Abstract
The upper airway is a complex muscular tube that is used by the respiratory and digestive systems. The upper airway is invested with several small and anatomically peculiar muscles. The muscle fiber orientations and their nervous innervation are both extremely complex, and how the activity of the muscles is initiated and adjusted during complex behaviors is poorly understood. The bulk of the evidence suggests that the entire assembly of tongue and laryngeal muscles operate together but differently during breathing and swallowing, like a ballet rather than a solo performance. Here we review the functional anatomy of the tongue and laryngeal muscles, and their neural innervation. We also consider how muscular activity is altered as respiratory drive changes, and briefly address upper airway muscle control during swallowing.
Keywords: control of breathing, larynx, pharynx, respiratory muscles, swallowing
controlling upper airway muscles is one of the most daunting problems that the mammalian central nervous system must solve. Patterns of muscle control must rapidly alternate between different system requirements for respiration, speech, swallowing, and upper airway protection. Breathing and swallowing, the subject of this brief review, are highly automated behaviors driven by central pattern generators. We review the functional anatomy and neural innervation of tongue and laryngeal muscles, and how the muscle activities are altered as respiratory drive changes and when swallowing is required. We also consider how muscle activity is altered by central nervous system state, chemoreceptor stimulation, changes in lung volume, and during swallowing. We also try to identify important gaps in our knowledge and address the hurdles that limit our understanding of these motor systems. Because this is a brief review, we had to be selective in the topics covered and in the use of citations. As a result, we regret that many outstanding studies of upper airway muscle function during breathing and swallowing could not be reviewed and that consideration of the crucial role played by muscles of the pharyngeal wall also had to be passed over.
MAMMALIAN TONGUE MUSCLES: FUNCTIONAL ANATOMY AND INNERVATION
The anatomy of mammalian tongue muscles has been reviewed extensively (13, 14, 83, 123, 147) and will be summarized briefly here. Four of the tongue muscles are extrinsic muscles, which originate on bony structures or connective tissue and insert into the tongue body (the genioglossus, hyoglossus, styloglossus, and palatoglossus),1 and four are intrinsic muscles (inferior longitudinalis, superior longitudinalis, transversus, and verticalis) in which the fibers are wholly contained within the tongue body, or blade (reviewed in 37, 38), though there is evidence that some intrinsic fibers attach to the hyoid bone (44, 46). The tongue and other upper airway muscles are small compared with other respiratory muscles, their fibers intermingle extensively within the tongue body, and their innervation is complex (Fig. 1, A and B) (see 37, 91, 92, 97, 98, 137, 140). Collectively, these unique muscles produce a variety of intricate but well-controlled movements, including retraction, protrusion, elevation, and depression of the tongue, as well as alterations in tongue shape and stiffness. Because the tongue is unattached on one end, it can move with almost infinite degrees of freedom, and force production by one muscle depends on the cocontraction of some or all of the other muscles.
Fig. 1.
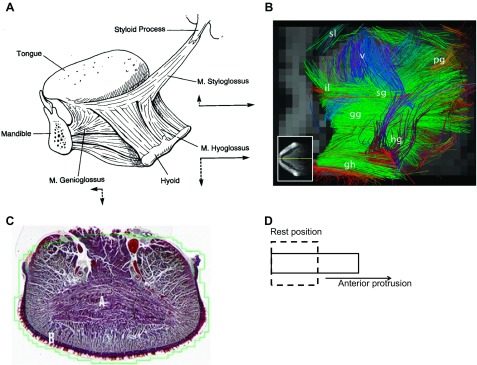
Anatomy of the human tongue musculature. A highlights the extrinsic musculature, with the arrows showing the approximate movement of the tongue when one of the muscles is stimulated in isolation. [Borrowed with permission from (38).] B shows extrinsic and intrinsic fibers of the human tongue using in vivo imaging with tractography. Sl, superior longitudinalis; il, inferior longitudinalis; pg, palatoglossus; v, verticalis; sg, styloglossus; gg, genioglossus; hg, hyoglossus; gh, geniohyoid. The intermingling of extrinsic and intrinsic fibers is obvious. Note that the transversus muscle is not easily seen in sagittal section, as is the case here. [Borrowed with permission from (44).] C: transverse section through the human tongue blade to show the transversus muscle fibers, which are labeled with an A. D is a simplified model of a muscular hydrostat. Activation of transversus and verticalis fibers (and likely vertically oriented fibers of the genioglossus and hyoglossus) reduces the lateral diameter of the box (i.e., the tongue) while increasing the length of its anterior-posterior axis, resulting in elongation with no change in volume. This change in shape, together with forward movement of the tongue base (driven largely by the genioglossus muscle) results in protrusion and anterior displacement of the tongue tip (see text).
The tongue muscles are typically divided functionally into protrudors or retractors, reflecting their principal actions. Retractor muscles pull the tongue backward, toward the posterior pharyngeal wall, and include the extrinsic hyoglossus and styloglossus, and the intrinsic inferior and superior longitudinalis muscles. Protrudor muscles move the tongue blade and base forward, and include the extrinsic genioglossus, and the intrinsic verticalis and transversus muscles. Although classifying the muscles in this way is convenient, precisely how the tongue moves when the muscles are stimulated in isolation is poorly understood. With care, selective stimulation of protrudor and retractor muscle groups, as well as some of the intrinsic muscles, can be done in rats (8, 40, 47) and human subjects (104, 129), but movements other than simple protrusion or retraction are difficult to quantify. It does appear that both the genioglossus and hyoglossus also cause depression of the tongue base, sharing this common action. This may explain why coactivating protrudor and retractor muscles both dilates and stiffens the pharyngeal airway in both rodent (41) and human (32, 104) models (see below). The styloglossus causes elevation of the sides of the tongue blade, as well as retraction.
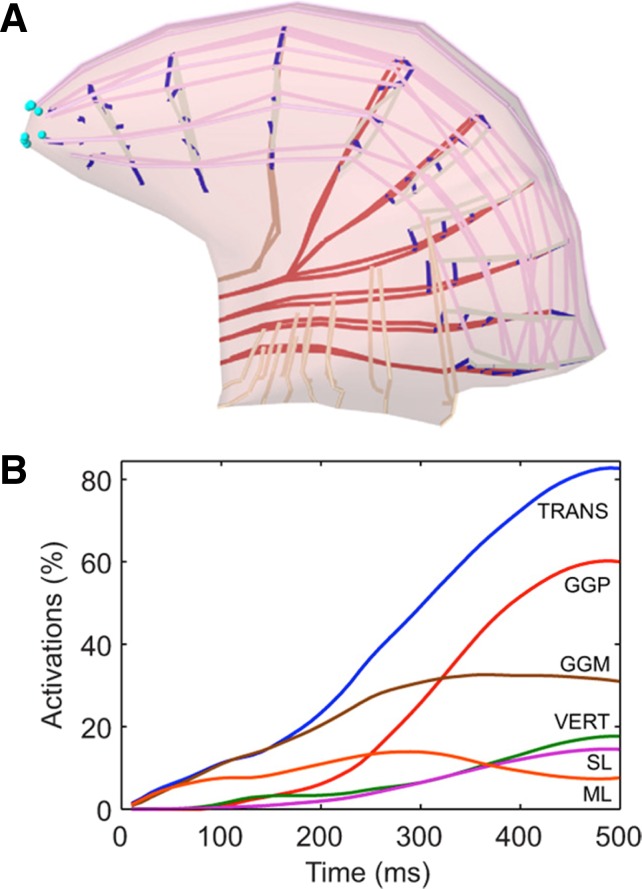
The intrinsic muscles are most often associated with changes in tongue shape (139), though they clearly assist in protrusion and retraction (see below). Most of what we know of intrinsic muscle actions has been inferred from the elegant modeling work of Kier and colleagues (65, 101, 65a), where they developed the notion of the tongue as a muscular hydrostat. A muscular hydrostat can move by displacing its tissues, while also providing the structural support that the movement requires. Hydrostats take advantage of their high water content, and thus incompressible nature, to elongate, shorten and stiffen without changing volume. In a constant volume structure, opposing changes along the anterior-posterior axis must be offset by changes along the lateral axis (Fig. 1C). As such, longitudinalis muscle contraction is purported to increase the lateral axis (making the tongue thicker in the transverse plane) while shortening the anterior-posterior axis and causing an effective retraction. The verticalis and transversus muscles are thought to shorten the lateral axis of the tongue, thereby increasing the anterior-posterior axis and causing an effective protrusion. As pointed out by Gaige and colleagues (44, 46), muscular hydrostats require fibers that are arranged in multiple orientations around the long axis. Moreover, the different fibers interdigitate extensively in the tongue body so activating motor units across the muscle fiber populations allows an enormous number of potential movements, and may afford the system with the ability to accomplish a given task with different sets of muscles and muscle fibers. Such redundancy could be important in conditions such as stroke or following carotid endarterectomy, wherein 5% or more of patients suffer hypoglossal nerve injury, but the ability to protect the airway, to chew, to swallow, and to speak are only mildly affected or can be relearned (12, 49). A major tenet of the muscular hydrostat hypothesis that is often overlooked is that the model requires that multiple muscles participate in all tongue movements. This thesis is confirmed in modeling studies, wherein anterior displacement of the tongue requires the bilateral activation of the genioglossus muscle but also activation of the intrinsic transversus, verticalis, and superior longitudinal muscles (144) (Fig. 2).
