Abstract
Although respiratory muscle motor units have been studied during natural breathing, simultaneous measures of muscle force have never been obtained. Tongue retractor muscles, such as the hyoglossus (HG), play an important role in swallowing, licking, chewing, breathing, and, in humans, speech. The HG is phasically recruited during the inspiratory phase of the respiratory cycle. Moreover, in urethane anesthetized rats the drive to the HG waxes and wanes spontaneously, providing a unique opportunity to study motor unit firing patterns as the muscle is driven naturally by the central pattern generator for breathing. We recorded tongue retraction force, the whole HG muscle EMG and the activity of 38 HG motor units in spontaneously breathing anesthetized rats under low-force and high-force conditions. Activity in all cases was confined to the inspiratory phase of the respiratory cycle. Changes in the EMG were correlated significantly with corresponding changes in force, with the change in EMG able to predict 53–68% of the force variation. Mean and peak motor unit firing rates were greater under high-force conditions, although the magnitude of discharge rate modulation varied widely across the population. Changes in mean and peak firing rates were significantly correlated with the corresponding changes in force, but the correlations were weak (r2 = 0.27 and 0.25, respectively). These data indicate that, during spontaneous breathing, recruitment of HG motor units plays a critical role in the control of muscle force, with firing rate modulation playing an important but lesser role.
Keywords: breathing, electrophysiology, hypoglossal, tongue muscles
muscles of the mammalian tongue participate in many important behaviors, including breathing, swallowing, mastication, licking, and facial expression and, in humans, speech. The tongue is mechanically complex, and its shape, stiffness and position in space are controlled by the combined actions of eight different muscles (Fregosi and Fuller 1997; Smith et al. 2005). Four of the muscles are extrinsic muscles, which originate on bony structures or connective tissue and insert into the tongue body [the genioglossus, hyoglossus (HG), styloglossus and palatoglossus], and four are intrinsic muscles (inferior longitudinalis, superior longitudinalis, transversus and verticalis) in which the fibers are wholly contained within the tongue body (Fregosi 2011; Fregosi and Fuller 1997; Smith et al. 2005). The tongue muscles are very small, their fibers intermingle extensively within the tongue body (Gaige et al. 2007), and their innervation is complex (see Fregosi 2011). Collectively, these unique muscles produce a variety of intricate but well-controlled movements, including retraction, protrusion, elevation and depression of the tongue, as well as alterations in tongue shape and stiffness.
Muscle contraction, and hence movement, depends on the activation of motor units. A motor unit is defined as an α-motoneuron and all of the muscle fibers that it innervates. While the force capacity of individual motor units depends primarily on the number of muscle fibers innervated by the parent motoneuron (Bodine et al. 1987; Kanda and Hashizume 1992), the force exerted by a motor unit depends on its firing rate (Kernell 2003). As such, the number and size of motor units recruited, and their individual firing rates, determine whole muscle force. Although respiratory muscle motor units from chest wall (Butler et al. 1999; Gandevia et al. 1999; Iscoe et al. 1976; Sieck et al. 1984) and upper airway muscles (Bailey 2011; Bailey et al. 2007; Butler and Gandevia 2008; John et al. 2005; Saboisky et al. 2006, 2010; Wilkinson et al. 2010) have been studied during natural breathing, simultaneous measures of muscle force have never been obtained. In previous work we showed that quasi-isometric tongue muscle force could be measured with reasonable accuracy in anesthetized rodents (Fuller et al. 1998). Additionally, recent work in the urethane-anesthetized rat shows that the central nervous system state waxes and wanes spontaneously, with corresponding changes in the output of the respiratory central pattern generator (Pagliardini et al. 2012). Our laboratory has long noted that tongue muscle force and EMG activity wax and wane spontaneously in urethane-anesthetized rats (Fregosi and Fuller 1997). Here we take advantage of this phenomenon to study HG motor unit discharge during spontaneous breathing, under conditions of high and low drive to the hypoglossal motoneuron pool.
MATERIALS AND METHODS
Surgical preparation.
