the transcriptome of cloned embryos during the first two cell cycles. Dev Biol 304: 75–89, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang X, Jafari N, Barnes RB, Confino E, Milad M, Kazer RR. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil Steril 83, Suppl 1: 1169–1179, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.