Fig. 2.
Schematic diagram showing the results of simulated tongue protrusion. A depicts forward displacement of the tongue tip and base from the resting position (dashed line). The fibers of different muscles are color coded and correspond to the nomenclature in B. B shows the percentage activation of each of the participating tongue muscles during the simulated protrusion. Note that three of the intrinsic muscles assist the posterior and medial fibers of the genioglossus. TRANS, transversus; GGP, genioglossus posterior; GGM, genioglossus medial; VERT, verticalis; SL, superior longitudinalis; ML, mylohyoid. [Borrowed with permission from (144).]
Branches of the hypoglossal nerve supply all of the tongue muscle fibers. The main nerve trunk bifurcates into large medial and lateral branches as it approaches the muscles, with the medial branch containing axons supplying the protrudor muscles (both intrinsic and extrinsic muscles) and the lateral branch the retractors. The intrinsic muscle branches tend to be distal and are very small and numerous, making selective denervation or stimulation extremely difficult. There have been several studies of the musculotopic organization of the hypoglossal motor nucleus, and some general rules emerge though there are likely important species differences (31, 47, 48, 70, 91, 92, 140, 148). Careful muscular isolation and tracer injection studies by McClung and Goldberg in adult rats (90, 91) show that the ventral subdivision of the nucleus contains a greater proportion of protrudor muscle motoneurons, while the dorsal subdivision contains more retractor motoneurons. Their data also show that intrinsic and extrinsic muscle motoneurons overlap extensively, and extend from about 0.9 mm below the obex to about 0.9 mm above, with the extrinsic column a few hundred microns longer than the intrinsic column (91). There are also interesting data showing that there may be some musculotopic organization within a tongue muscle. For example, the horizontal fibers of the genioglossus muscle, which pull the base of the tongue forward, and the vertical fibers which likely participate in tongue shaping, are innervated by spatially distinct populations of motoneurons (92). Since the dendrites of hypoglossal motoneurons branch extensively and extend for at least 500 μm even in juvenile rats (102), it is easy to envision synaptic coupling between motoneurons that are separated by long distances; thus the functional significance of musculotopic organization of the hypoglossal motoneuron pool is unknown.
When the complexity of the muscular and nervous anatomy of the tongue is considered, it is clear why accessibility to either nerves or muscles has dictated the nature of physiologic studies on the tongue motor system and therefore what we know about it. For example, the great majority of all experiments of respiration-related control of the tongue muscles have used the genioglossus muscle or the main trunk of the hypoglossal nerve as the index of respiration-related motor drive to the tongue musculature. As a result, we have learned a lot about the function of the genioglossus muscle or how all hypoglossal motoneurons behave in concert, but our understanding of tongue function in breathing and swallowing is incomplete, simply because the actions of the majority of its muscles have not been studied. Future work should be focused on enhancing our understanding of the function of all tongue muscles, including how the different muscles interact to produce the complex movements observed in breathing, swallowing, mastication, and speech. Similarly, studies of whole hypoglossal nerve activity or hypoglossal motoneurons without identifying their muscular targets provides no information about the activation of a specific muscle (see 37).
Finally, although the general features of tongue muscle anatomy, innervation, and function are similar in commonly used experimental mammals, such as rats, cats, and mice, the human tongue musculature and its innervation do differ in some respects. For example, the human tongue musculature tends to have a relatively dense neural innervation, variable patterns of motor end-plate morphology and density, and in some muscles, including the genioglossus, examples of neuromuscular compartmentalization. However, the majority of the specializations in human tongue muscle innervation are found in the intrinsic muscles (97). The authors of this work (97) suggest that these observations are consistent with improved control of tongue shape in humans compared with lower mammals, likely in support of speech, as has long been recognized (145). In the context of the present review, these specializations may lead to species differences in the regulation of pharyngeal airway size and/or stiffness by tongue muscle contraction. A variety of studies suggest that qualitative differences, however, are few, despite notable differences in pharyngeal airway size and shape (and the distance between the epiglottis and the tip of the soft palate) between humans and lower mammals. For example, chemoreceptor stimulation coactivates tongue protrudor and retractor muscles in humans (88) and rats (40); the control of pharyngeal constrictor muscles during chemoreceptor stimulation is similar in cats and human subjects (74); and coactivation of protrudor and retractor muscles similarly stiffens and dilates the pharynx in humans (32, 104) and rats (41). Thus the tongue musculature, its general neural innervation patterns (97), and, importantly, its mechanical actions are remarkably well conserved. Accordingly, in the following sections species differences will be addressed only when relevant.
MAMMALIAN TONGUE MUSCLES: RESPONSE TO CHANGES IN RESPIRATORY DRIVE
The hypoglossal motor nucleus receives both excitatory and inhibitory synaptic input from many brain regions. Direct inputs originate in the nucleus of the solitary tract, the majority of the reticular nuclei, the principal and spinal trigeminal nuclei, reticularis subcoeruleus, the caudal Raphe nucleus, and the Kolliker-Fuse nucleus (149). These regions, in turn, receive higher order inputs from the frontal, insular and parietal cortices, several cerebellar nuclei, the substantia nigra, the superior colliculus, and the mesencephalic trigeminal nucleus (149). Many of these regions modulate ventilatory drive, with the reticular formation containing the interneurons that link the central respiratory drive from the pre-Bötzinger complex with the hypoglossal motor nucleus (68, 69). The activity in most of these brain regions is highly dependent on central nervous system state, with the wakefulness drive reduced in sleep, and increased in exercise.
The role of central nervous system state in controlling drive to hypoglossal motoneurons, and thus tongue muscles, has attracted considerable interest since reductions in genioglossus muscle electromyographic (EMG) activity during sleep was first described (116, 128). This topic has been reviewed extensively in recent years (29, 43, 51, 71, 85), and there is an emerging consensus that non-rapid eye movement sleep is associated with reductions in excitatory neuromodulatory inputs primarily from noradrenergic (1, 36), serotonergic (19, 36, 80, 141), and cholinergic neurons (16, 24, 82), while during REM sleep there is also direct synaptic inhibition of hypoglossal motoneurons (33, 42). These observations have, in turn, led to several studies of the influence of hypercapnia, hypoxia, and lung volume–related reflexes on the drive to hypoglossal motoneurons and tongue muscles.