The Institutional Animal Care and Use Committee at the University of Arizona approved all surgical and experimental procedures reported here. We used 17 spontaneously breathing adult male rats of either sex (Sprague-Dawley, 240–340 g), anesthetized initially with 2–4% isoflurane mixed in O2. Femoral artery catheterization was performed, and animals were slowly weaned off the isoflurane in exchange for urethane, which was injected via a femoral artery catheter to a final dose of 1.3 g/kg. Supplemental doses (0.3 g/kg) of urethane were given as needed to maintain deep anesthesia (no reaction to paw pinch). A tracheal cannula was inserted below the larynx to maintain a patent airway and for delivery of humidified oxygen (50% O2, 50% N2). A heating pad was used to maintain rectal temperature between 36.5 and 38.5°C.
The digastric, mylohyoid, and geniohyoid muscles were removed bilaterally to expose the hypoglossal nerves and the HG muscles, as described in detail previously (Bailey and Fregosi 2004; Bailey et al. 2001, 2005; Fuller et al. 1999; John et al. 2005). The hypoglossal nerves bifurcate into medial and lateral branches: the former innervates the tongue protrudor muscles (genioglossus, intrinsic tongue protrudor muscles), and the latter innervates the retractor muscles (HG, styloglossus and intrinsic retractor muscles). Because our focus was on tongue retraction force, in particular that produced by the HG muscle, the medial branches of the hypoglossal nerves were severed at the bifurcation to eliminate protrusion force.
Rats were placed supine in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) and secured with ear bars. The maxilla was secured to the frame with flexible tubing to minimize any respiratory artifact in the recordings and to ensure that the mouth remained open throughout the experiments. The tongue was attached to a force displacement transducer (FT-03, Grass Instruments, West Warwick, RI) by a silk loop sutured through the center of the tongue, as described in detail previously (Fuller et al. 1998; Gilliam and Goldberg 1995). The force transducer was configured without springs, providing a reliable force resolution of 2 mg to 50 g, and was driven by a DC strain-gauge amplifier (Grass Instruments P 122, West Warwick, RI). The force transducer was secured to a micromanipulator, which allowed for fine control of the position and tension of the tongue, as described in detail previously (Fuller et al. 1998, 1999; Fuller and Fregosi 2000). At the beginning of each experiment the optimal length-tension relationship of the muscle was determined by systematically manipulating tongue position, and thus muscle length, until peak, respiration-related force was achieved (Fuller et al. 1998, 1999; Fuller and Fregosi 2000). The optimal length obtained in this manner was maintained throughout the experiment, allowing us to standardize the force measurements both within and between experiments.
HG muscle motor unit and whole muscle EMG recordings.
We recorded the respiration-related firing rate of 38 HG motor units. The number of motor units recorded from each animal ranged from one to four, with a median of two units per animal. Motor unit potentials were recorded with high-impedance (10 MΩ) tungsten microelectrodes (Frederick Haer, Bowdoin, ME) that were inserted into the muscle belly using a manually operated micromanipulator (Narishige, Tokyo, Japan). The recording electrode was referenced to a second electrode inserted into adjacent skin flaps, and both electrodes were referenced to a common ground. As done previously (John et al. 2005), the EMG was differentially amplified (model 7WU16K; Grass Instruments, West Warwick, RI), filtered between 300 and 10,000 Hz, and monitored on a storage oscilloscope and computer screen. When a single motor unit potential was clearly and unambiguously distinguished, the animal was allowed to breathe undisturbed while the motor unit activity and tongue muscle retraction force were recorded for 15–30 min. If a motor unit could not be clearly distinguished with visual inspection under both low- and high-force conditions, it was excluded from analysis. Voltage outputs from the force and microelectrode amplifiers were fed to an analog-to-digital converter, which sampled motor unit potentials at 20,000 Hz and force at 5,000 Hz.