Whole hypoglossal nerve activity, which provides an index of the summed activity of all motor axons in electrical contact with the recording electrode, increases with hypercapnia and hypoxia in animal models (15, 21, 56, 73, 96, 142, 154). EMG recordings also consistently show that hypercapnia and hypoxia increase drive to the genioglossus muscle in various species (5, 9, 40, 53, 94, 130, 150), including humans (62, 66, 88, 103, 105, 107–109, 143). More recent studies have shown that drive to both the hyoglossus and genioglossus muscles increases in parallel with hypercapnia or hypoxia in animal models (9, 10, 40, 58) and in humans (88). Similarly, intrinsic tongue muscles are also coactive with extrinsic muscles, with both populations increasing their activity in hypercapnia (5) and hypoxia (9). Taken together, these results are consistent with excitation of a large fraction of the hypoglossal motor neuron pool with stimulation of central and/or peripheral chemoreceptors.
Tracheal occlusion in animal models or breath holding in human subjects also coactivates genioglossus and hyoglossus muscles, hyoglossus (88), and the activity recorded from the medial and lateral hypoglossal nerve branches in rodents (40, 81), consistent with strong, broadly distributed inhibition of hypoglossal motoneurons by pulmonary stretch receptor afferents (10, 55, 72). Similarly, experimentally removing lung volume feedback with brief (1–2 respiratory cycles) airway occlusions in animal models increases both genioglossus, hyoglossus, and intrinsic tongue muscle activities (5, 6, 9, 10), showing that lung inflation mediated inhibition of hypoglossal motoneurons is distributed broadly to motoneurons innervating multiple tongue muscles. Both genioglossus and hyoglossus activities are also markedly inhibited following spontaneously augmented breaths (59), indicating that the results of studies using airway occlusions do not reflect experimental artifact. In addition to inputs from the pulmonary airways and central and peripheral chemoreceptors, hypoglossal motoneurons also receive afferent inputs from mechanoreceptors in the upper airway mucosa, both in animal models and in human subjects (25, 52, 87, 89, 118, 133). Recent studies show that negative pressure pulses applied to the pharynx results in coactivation of the genioglossus and hyoglossus muscles in anesthetized rats (119), again suggesting that afferent inputs to hypoglossal motoneurons are distributed throughout the motoneuron pool.
Although the bulk of the work on central nervous system state changes and neural drive to tongue muscles has focused on differences between wakefulness and sleep, exercise affords an opportunity to examine the influence of a heightened central nervous system state on neural drive to the tongue muscles. Studies in exercising rodents are equivocal, with one study showing that treadmill exercise training (5 days/wk for 8 wk) altered the balance of myosin heavy chain subtypes in the genioglossus muscle (67), consistent with muscle activation during the exercise training sessions. However, another group did not find changes in myosin heavy chain phenotype or oxidative capacity in the genioglossus following treadmill exercise performed 4 times per week for 12 wk (151). Although these observations in rodents suggest that the tongue muscles may not be vigorously activated during exercise, we are unaware of any measures of rodent tongue muscle activities (e.g., EMG) during exercise. In other words, the extent of upper airway muscle activation in rodents may be insufficient to induce cellular changes in the muscle fibers. In exercising horses, bilateral hypoglossal nerve block induced nasopharyngeal narrowing, suggesting that the tongue muscles play an important role in equine airway defense during exercise (27). Studies in human subjects show that bicycle exercise increases the genioglossus muscle EMG activity in an intensity-dependent manner (134, 156), with the EMG reaching 40% of its maximal value at peak exercise intensities (156). Interestingly, the drive to the genioglossus was shown to be the same whether the subjects exercised while upright or supine (156), but the activity did increase briskly as soon as subjects switched from nasal to oronasal breathing. The latter observation suggests that drive to the genioglossus muscle depends importantly on changes in jaw position (84) and/or activation of pressure-sensitive mechanoreceptors in the mouth and pharynx.
Studies of the respiration-related activities of single hypoglossal motoneurons or genioglossus muscle motor units have been reviewed recently (4, 45). The discharge pattern in animal models tends to be very strongly inspiratory modulated (19, 54, 55, 61, 95, 135), though limited numbers of neurons with other discharge patterns have been observed in cats (54, 55, 95, 135). In contrast, recordings of motor unit discharge in human subjects do show phasic inspiratory discharge, but many of these units show mainly tonic discharge, with an increase in their firing rate during the inspiratory phase (4, 7, 117). Saboisky et al. (120) have described units with apparently rigid discharge patterns that confine them to fire in expiration, in inspiration or tonically. On the basis of these observations, they suggested that there must be unique premotor inputs that also have one of these three stereotyped discharge patterns and convey this information, apparently unaltered, to hypoglossal motoneurons. Of course, an alternate interpretation is that factors such as posture, central nervous system state, and upper airway mechanics dictate whether excitatory synaptic input, and thus motoneuron activation, is phasic or tonic (4), providing units with the flexibility to switch their discharge pattern. Support for this latter point is nicely illustrated in exercising human subjects (152).
Increasing respiratory drive with hypercapnia or hypoxia tends to increase hypoglossal motoneuron discharge frequency, with changes in firing rate more consistent in cells where discharge is confined to the inspiratory phase (54, 55, 61, 95). Both hypoxia and hypercapnia also recruit motoneurons that were inactive under baseline conditions (54, 55, 61, 95). Although it is difficult to quantify the relative contributions of rate coding and recruitment to the overall neural drive to a muscle, estimates suggest that recruitment of motor units accounts for about 75% of the total increase in genioglossus muscle EMG between baseline and maximal hypercapnic stimulation (61). Hypoglossal motoneurons strongly increase their firing rate when lung inflation is withheld for a single respiratory cycle (55, 135). The latter observation suggests that suppression of motoneuron firing rate underlies the inhibition of hypoglossal motoneuron population output or drive to individual tongue muscles by lung inflation discussed above, though the extent to which lung inflation suppresses motoneuron recruitment is unknown.
Studies in human subjects, all based on the recording of motor units from the genioglossus muscle, generally confirm the findings in animal models in that recruitment seems to dominate the increase in drive to the muscle evoked by chemoreceptor stimulation (100, 117, 121). Similarly, the increase in drive to the genioglossus muscle with arousal is dominated by the recruitment of units that were inactive during sleep, while the decrease in drive with the transition from wakefulness to sleep (7, 155) is dominated by a drop out of motor units with either very little or no change in discharge rate. Consistent with these observations, recent studies of genioglossus motor unit activity during exercise show that the increase in the magnitude of multiunit EMG activity is due largely to motor unit recruitment as exercise intensity increases. Although there was evidence of within-breath firing rate modulation that averaged about 8 Hz, the modulation in this study was the result of a drop in the minimum firing rate (expiration), rather than an increase in peak firing rate (inspiration). Thus during exercise in healthy human subjects the increase in inspiratory-related drive to the genioglossus muscle is the result of recruitment of previously silent motor units and a shift in the firing behavior of already active units to inspiratory-phase-dependent bursting (153). In summary, increased respiration-related neural drive to the tongue muscles is mediated largely, though not entirely, by motor unit recruitment. This is likely a task-dependent feature of the system, as animal studies clearly show that individual motoneurons are capable of increasing their firing rate. Moreover, volitional increases in drive to the human genioglossus muscle are associated with marked increases in firing rate (11, 114). These observations coupled with the findings showing that multiple tongue muscles are coactivated in phase with inspiration, as discussed above, suggest that drive to the hypoglossal motor nucleus is distributed widely, both within and across muscle motoneuron pools.
MAMMALIAN TONGUE MUSCLES: ROLE IN SWALLOWING
In contrast with respiration requiring dilation of the oropharynx, swallowing requires compression of the oropharynx to propel the bolus (liquid or food) through the hypopharynx and into the upper esophageal sphincter. In the human, forceful retraction of the tongue base coordinated with three dimensional pharyngeal compression moves the bolus into and through the upper esophageal sphincter (99). Due to the angle of the oropharynx in the human, anterior and superior motion of the hyoid and larynx pull the laryngeal vestibule up behind the epiglottis to reduce airway entry. These actions may depend upon the geniohyoid and mylohyoid, respectively (113). Bolus propulsion to move the bolus through the hypopharynx depends upon a complex cocontraction of intrinsic and extrinsic tongue muscles: the hyoglossus, geniohyoid, styloglossus, and intrinsic tongue muscles (35).