Whole muscle EMG of the HG was recorded in nine of the animals using two fine hook wire electrodes inserted into the muscle belly and spaced about 2 mm apart, as described previously (Bailey and Fregosi 2004; Bailey et al. 2005; Fuller et al. 1998; Fuller and Fregosi 2000; Janssen and Fregosi 2000; Janssen et al. 2000). The signal was amplified and filtered (30–300 Hz) and sent in parallel to an oscilloscope and an analog-to-digital converter, and sampled at 5,000 Hz. All recorded data were stored on a hard drive and subsequently backed up on CD-ROM disks. Data were analyzed offline by identifying 20 consecutive respiratory cycles where peak inspiratory tongue retraction force, an index of respiratory drive (Fuller et al. 1998), was stable. We then searched the record for another period of stable retraction force that was at least 30% different than the first analyzed data set. This was possible because respiratory drive transiently changes in urethane-anesthetized rats (Fregosi and Fuller 1997; Janssen et al. 2000; Pagliardini et al. 2012), and we took advantage of this to make comparisons at two levels of respiratory drive to the HG motor unit pool, which we refer to as low-force and high-force conditions.
For each of the 20 breath cycles analyzed in a sequence, we computed the peak tongue retraction force and the peak, area and average (area/duration) of the rectified and low-pass-filtered whole muscle EMG, which we will refer to as the integrated EMG (iEMG). For each motor unit recording, we computed the average and peak motor unit firing rate, the duration of the motor unit spike train, the force at spike onset and the number of action potentials generated in each respiratory cycle (N/cycle). In addition, we calculated the coefficient of variation (CV) of the interspike intervals (ISIs) as an index of motor unit discharge rate variability. Because the firing rates of motor units driven by a central pattern generator follow a stereotyped trend that could bias measures of variability, we computed a detrended CV of ISIs using the following method, which is based on a “floating” standard deviation (Nelson et al. 1984, 1983). To determine the detrended CV, we computed a running average ISI using a five-spike moving window for all motor units that had a minimum of five spikes in each respiratory cycle in both low-force and high-force conditions. The error of each ISI was then computed relative to the five-spike moving window centered on each ISI. These ISI error values were squared and then summed to get the sum of squares. The sum of squares was then used to calculate the standard deviation (SD), and the detrended CV was taken as the SD divided by the mean ISI within each respiratory burst.
To arrive at the mean value for each motor unit, we computed the average value over 20 respiratory cycles for each of the measured variables. We then computed the grand mean for the 38-motor unit population. The grand means in low-force and high-force conditions were compared with paired t-tests, using P < 0.05 as the threshold for statistical significance. We also used linear regression analysis to examine relations between tongue retraction force and select motor unit firing properties, and between force and the iEMG.
RESULTS
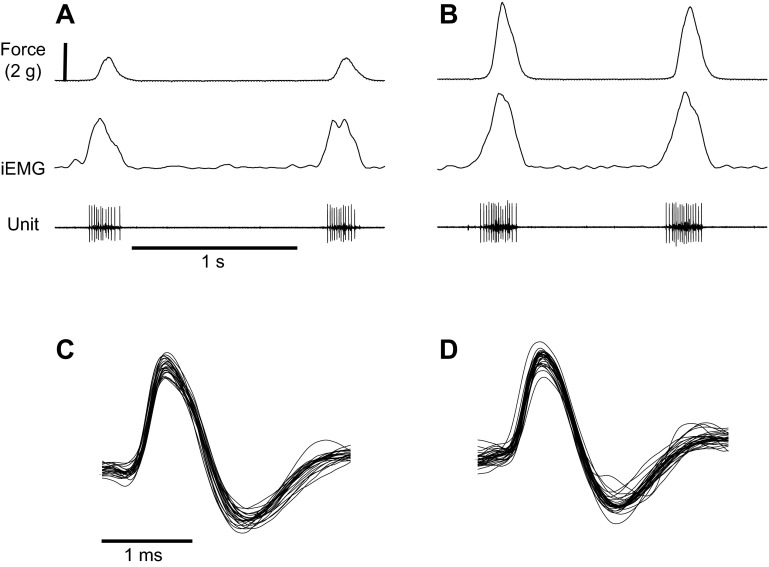
We recorded the activity of 38 HG muscle motor units in spontaneously breathing, urethane-anesthetized rats. For each unit, we computed motor unit firing characteristics under low-force and high-force conditions, which occurred spontaneously as respiratory drive to the muscle changed randomly, as is often observed in urethane-anesthetized adult rats (Fregosi and Fuller 1997; Pagliardini et al. 2012). Figure 1 shows a representative recording of tongue muscle retraction force, the HG iEMG and the activity of a HG motor unit under low (panel A) and high (panel B) force conditions. The higher force condition was associated with an increase in iEMG, a modest increase in motor unit firing rate, and an increase in spike train duration. Figure 1, C and D, show all of the spikes shown in panel A and panel B, respectively, overlaid on top of one another. This analysis shows that the motor unit was well discriminated, and that the recording was stable across low- and high-force conditions.