MAMMALIAN LARYNGEAL MUSCLES: FUNCTIONAL ANATOMY AND INNERVATION
The laryngeal muscles must control the vocal folds for multiple functions, opening for inspiration, closing for airway protection during swallow, and rapid opening and closing to build up pressure for airway clearance during cough. For vocalization the two folds must be held in the midline to allow for vibration on expiratory airflow. Speech and singing require rapid opening and closing of the vocal folds with precise regulation of length and tension to control the frequency of vibration and vocal intensity in coordination with expiratory airflow. The motoneurons for the laryngeal muscles are arranged in the nucleus ambiguus, from the rostral end for the cricothyroid muscle to the posterior cricoarytenoid, lateral cricoarytenoid, and thyroarytenoid in the more caudal region (28). The laryngeal muscles are controlled by brain stem systems for respiration and swallowing (30). For respiration, both the pre-Bötzinger complex for inspiratory pacing and the retrotrapezoid nucleus-parafacial respiratory group in the medulla have inputs to the laryngeal motoneurons for inspiratory and expiratory output, respectively (34). For swallowing, neurons in the ventral swallowing group have inputs to the laryngeal motoneurons (60). During respiration, the vocal folds are actively opened to reduce airflow resistance during inspiration similar to the dilation that occurs in the oropharynx and pharyngeal musculature. During expiration, the vocal folds partially close to provide some resistance during expiratory flow by active respiratory control of the laryngeal motoneurons (26, 76). During swallowing, the larynx closes for airway protection to prevent food or liquid from passing into the trachea in the middle of swallowing at three levels, the vocal folds, the ventricular/false folds, and the epiglottis (64). The laryngeal muscles are also controlled for mammalian vocalization by an innately controlled network in the midbrain and brainstem (63). In humans, fine control of the laryngeal muscles for speech develops in the cortex, cerebellum, and basal ganglia (136). For singing, highly skilled control of the laryngeal muscles emerges with training that involves these same brain regions, as well as auditory integration (106).
The cricoarytenoid joint limits the actions of the laryngeal muscles. The arytenoid cartilages articulate with the posterior cricoarytenoid joint facet on the posterior rim of the cricoid cartilage. The joint facet limits rotation and sliding to a minimum and allows rocking of the arytenoid on the cricoid rim from down and inward for adduction of the folds, to up and outward for abduction of the folds (Fig. 3A). Thus the action is two dimensional, but because most views of the larynx are superior only, the superior-inferior dimension is erroneously interpreted as a swinging of the vocal process from lateral for opening to medial for closing (132) (Fig. 3B).
Fig. 3.
A shows the cricoarytenoid joint facet on the rim of the cricoid cartilage on the left (CA) and the arytenoid cartilage on the joint facet on the right (AC) with the muscular process (MP) in the back, the point of attachment of the posterior cricoarytenoid and lateral cricoarytenoid muscles and the vocal process (VP) in the front with the motion of the vocal process for abduction moving up and outward. [Borrowed with permission from (132).] B shows how the superior view of the larynx reduces the two-dimensional motion of the vocal process to a lateral medial motion (132). Ci is reconstructed from MRI scans of a human larynx with the vectors of the posterior cricoarytenoid (PCA) and lateral cricoarytenoid (LCA) muscles attached from the muscular process of the arytenoid to different points on the cricoid cartilage. [Borrowed with permission from (131).] Cii shows the vectors of the thyroarytenoid (TA) muscle and the vocalis (VF) attaching the front aspect of the arytenoid cartilage to the thyroid cartilage (131).
The laryngeal muscles can be grouped by function and innervation. Only one muscle opens or abducts the vocal folds, the posterior cricoarytenoid muscle. As it shortens with contraction it pulls the muscular process of the arytenoid cartilage backward on the rim of the cricoid cartilage; this action results in motion of the vocal process of the arytenoid cartilage lateral and upward to open the vocal folds (132) (Fig. 3C). The cricothyroid muscle stretches the vocal fold by pulling the thyroid cartilage forward and downward over the cricoid cartilage increasing the distance from the anterior insertion of the vocal fold to its insertion on the voice process of the arytenoid. Some have also suggested that with the action of the cricothyroid muscle to pull the thyroid cartilage downward over the cricoid, it widens the vocal fold opening as the thyroid descends over the cricoid cartilage, further adding to vocal fold abduction action by the cricothyroid muscle in the human (20). Three other intrinsic laryngeal muscles are all thought to add to vocal fold closing but with different actions. The thyroarytenoid muscle comprises the vocal fold attaching the anterior surface of the arytenoid cartilage to the inner surface of the thyroid cartilage and contains two compartments, one the vocalis and another lateral to the vocalis, the thyroarytenoid (125). As the thyroarytenoid contracts, it shortens the vocal fold and pulls the vocal process down and inward toward the midline adding to vocal fold closure (Fig. 3C). The lateral cricoarytenoid and the interarytenoid muscles also close the vocal fold. (Fig. 3C).
Innervation of the laryngeal muscles involves two nerves, the external branch of the superior laryngeal nerve, which innervates the cricothyroid, and the recurrent laryngeal nerve, which innervates the other four muscles. The external branch of the superior laryngeal nerve separates laterally from the internal branch before the internal branch enters the larynx through the thyrohyoid membrane to branch under the surface of the epiglottis, sending separate branches to the mucosa in the laryngeal vestibule, particularly over the surface covering the arytenoid cartilages (124). The external branch enters the cricothyroid muscle, which is thought to involve at least two separate compartments likely innervated by different branches of the nerve: the rectus in the anterior portion pulling the thyroid cartilage downward over the cricoid and the oblique which is more lateral and pulls the thyroid cartilage forward relative to the cricoid. An additional lateral compartment has been proposed in the canine (122). The external branch of the superior laryngeal nerve innervates the cricothyroid muscle. Some have proposed that the internal branch of the superior laryngeal nerve also innervates the interarytenoid muscle in the human (124), although no physiological evidence of this has been reported.
The other intrinsic laryngeal muscles are innervated by the recurrent laryngeal nerve: the posterior cricoarytenoid, the interarytenoid, the lateral cricoarytenoid, and the thyroarytenoid. The recurrent laryngeal nerve ascends toward the larynx in the trachea-esophageal groove as the inferior laryngeal nerve entering the larynx from the posterior aspect. It first sends fibers to innervate the posterior cricoarytenoid, then the lateral cricoarytenoid, while the anterior branch travels laterally to innervate the thyroarytenoid (126).
The activation patterns of the laryngeal muscles vary between respiration, swallowing, cough, and voice. The only abductor muscle, the posterior cricoarytenoid, is the only muscle that has a similar action regardless of the tasks being performed. Posterior cricoarytenoid muscle activation is consistently associated with vocal fold opening for all tasks including inspiration, sniff, and cough (17, 115). Three compartments have been proposed for this muscle: vertical, oblique, and horizontal in the canine (122). The oblique and horizontal compartments may contribute to abduction for inspiration. Activation of the vertical compartment could account for rocking backward to oppose the tensing and shortening actions of the cricothyroid and thyroarytenoid in high-pitched voice production (122).
Each of the laryngeal muscles acts interactively with other muscles during different tasks. For example, the cricothyroid muscle, which serves to lengthen the vocal folds, may augment vocal fold opening when it cocontracts with the posterior cricoarytenoid muscle during inspiration and sniff in humans (3, 115). However, cricothyroid contraction has not consistently been associated with changes in vocal fold adduction or abduction (157–159). The three adductor muscles (the thyroarytenoid, lateral cricoarytenoid, and interarytenoid) interact during vocal fold adduction. The most information is available on the thyroarytenoid muscle, likely due to ease of access to the main body of the vocal fold via needle insertion through the cricothyroid membrane. Both the thyroarytenoid and lateral cricoarytenoid are active during the vocal fold closure phase of cough but are not as clearly associated with vocal fold closing during speech (115). Possibly the use of the laryngeal muscles for speech is developed somewhat idiosyncratically by infants as they learn to emulate the auditory targets of speech sounds from the language in their environment. Perhaps muscle activation patterns for speech are more individualized due to implicit learning while muscle patterning during respiration, cough, and swallow are under the control of innate central pattern generators in the brain stem.
Several constraints have limited human research on the laryngeal muscles and laryngeal biomechanics. In humans, the larynx can only be visualized with a transoral rigid laryngoscope prohibiting speech, cough, and swallow or with nasoendoscopes for only a few minutes at a time. Electromyographic access to the laryngeal muscles requires insertions of needles between the cricoid and thyroid cartilages (50) and verification gestures to identify muscle location (50, 86). Although visualization and placement via a nasoendoscope allows visualization of electrode insertion (77), anesthesia is required, thus altering muscle function. Endoscopic techniques allow recording from the posterior cricoarytenoid, although these are less accurate than hooked wire electrodes (39). Finally, during swallowing the larynx moves markedly and electrodes are easily dislodged. As a result, relatively few studies of laryngeal electromyography during speech, respiration, and cough have been conducted in humans (115).
MAMMALIAN LARYNGEAL MUSCLES: RESPONSE TO CHANGES IN RESPIRATORY DRIVE
During quiet respiration, the posterior cricoarytenoid is active for inspiration to open the vocal folds with phasic firing of some units prior to the onset of inspiration and tonic firing throughout the cycle (75). The thyroarytenoid muscle is active during quiet respiration, with both phasic and tonic firing patterns being higher during inspiration but also present during expiration (26). Increasing inspiratory drive increases posterior cricoarytenoid activity as it adds to widening of the upper airway from the nasal alae downward (18, 127, 146). With hypercapnia, not only does the posterior cricoarytenoid muscle activity increase to widen vocal fold opening during inspiration to reduce airway resistance, but also the thyroarytenoid muscle increases in level of activation during expiration to reduce airflow and maintain alveolar expansion (2, 57, 76, 79). Thus both the vocal fold abductor and adductor muscles are active in relation to respiratory drive with tonic and phasic motor unit firing patterns associated with vocal fold valving during inspiration and expiration for both involuntary and voluntary changes in breathing (75, 78). Further, voluntary sniff maximally opens the vocal folds with rapid phasic recruitment of both the posterior cricoarytenoid and the cricothyroid muscles in humans which is used clinically to test for abductor muscle function (115).
INFLUENCE OF SWALLOWING ON THE LARYNGEAL AND TONGUE MUSCLES
Swallowing requires rapid and complete closure of the entry to the laryngeal vestibule while allowing the opening of the upper esophageal sphincter for the liquid or food to enter the esophagus and clear the hypopharynx. Extrinsic laryngeal muscles including the suprahyoid muscles such as the geniohyoid, mylohyoid, and hyoglossus elevate with the hyoid while the thyrohyoid, suprahyoid, and the long pharyngeal muscles (111) pull the larynx upward toward the hyoid to assist in epiglottic inversion as the arytenoid cartilages are pulled forward underneath the epiglottis to assist with closing the upper entry to the vestibule. This motion is coincident with posterior motion of the posterior tongue to propel the food into the posterior oropharynx and provide downward pressure coincident with pharyngeal contraction. Although the use of suprahyoid and thyrohyoid muscles to elevate the hyo-laryngeal complex have been examined (22, 23), the interaction of the simultaneous motion of tongue posterior pressure and hyo-laryngeal motions have not been examined for biomechanical interactions. The anterior elevation of the larynx is thought to add to relaxation of the upper esophageal sphincter to allow the food and liquid to clear from the hypopharynx (111–113). However, the coincident motions of tongue retraction along with hyo-laryngeal elevation and pharyngeal squeezing have not yet been modeled to establish the muscle contraction timing and forces needed to effectively clear the bolus from the hypopharynx during swallowing.
RELATIONSHIP BETWEEN RESPIRATION AND SWALLOWING
Respiration and swallowing have opposing biomechanical effects on upper airway musculature; dilation for inspiration to allow unobstructed air intake into the lungs contrasted with oropharyngeal compression for squeezing the bolus through the pharynx and the upper esophageal sphincter. The control of these two systems is coordinated in neurons involved in these two integrative systems in the brain stem (60). For example, a neuron contained in the nucleus tractus solitarius showing cyclic depolarization with inspiration, altered its firing pattern during fictive swallowing to be coincident with phases of swallowing (60). Swallowing interrupts the respiratory cycle during an apneic period in the pharyngeal phase when the larynx is closed, and the bolus is moved through the pharynx into the esophagus similarly in mammals (93) and humans (110). Further, swallowing also causes a resetting of the respiratory rhythm demonstrating a modification of respiratory cycling by swallowing (110). As swallowing onset close to the onset of inspiration can increase the risk of aspiration of substances with the coincidence of dilation and compression in the upper airway, the relationship between these two systems needs to be well controlled for survival (93).
DISCLOSURES
Dr. Ludlow serves as a consultant to Passy Muir, Inc., and is an inventor on 2 patents for devices for treating swallowing disorders.
AUTHOR CONTRIBUTIONS
Author contributions: R.F.F. and C.L.L. prepared figures; R.F.F. and C.L.L. drafted manuscript; R.F.F. and C.L.L. edited and revised manuscript; R.F.F. and C.L.L. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants DC 007692, U54 NS065701, and 1R43 DC012754-01.
Footnotes
Because the palatoglossus originates on the palatine aponeurosis and inserts into the posterolateral tongue, it is sometimes considered a palatal muscle and sometimes a tongue muscle. The motor innervation of palatoglossus is via the pharyngeal plexus, not the hypoglossal nerve. Thus, the tongue is often considered to have three extrinsic muscles.
REFERENCES
- 1.Adachi T, Robinson DM, Miles GB, Funk GD. Noradrenergic modulation of XII motoneuron inspiratory activity does not involve alpha2-receptor inhibition of the Ih current or presynaptic glutamate release. J Appl Physiol 98: 1297–1308, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Adachi T, Umezaki T, Matsuse T, Shin T. Changes in laryngeal muscle activities during hypercapnia in the cat. Otolaryngol Head Neck Surg 118: 537–544, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Amis TC, Brancatisano A, Tully A. Thyroid cartilage movements during breathing. J Appl Physiol 78: 441–448, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bailey EF. Activities of human genioglossus motor units. Respir Physiol Neurobiol 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 96: 440–449, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol 101: 609–617, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol 98: 3284–3291, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bailey EF, Huang Y, Fregosi RF. The anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bailey EF, Janssen PL, Fregosi RF. PO2-dependent changes in intrinsic and extrinsic tongue muscle activities in the rat. Am J Respir Crit Care Med 171: 1403–1407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 525–534, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol 97: 933–936, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Ballotta E, Da Giau G, Renon L. Early and late outcomes of young patients after carotid endarterectomy. Surgery 125: 581–586, 1999 [PubMed] [Google Scholar]
- 13.Barnwell YM. Human lingual musculature: an historical review. Int J Oral Myol 2: 31–41, 1976 [PubMed] [Google Scholar]
- 14.Bartlett D., Jr. Upper airway motor systems. In: Handbook of Physiology Section 3:The respiratory system, edited by Cherniack NS, Widdicombe J. G. Washington DC: American Physiological Society, 1986, p. 223–245 [Google Scholar]