Fig. 1.
Respiration-related tongue muscle force output and the discharge of a hyoglossus muscle motor unit, showing two successive breath cycles, under low-force (A) and high-force (B) conditions. From top down, traces include tongue retraction force, the rectified and integrated EMG (iEMG), and single motor unit potentials recorded with a high-impedance microelectrode. In this example, the average firing rate was 63 Hz and 74 Hz at low-and high-force conditions, respectively, a bit higher than the average values shown in Fig. 4 and Table 1. C and D: overlays of all successive spikes from the two breath cycles shown in A and B, respectively. The averaged potential in C consists of 23 spikes, while that in D consists of 31 spikes. Note the consistency of motor unit discharge, even though the muscle was actively contracting.
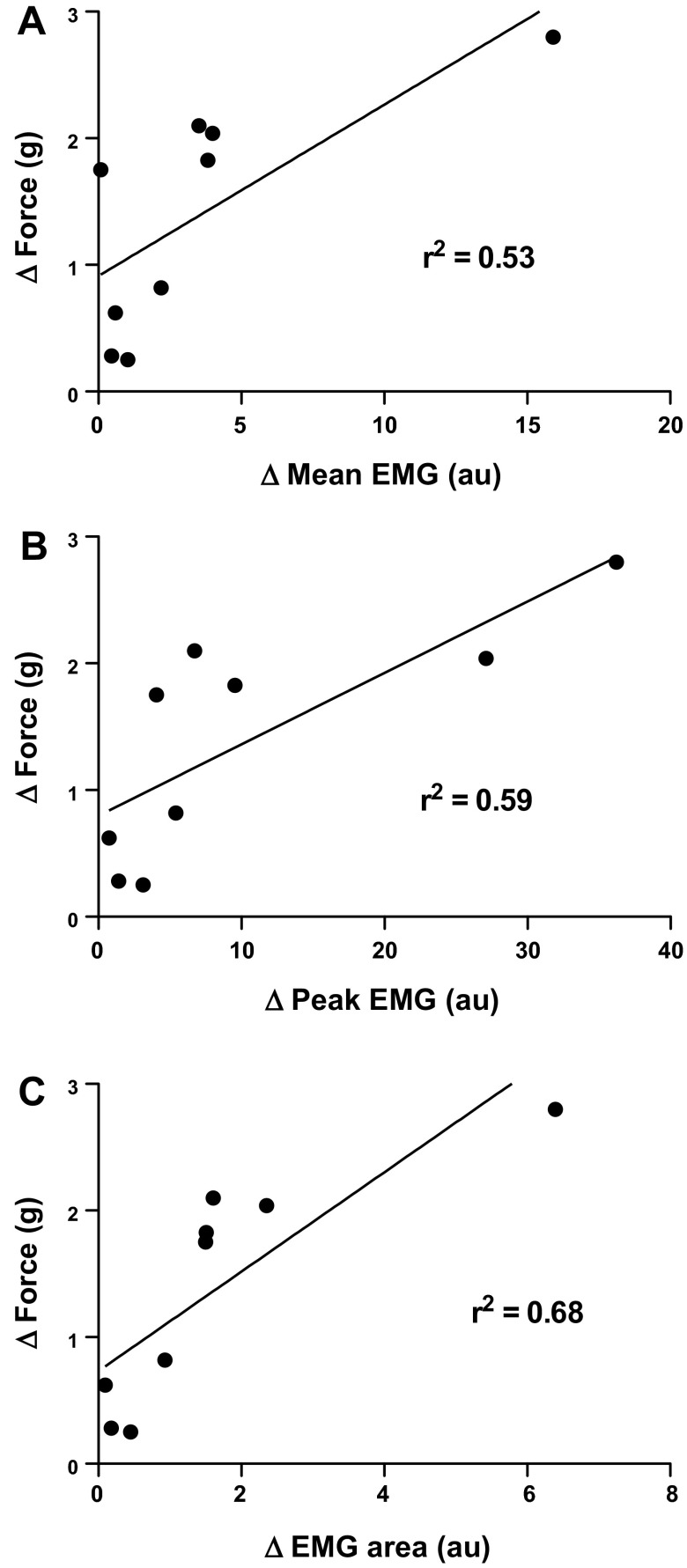
Figure 1 also shows that the change in peak tongue retraction force from the low- to the high-force condition exceeded the change in the peak HG iEMG. To estimate the contribution of the HG muscle to the total, measured tongue retraction force, we used correlation analyses between changes in force and three different iEMG variables for the nine animals in which we had recordings of whole muscle EMG, as shown in Fig. 2. Changes in mean iEMG (top panel; y = 0.14x + 0.9, F = 7.7, P = 0.027), peak iEMG activity (middle panel; y = 0.05x + 0.8, F = 10.3, P = 0.015) and iEMG area (bottom panel; y = 0.39x + 0.7, F = 15, P = 0.006) all correlated significantly with the change in tongue retraction force and could explain between 53 and 68% of the change in force (refer to the r2 values in Fig. 2). Because one animal had a relatively large change in force (2.8 g), we were concerned that this data point would have an excessively large influence on the correlation analysis. Accordingly, we removed this data point from all three of the correlation analyses. We found that for the data shown in Fig. 2A (mean EMG-force), the r2 drops from 0.53 to 0.47; for panel B (peak EMG-force), the r2 drops from 0.59 to 0.39; and for panel C (EMG area-force), the r2 actually increased, from 0.68 to 0.86. Thus the influence of this single data point on the linear regression analysis is not large, and it actually weakens or strengthens the correlation, depending on which EMG variable is used.
Fig. 2.
In 9 of the 28 animals used, we also recorded hyoglossus muscle EMG with hook wire electrodes, to estimate the muscle's motor unit population activity at low- and high-force levels. In this figure we look at the change (Δ) in three EMG variables as a function of the change in tongue retraction force, from the low- to the high-force condition. All three variables [mean EMG (A), peak EMG (B), and EMG area (C)] were computed from the rectified and smoothed EMG (see materials and methods). The correlation values could explain between 53 and 68% of the variance in force, depending on which variable was used for the estimate. au, Arbitrary units.