- 15.Bartlett D, Jr, Knuth SL, Ward DK. Influence of extreme hypercapnia on respiratory motor nerve activity in cats. Respir Physiol 70: 173–181, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol 76: 3758–3770, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Brancatisano TP, Dodd DS, Engel LA. Respiratory activity of posterior cricoarytenoid muscle and vocal cords in humans. J Appl Physiol 57: 1143–1149, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Brancatisano TP, Dodd DS, Engel LA. Responses of the posterior cricoarytenoid and alae nasi muscles to increased chemical drive in man. Respir Physiol 64: 177–189, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Brandes IF, Zuperku EJ, Stucke AG, Jakovcevic D, Hopp FA, Stuth EA. Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs. J Neurophysiol 95: 3449–3459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broncatisano T. The role of the laryngeal muscles in respiratory control in the human. In: Controlling Complexity and Chaos: 9th Vocal Fold Physiology Symposium, edited by Fletcher N, Davis P. San Diego: Singular Press, 1995. In press [Google Scholar]
- 21.Bruce EN, Mitra J, Cherniack NS. Central and peripheral chemoreceptor inputs to phrenic and hypoglossal motoneurons. J Appl Physiol 53: 1504–1511, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Burnett TA, Mann EA, Bidus K, Ludlow CL. An evaluation of muscle stimulation for laryngeal elevation in humans. Dysphagia 16: 146, 2001 [Google Scholar]
- 23.Burnett TA, Mann EA, Cornell SA, Ludlow CL. Laryngeal elevation achieved by neuromuscular stimulation at rest. J Appl Physiol 94: 128–134, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Chamberlin NL, Bocchiaro CM, Greene RW, Feldman JL. Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience 115: 861–870, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579: 515–526, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanaud CM, Ludlow CL. Single motor unit activity of human intrinsic laryngeal muscles during respiration. Ann Otol Rhinol Laryngol 101: 832–840, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Cheetham J, Pigott JH, Hermanson JW, Campoy L, Soderholm LV, Thorson LM, Ducharme NG. Role of the hypoglossal nerve in equine nasopharyngeal stability. J Appl Physiol 107: 471–477, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Davis PJ, Nail BS. On the location and size of laryngeal motoneurons in the cat and rabbit. J Comp Neurol 230: 13–32, 1984 [DOI] [PubMed] [Google Scholar]
- 29.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dick TE, Oku Y, Romaniuk JR, Cherniack NS. Interaction between central pattern generators for breathing and swallowing in the cat. J Physiol 465: 715–730, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol 357: 376–394, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 123: 57–61, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Engelhardt JK, Silveira V, Morales FR, Pose I, Chase MH. Serotoninergic control of glycinergic inhibitory postsynaptic currents in rat hypoglossal motoneurons. Brain Res 1345: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felton SM, Gaige TA, Reese TG, Wedeen VJ, Gilbert RJ. Mechanical basis for lingual deformation during the propulsive phase of swallowing as determined by phase-contrast magnetic resonance imaging. J Appl Physiol 103: 255–265, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res 14: 419–429, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Fregosi RF. Respiratory related control of hypoglossal motoneurons–Knowing what we do not know. Respir Physiol Neurobiol 179: 43–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol 110: 295–306, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Fujita M, Ludlow CL, Woodson GE, Naunton RF. A new surface electrode for recording from the posterior cricoarytenoid muscle. Laryngoscope 99: 316–320, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 507 (Pt 1): 265–276, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 519 Pt 2: 601–613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fung SJ, Yamuy J, Xi MC, Engelhardt JK, Morales FR, Chase MH. Changes in electrophysiological properties of cat hypoglossal motoneurons during carbachol-induced motor inhibition. Brain Res 885: 262–272, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Funk GD, Zwicker JD, Selvaratnam R, Robinson DM. Noradrenergic modulation of hypoglossal motoneuron excitability: developmental and putative state-dependent mechanisms. Arch Ital Biol 149: 426–453, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Gaige TA, Benner T, Wang R, Wedeen VJ, Gilbert RJ. Three dimensional myoarchitecture of the human tongue determined in vivo by diffusion tensor imaging with tractography. J Magn Reson Imaging 26: 654–661, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol 147: 159–176, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Gilbert RJ, Napadow VJ, Gaige TA, Wedeen VJ. Anatomical basis of lingual hydrostatic deformation. J Exp Biol 210: 4069–4082, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol 74: 547–555, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Guo Y, Goldberg SJ, McClung JR. Compartmental organization of styloglossus and hyoglossus motoneurons in the hypoglossal nucleus of the rat. Brain Res 728: 277–280, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Gutrecht JA, Jones HR., Jr. Bilateral hypoglossal nerve injury after bilateral carotid endarterectomy. Stroke 19: 261–262, 1988 [DOI] [PubMed] [Google Scholar]
- 50.Hirano M, Ohala J. Use of hooked-wire electrodes for electromyography of the intrinsic laryngeal muscles. J Speech Hear Res 12: 362–373, 1969 [DOI] [PubMed] [Google Scholar]
- 51.Horner RL. Impact of brainstem sleep mechanisms on pharyngeal motor control. Respir Physiol 119: 113–121, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Horner RL, Innes JA, Guz A. Reflex pharyngeal dilator muscle activation by stimuli of negative airway pressure in awake man. Sleep 16: S85–86, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO(2) in rats. J Appl Physiol 92: 878–887, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Hwang JC, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol 55: 793–798, 1983 [DOI] [PubMed] [Google Scholar]
- 55.Hwang JC, St John WM. Alterations of hypoglossal motoneuronal activities during pulmonary inflations. Exp Neurol 97: 615–625, 1987 [DOI] [PubMed] [Google Scholar]
- 56.Hwang JC, St John WM, Bartlett D., Jr. Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol 55: 785–792, 1983 [DOI] [PubMed] [Google Scholar]
- 57.Insalaco G, Kuna ST, Cibella F, Villeponteaux RD. Thyroarytenoid muscle activity during hypoxia, hypercapnia, and voluntary hyperventilation in humans. J Appl Physiol 69: 268–273, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol 89: 1345–1351, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Janssen PL, Williams JS, Fregosi RF. Consequences of periodic augmented breaths on tongue muscle activities in hypoxic rats. J Appl Physiol 88: 1915–1923, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969., 2001 [DOI] [PubMed] [Google Scholar]
- 61.John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med 172: 1331–1337, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jordan AS, Catcheside PG, O'Donoghue FJ, Saunders NA, McEvoy RD. Genioglossus muscle activity at rest and in response to brief hypoxia in healthy men and women. J Appl Physiol 92: 410–417, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Jurgens U. The neural control of vocalization in mammals: a review. J Voice 23: 1–10, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Kawasaki A, Fukuda H, Shiotani A, Kanzaki J. Study of movements of individual structures of the larynx during swallowing. Auris Nasus Larynx 28: 75–84, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Kier WM. The diversity of hydrostatic skeletons. J Exp Biol 215: 1247–1257, 2012 [DOI] [PubMed] [Google Scholar]
- 65a.Kier WM, Smith KK. Tongue tentacles and trunks: the biomechanics of movement in muscular hydrostats. Zool. J Linn. Soc. 83: 307–324, 1985 [Google Scholar]
- 66.Klawe JJ, Tafil-Klawe M. Age-related response of the genioglossus muscle EMG-activity to hypoxia in humans. J Physiol Pharmacol 54 Suppl 1: 14–19, 2003 [PubMed] [Google Scholar]
- 67.Kletzien H, Russell JA, Leverson GE, Connor NP. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J Appl Physiol 114: 472–481, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koizumi H, Koshiya N, Chia JX, Cao F, Nugent J, Zhang R, Smith JC. Structural-functional properties of identified excitatory and inhibitory interneurons within pre-Botzinger complex respiratory microcircuits. J Neurosci 33: 2994–3009, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci 28: 2353–2365, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krammer EB, Rath T, Lischka MF. Somatotopic organization of the hypoglossal nucleus: a HRP study in the rat. Brain Res 170: 533–537, 1979 [DOI] [PubMed] [Google Scholar]
- 71.Krimsky WR, Leiter JC. Physiology of breathing and respiratory control during sleep. Semin Respir Crit Care Med 26: 5–12, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Kuna ST. Inhibition of inspiratory upper airway motoneuron activity by phasic volume feedback. J Appl Physiol 60: 1373–1379, 1986 [DOI] [PubMed] [Google Scholar]
- 73.Kuna ST. Interaction of hypercapnia and phasic volume feedback on motor control of the upper airway. J Appl Physiol 63: 1744–1749, 1987 [DOI] [PubMed] [Google Scholar]
- 74.Kuna ST. Respiratory-related activation and mechanical effects of the pharyngeal constrictor muscles. Respir Physiol 119: 155–161, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Kuna ST, Day RA, Insalaco G, Villeponteaux RD. Posterior cricoarytenoid activity in normal adults during involuntary and voluntary hyperventilation. J Appl Physiol 70: 1377–1385, 1991 [DOI] [PubMed] [Google Scholar]
- 76.Kuna ST, Insalaco G. Respiratory-related intrinsic laryngeal muscle activity in normal adults. Prog Clin Biol Res 345: 117–123; discussion 123–114, 1990 [PubMed] [Google Scholar]
- 77.Kuna ST, Insalaco G, Villeponteaux DR, Vanoye CR, Smickley JS. Effect of hypercapnia and hypoxia on arytenoideus muscle activity in normal adult humans. J Appl Physiol 75: 1781–1789, 1993 [DOI] [PubMed] [Google Scholar]
- 78.Kuna ST, Insalaco G, Villeponteaux RD. Arytenoideus muscle activity in normal adult humans during wakefulness and sleep. J Appl Physiol 70: 1655–1664, 1991 [DOI] [PubMed] [Google Scholar]
- 79.Kuna ST, Smickley JS, Insalaco G. Posterior cricoarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol 68: 1746–1754, 1990 [DOI] [PubMed] [Google Scholar]
- 80.Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci 21: 7384–7391, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Influence of vagal afferents on supraspinal and spinal respiratory activity following cervical spinal cord injury in rats. J Appl Physiol 109: 377–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, Sood S, Liu H, Horner RL. Opposing muscarinic and nicotinic modulation of hypoglossal motor output to genioglossus muscle in rats in vivo. J Physiol 565: 965–980, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lowe AA. The tongue and airway. Otolaryngol Clin North Am 23: 677–698, 1990 [PubMed] [Google Scholar]
- 84.Lowe AA, Sessle BJ. Tongue activity during respiration, jaw opening, and swallowing in cat. Can J Physiol Pharmacol 51: 1009–1011, 1973 [DOI] [PubMed] [Google Scholar]
- 85.Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir Physiol Neurobiol 147: 191–203, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Ludlow CL, Yeh J, Cohen LG, Van Pelt F, Rhew K, Hallett M. Limitations of electromyography and magnetic stimulation for assessing laryngeal muscle control. Ann Otol Rhinol Laryngol 103: 16–27, 1994 [DOI] [PubMed] [Google Scholar]
- 87.Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D, White DP. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med 165: 71–77, 2002 [DOI] [PubMed] [Google Scholar]
- 88.Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 160: 1976–1982, 1999 [DOI] [PubMed] [Google Scholar]
- 89.Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol 52: 438–444, 1982 [DOI] [PubMed] [Google Scholar]
- 90.McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec 260: 378–386, 2000 [DOI] [PubMed] [Google Scholar]
- 91.McClung JR, Goldberg SJ. Organization of motoneurons in the dorsal hypoglossal nucleus that innervate the retrusor muscles of the tongue in the rat. Anat Rec 254: 222–230, 1999 [DOI] [PubMed] [Google Scholar]
- 92.McClung JR, Goldberg SJ. Organization of the hypoglossal motoneurons that innervate the horizontal and oblique components of the genioglossus muscle in the rat. Brain Res 950: 321–324, 2002 [DOI] [PubMed] [Google Scholar]
- 93.McFarland DH, Lund JP. An investigation of the coupling between respiration, mastication, and swallowing in the awake rabbit. J Neurophysiol 69: 95–108, 1993 [DOI] [PubMed] [Google Scholar]
- 94.Megirian D, Hinrichsen CF, Sherrey JH. Respiratory roles of genioglossus, sternothyroid, and sternohyoid muscles during sleep. Exp Neurol 90: 118–128, 1985 [DOI] [PubMed] [Google Scholar]
- 95.Mitra J, Cherniack NS. The effects of hypercapnia and hypoxia on single hypoglossal nerve fiber activity. Respir Physiol 54: 55–66, 1983 [DOI] [PubMed] [Google Scholar]
- 96.Mitra J, Prabhakar NR, Haxhiu M, Cherniack NS. Comparison of the effects of hypercapnia on phrenic and hypoglossal activity in anesthetized decerebrate and decorticate animals. Brain Res Bull 17: 181–187, 1986 [DOI] [PubMed] [Google Scholar]
- 97.Mu L, Sanders I. Human tongue neuroanatomy: Nerve supply and motor endplates. Clin Anat 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mu L, Sanders I. Neuromuscular organization of the canine tongue. Anat Rec 256: 412–424, 1999 [DOI] [PubMed] [Google Scholar]
- 99.Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Biomechanical basis for lingual muscular deformation during swallowing. Am J Physiol Gastrointest Liver Physiol 277: G695–G701, 1999 [DOI] [PubMed] [Google Scholar]
- 100.Nicholas CL, Bei B, Worsnop C, Malhotra A, Jordan AS, Saboisky JP, Chan JK, Duckworth E, White DP, Trinder J. Motor unit recruitment in human genioglossus muscle in response to hypercapnia. Sleep 33: 1529–1538, 2010 [PMC free article] [PubMed] [Google Scholar]
- 101.Nishikawa KC, Kier WM, Smith KK. Morphology and mechanics of tongue movement in the African pig-nosed frog Hemisus marmoratum: a muscular hydrostatic model. J Exp Biol 202: 771–780, 1999 [DOI] [PubMed] [Google Scholar]
- 102.Nunez-Abades PA, Cameron WE. Relationship between membrane properties and cell size of developing rat genioglossal motoneurons studied in vitro. Neurosci Lett 223: 41–44, 1997 [DOI] [PubMed] [Google Scholar]
- 103.Okabe S, Chonan T, Hida W, Satoh M, Kikuchi Y, Takishima T. Role of chemical drive in recruiting upper airway and inspiratory intercostal muscles in patients with obstructive sleep apnea. Am Rev Respir Dis 147: 190–195, 1993 [DOI] [PubMed] [Google Scholar]
- 104.Oliven A, Odeh M, Geitini L, Oliven R, Steinfeld U, Schwartz AR, Tov N. Effect of coactivation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol 103: 1662–1668, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to isocapnic hypoxia in humans. Am Rev Respir Dis 124: 215–217, 1981 [DOI] [PubMed] [Google Scholar]
- 106.Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage 33: 628–635, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Parisi RA, Neubauer JA, Frank MM, Edelman NH, Santiago TV. Correlation between genioglossal and diaphragmatic responses to hypercapnia during sleep. Am Rev Respir Dis 135: 378–382, 1987 [DOI] [PubMed] [Google Scholar]
- 108.Parisi RA, Santiago TV, Edelman NH. Genioglossal and diaphragmatic EMG responses to hypoxia during sleep. Am Rev Respir Dis 138: 610–616, 1988 [DOI] [PubMed] [Google Scholar]
- 109.Patrick GB, Strohl KP, Rubin SB, Altose MD. Upper airway and diaphragm muscle responses to chemical stimulation and loading. J Appl Physiol 53: 1133–1137, 1982 [DOI] [PubMed] [Google Scholar]
- 110.Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol 483 (Pt 1): 273–288, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pearson WG, Jr, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys 85: 735–740, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pearson WG, Jr, Langmore SE, Yu LB, Zumwalt AC. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia 27: 445–451, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pearson WG, Jr, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia 26: 345–351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pittman LJ, Bailey EF. Genioglossus and intrinsic electromyographic activities in impeded and unimpeded protrusion tasks. J Neurophysiol 101: 276–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poletto CJ, Verdun LP, Strominger R, Ludlow CL. Correspondence between laryngeal vocal fold movement and muscle activity during speech and nonspeech gestures. J Appl Physiol 97: 858–866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978 [DOI] [PubMed] [Google Scholar]