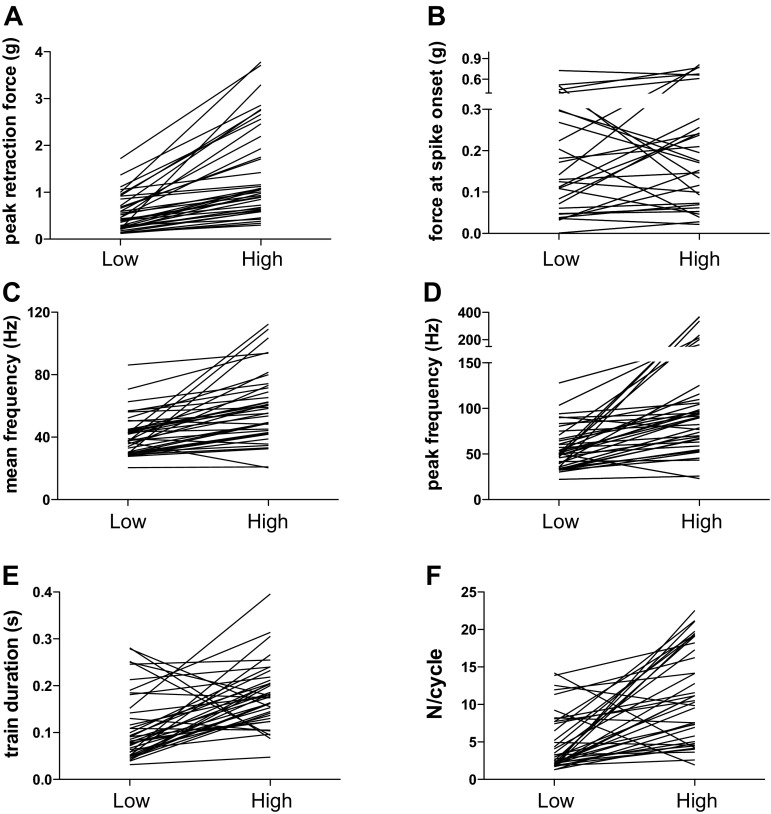
Quantitative analyses of motor unit firing characteristics under low- and high-force conditions are shown in Table 1 and Fig. 3. Peak tongue retraction force averaged 0.5 and 1.4 g in the low- and high-force condition, respectively (Table 1). The difference in peak tongue retraction force between low- and high-force conditions varied widely, as shown in Fig. 3A. Similarly, the 38 motor units that were studied showed a wide range of recruitment thresholds (force at spike onset, Fig. 3B), and the thresholds did not change significantly between low- and high-force conditions (Table 1). Mean and peak firing rates were greater in the high-force condition (Table 1, Fig. 3, C and D), although the difference varied widely across the sampled population. Nonetheless, all but three motor units increased their firing rate, with the three outliers showing very slight declines in both peak and mean firing rate. The duration of the motor unit spike train was significantly longer in the high-force condition (Table 1, Fig. 3E), with 30 of 38 motor units showing increased train duration. The number of spikes generated in each respiratory cycle (N/cycle) also rose significantly from the low- to the high-force condition (Table 1, Fig. 3F).
Table 1.
Analysis of hyoglossus motor unit firing rates under low-force and high-force conditions
| Low Force | High Force | P Value | |
|---|---|---|---|
| Peak retraction force, g | 0.5 ± 0.4 (0.1–1.7) | 1.4 ± 1 (0.3–3.8) | <0.0001 |
| Force at spike onset, g | 0.2 ± 0.2 (0.0–0.8) | 0.3 ± 0.2 (0.0–0.8) | 0.42 |
| Mean frequency, Hz | 41.4 ± 13.3 (20.4–86.1) | 59.1 ± 22.7 (20.4–112.3) | <0.0001 |
| Peak frequency, Hz | 55.7 ± 23.4 (22.2–127.9) | 112.9 ± 85.9 (22.9–369.0) | 0.0002 |
| N/cycle | 4.9 ± 3.8 (1–14) | 11.1 ± 6.2 (2–22) | <0.0001 |
| Spike train duration, s | 0.11 ± 0.07 (0.03–0.28) | 0.18 ± 0.07 (0.05–0.4) | 0.0001 |
| CV of ISI | 0.23 ± 0.06 (0.12–0.33) | 0.24 ± 0.08 (0.14–0.46) | 0.16 |
Values are means ± SD (range). N/cycle, number of spikes per respiratory cycle; CV, coefficient of variation; ISI, interspike interval. P values were derived from paired, two-tailed t-tests comparing low and high force for each variable.
Fig. 3.
Motor unit discharge variables, showing average data recorded at low force and high force for each of the 38 motor units that were studied. A: peak retraction force in grams. B: tongue retraction force recorded at spike onset. C: mean motor unit discharge frequency. D: peak motor unit discharge frequency. E: duration of the motor unit spike train. F: number of spikes per respiratory cycle (N/cycle).