- 117.Richardson PA, Bailey EF. Tonically discharging genioglossus motor units show no evidence of rate coding with hypercapnia. J Neurophysiol 103: 1315–1321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ryan S, McNicholas WT, O'Regan RG, Nolan P. Reflex respiratory response to changes in upper airway pressure in the anaesthetized rat. J Physiol 537: 251–265, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ryan S, Nolan P. Long-term facilitation of upper airway muscle activity induced by episodic upper airway negative pressure and hypoxia in spontaneously breathing anaesthetized rats. J Physiol 587: 3343–3353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 95: 2213–2221, 2006 [DOI] [PubMed] [Google Scholar]
- 121.Saboisky JP, Jordan AS, Eckert DJ, White DP, Trinder JA, Nicholas CL, Gautam S, Malhotra A. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol 109: 1939–1949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sanders I, Jacobs I, Wu BL, Biller HF. The three bellies of the canine posterior cricoarytenoid muscle: implications for understanding laryngeal function. Laryngoscope 103: 171–177, 1993 [DOI] [PubMed] [Google Scholar]
- 123.Sanders I, Mu L. A three-dimensional atlas of human tongue muscles. Anat Rec (Hoboken) 296: 1102–1114, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanders I, Mu L. Anatomy of the human internal superior laryngeal nerve. Anat Rec 252: 646–656, 1998 [DOI] [PubMed] [Google Scholar]
- 125.Sanders I, Rai S, Han Y, Biller HF. Human vocalis contains distinct superior and inferior subcompartments: possible candidates for the two masses of vocal fold vibration. Ann Otol Rhinol Laryngol 107: 826–833, 1998 [DOI] [PubMed] [Google Scholar]
- 126.Sanders I, Wu BL, Mu L, Li Y, Biller HF. The innervation of the human larynx. Arch Otolaryngol Head Neck Surg 119: 934–939, 1993 [DOI] [PubMed] [Google Scholar]
- 127.Sant'Ambrogio FB, Mathew OP, Clark WD, Sant'Ambrogio G. Laryngeal influences on breathing pattern and posterior cricoarytenoid muscle activity. J Appl Physiol 58: 1298–1304, 1985 [DOI] [PubMed] [Google Scholar]
- 128.Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol 51: 160–170, 1976 [DOI] [PubMed] [Google Scholar]
- 129.Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith PL. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol 81: 643–652, 1996 [DOI] [PubMed] [Google Scholar]
- 130.Schwartz AR, Thut DC, Brower RG, Gauda EB, Roach D, Permutt S, Smith PL. Modulation of maximal inspiratory airflow by neuromuscular activity: effect of CO2. J Appl Physiol 74: 1597–1605, 1993 [DOI] [PubMed] [Google Scholar]
- 131.Selbie WS, Gewalt SL, Ludlow CL. Developing an anatomical model of the human laryngeal cartilages from MRI. J Acoust Soc Am 112: 1077–1090, 2002 [DOI] [PubMed] [Google Scholar]
- 132.Selbie WS, Zhang L, Levine WS, Ludlow CL. Using joint geometry to determine the motion of the cricoarytenoid joint. J Acoust Soc Am 103: 1115–1127, 1998 [DOI] [PubMed] [Google Scholar]
- 133.Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol 520 Pt 3: 897–908, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi YX, Seto-Poon M, Wheatley JR. Breathing route dependence of upper airway muscle activity during hyperpnea. J Appl Physiol 84: 1701–1706, 1998 [DOI] [PubMed] [Google Scholar]
- 135.Sica AL, Cohen MI, Donnelly DF, Zhang H. Hypoglossal motoneuron responses to pulmonary and superior laryngeal afferent inputs. Respir Physiol 56: 339–357, 1984 [DOI] [PubMed] [Google Scholar]
- 136.Simonyan K, Herscovitch P, Horwitz B. Speech-induced striatal dopamine release is left lateralized and coupled to functional striatal circuits in healthy humans: a combined PET, fMRI and DTI study. Neuroimage 70: 21–32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith JC, Goldberg SJ, Shall MS. Phenotype and contractile properties of mammalian tongue muscles innervated by the hypoglossal nerve. Respir Physiol Neurobiol 147: 253–262, 2005 [DOI] [PubMed] [Google Scholar]
- 139.Sokoloff AJ, Burkholder T. Tongue structure and function. In: Carniofacial muscles: A new framework for understanding the effector side of craniofacial muscle control, edited by McLoon LK, Andrade F. New York: Springer, 2012 [Google Scholar]
- 140.Sokoloff AJ, Deacon TW. Musculotopic organization of the hypoglossal nucleus in the cynomolgus monkey, Macaca fascicularis. J Comp Neurol 324: 81–93, 1992 [DOI] [PubMed] [Google Scholar]
- 141.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med 172: 1338–1347, 2005 [DOI] [PubMed] [Google Scholar]
- 142.St John WM, Knuth KV, Rist KE. Dynamic changes of hypoglossal and phrenic activities by hypoxia and hypercapnia. Respir Physiol 56: 237–244, 1984 [DOI] [PubMed] [Google Scholar]
- 143.Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med 165: 945–949, 2002 [DOI] [PubMed] [Google Scholar]
- 144.Stavness I, Lloyd JE, Fels S. Automatic prediction of tongue muscle activations using a finite element model. J Biomech 45: 2841–2848, 2012 [DOI] [PubMed] [Google Scholar]
- 145.Stone M, Lundberg A. Three-dimensional tongue surface shapes of English consonants and vowels. J Acoust Soc Am 99: 3728–3737, 1996 [DOI] [PubMed] [Google Scholar]
- 146.Strohl KP, Hensley MJ, Hallett M, Saunders NA, Ingram RHJ. Activation of upper airway muscles before onset of inspiration in normal humans. J Appl Physiol RespirEnviron Exerc Physiol 49: 638–642, 1980 [DOI] [PubMed] [Google Scholar]
- 147.Takemoto H. Morphological analyses of the human tongue musculature for three-dimensional modeling. J Speech Lang Hear Res 44: 95–107, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Uemura-Sumi M, Itoh M, Mizuno N. The distribution of hypoglossal motoneurons in the dog, rabbit and rat. Anat Embryol (Berl) 177: 389–394, 1988 [DOI] [PubMed] [Google Scholar]
- 149.Ugolini G. Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol 356: 457–480, 1995 [DOI] [PubMed] [Google Scholar]
- 150.van Lunteren E, Strohl KP, Parker DM, Bruce EN, Van de Graaff WB, Cherniack NS. Phasic volume-related feedback on upper airway muscle activity. J Appl Physiol 56: 730–736, 1984 [DOI] [PubMed] [Google Scholar]
- 151.Vincent HK, Shanely RA, Stewart DJ, Demirel HA, Hamilton KL, Ray AD, Michlin C, Farkas GA, Powers SK. Adaptation of upper airway muscles to chronic endurance exercise. Am J Respir Crit Care Med 166: 287–293, 2002 [DOI] [PubMed] [Google Scholar]
- 152.Walls CE, Laine CM, Kidder IJ, Bailey EF. Human hypoglossal motor unit activities in exercise. J Physiol 591: 3579–3590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Walls CE, Laine CM, Kidder IJ, Bailey EF. Human hypoglossal motor unit activities in exercise. J Physiol 591 (pt 14): 3579–3590 In Press: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weiner D, Mitra J, Salamone J, Cherniack NS. Effect of chemical stimuli on nerves supplying upper airway muscles. J Appl Physiol 52: 530–536, 1982 [DOI] [PubMed] [Google Scholar]
- 155.Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep 31: 525–533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Williams JS, Janssen PL, Fuller DD, Fregosi RF. Influence of posture and breathing route on neural drive to upper airway dilator muscles during exercise. J Appl Physiol 89: 590–598, 2000 [DOI] [PubMed] [Google Scholar]
- 157.Woodson GE. Configuration of the glottis in laryngeal paralysis. II: Animal experiments. Laryngoscope 103: 1235–1241, 1993 [DOI] [PubMed] [Google Scholar]
- 158.Woodson GE, Murry MP, Schweizer V, Hengesteg A, Chen N, Yeung D. Unilateral cricothyroid contraction and glottic configuration. J Voice 12: 335–339, 1998 [DOI] [PubMed] [Google Scholar]
- 159.Woodson GE, Sant'Ambrogio F, Mathew O, Sant'Ambrogio G. Effects of cricothyroid muscle contraction on laryngeal resistance and glottic area. Ann Otol RhinolLaryngol 98: 119–124, 1989 [DOI] [PubMed] [Google Scholar]