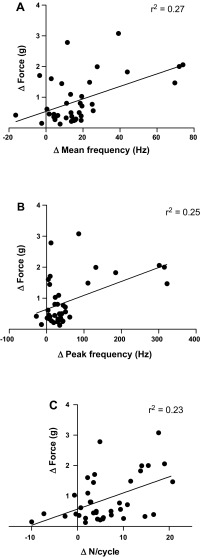
Figure 4, A and B, shows the results of correlation analyses between the change in tongue retraction force, as a function of mean (panel A) and peak (panel B) motor unit firing rate, and N/cycle (panel C). For this analysis we assume that peak tongue retraction force is a reliable index of the inspiration-related neural drive to the HG muscle motoneuron pool (Fuller et al. 1998), although as indicated above not all of the measured force is attributable to the HG muscles. All three variables correlated significantly with the change in force, although the correlations were weak (see the r2 values in Fig. 4). Results of the correlation analyses are as follows: mean frequency, y = 0.02x + 0.54, F = 13.50, P = 0.0008; peak frequency, y = 0.004x + 0.64, F = 12.1, P = 0.0013; N/cycle, y = 0.05x + 0.57, F = 10.9, P = 0.002.
Fig. 4.
Linear regression analysis of the change in three different motor unit firing properties [mean frequency (A); peak frequency (B); N/cycle (C)] as a function of the change in force between low-force and high-force conditions. Although significant, the r2 values show that these three firing rate properties are poor predictors of the change in tongue muscle force output.
The change in firing rate variability at low and high force, which was estimated by calculating the detrended CV of the ISIs, is shown in Table 1. For this analysis we report data on only 13 of the 38 motor units because the remaining units had fewer than 5 spikes per respiratory cycle in the low-force condition, and the latter is the minimum number of spikes required for an accurate analysis (see materials and methods). Nonetheless, the CV ISI was low and nearly identical in the low- and high-force condition. To confirm the low spike train variability, we analyzed the change in mean firing rate as a function of the change in peak firing rate between the high- and low-force conditions, for all 38 motor units. The correlation was very high (y = 4.2x − 16.4, r2 = 0.91, P < 0.0001), consistent with low spike train variability (data not shown).
DISCUSSION
Summary.
By taking advantage of spontaneous changes in drive to the HG muscle motoneuron pool (see below), we were able to examine the respiration-related activity of HG motor units under a low and a higher force condition. Although force output differed by almost threefold in low- and high-force conditions, mean and peak firing rates increased more modestly (by 43 and 100%, respectively). Indeed, correlation analyses suggest that changes in peak or mean frequency, or N/cycle, can explain only 23–27% of the corresponding change in muscle force. In contrast, changes in whole muscle HG iEMG activity could explain up to 68% of the change in muscle force. Taken together, these observations suggest that a significant portion of the increase in iEMG activity in the high-force condition was due to the recruitment of motor units that were inactive under low-force conditions. Quantifying the contributions of recruitment and firing rate to the change in force, however, is not possible given that we were unable to attribute the entire change in force to actions of the HG muscle (see below). Nonetheless, correlation analyses suggest that motor unit recruitment is more important than increases in firing rate for regulating the respiration-related force output of the HG muscle, at least under conditions of low-to-moderate levels of muscle force.
Critique of methods.
Thoracic traction on the pharyngeal airway, as elegantly studied and reported by Van de Graaff (1988), pulls the pharynx toward the lungs and could contribute to the tongue retraction force that we measured. However, for four reasons we believe that that this effect, if it occurred at all, was minimal. First, we previously showed that cutting all hypoglossal nerve branches abolishes tongue muscle force in the rat (see Fig. 6 in Fuller et al. 1998). Second, in our model the trachea is severed so it is unable to apply significant traction to the upper airway. Although other extrathoracic soft tissues such as the esophagus and mediastinal tissues could pull the pharynx caudally as the lungs inflate, in his paper Van de Graaff (1988) indicates clearly that the trachea is the most important structure contributing to tracheal traction, and that studies wherein the trachea or other lower cervical structures are severed likely fail to capture the influence of longitudinal tracheal traction or thoracic activity on the pharynx. Third, we rigidly fixed the rat's head in a stereotaxic frame which greatly minimizes the influence of thoracic traction on the pharynx. Indeed, the dogs studied by Van de Graaff (1988) were not stabilized in a stereotaxic frame, but when they clamped the lower cervical spine in three animals to minimize head and neck movements, the influence of tracheal traction was abolished. Fourth, in our dissection, the digastric, mylohyoid, and geniohyoid muscles are removed bilaterally to expose the hypoglossal nerves and the HG muscles, and this would also minimize extraneous forces that might either protrude or retract the tongue.
Another methodological issue that needs to be explored is our use of the whole muscle EMG to estimate respiration-related neural drive to the muscle. The correlation values computed from the relation between the change in three different EMG variables and corresponding changes in HG muscle force (Fig. 2) are significant, but it is clear that the EMG is unable to perfectly predict muscle force. This is likely due to a combination of factors, principally the activity of other tongue retractor muscles such as the styloglossus and the intrinsic retractor muscles. Other factors that weaken the correlation between muscle force and EMG activity include low-pass filtering of the EMG by soft tissues (Clancy et al. 2002), contamination by volume conduction from adjacent muscles, motor unit action potential cancellation (McDonald et al. 2013) and even the type of the contraction studied (Staudenmann et al. 2010). Interestingly, the data demonstrate that the change in EMG area is the best predictor of the change in muscle force, which is consistent with data in rat limb muscle (Enoka et al. 1989).
We also note that the contribution of motor unit recruitment to the spontaneous changes in muscle force were estimated, rather than directly measured. Specifically, we estimated recruitment by correlating the changes in motor unit discharge rate with corresponding changes in muscle force, assuming that a correlation coefficient of 1.0 would indicate that the entire change in force was due to rate coding. The high-impedance electrodes used here are designed to clearly discriminate single motor unit potentials with very high signal-to-noise ratios (Fig. 1). The high selectivity of these electrodes allowed us to track motor units reliably as muscle force changed modestly. But the high selectivity also prohibited the observation of recruitment, as the electrodes did not detect the activity of other motor units that may have been near the electrode tip and recruited as muscle force increased. In our laboratory's previous study (John et al. 2005) of genioglossus motor units during hypercapnia, we found that hypercapnia often led to the recruitment of many motor units, precluding our ability to discriminate the target neuron from the freshly recruited ones. As a result, in that study as here, we had to estimate recruitment by using muscle EMG activity and correlation analyses. Studies designed to rigorously measure motor unit recruitment in mammalian tongue muscles are needed.
HG muscle and motor unit discharge patterns, and the contributions of rate coding and recruitment to muscle force output.
We confirmed earlier work indicating that the rodent HG muscle contracts primarily during the inspiratory phase of the respiratory cycle in rats (Bailey and Fregosi 2004; Bailey et al. 2005; Fuller et al. 1998; Fuller and Fregosi 2000; Janssen and Fregosi 2000; Janssen et al. 2000) and in human subjects studied during hypercapnia (Mateika et al. 1999). This is consistent with our findings here, showing that all 38 motor units discharged during the inspiratory phase of the respiratory cycle. The rat genioglossus muscle also shows strong inspiratory phasic discharge, with the majority of the motor units also strongly inspiratory modulated (John et al. 2005). Similarly, rodent intrinsic tongue muscles also discharge during the inspiratory phase (Bailey et al. 2005), although intrinsic muscle single motor unit activities have not been examined. Thus, in rodent tongue muscles, motor unit discharge is confined almost exclusively to the inspiratory phase of the breathing cycle.
Other motor unit discharge patterns in the genioglossus muscle of human subjects have been observed (Bailey et al. 2007; Saboisky et al. 2006), although inspiratory phasic discharge and/or tonic discharge with inspiratory phase firing rate modulation are by far the most common respiration-related discharge patterns reported. Similarly, two or more respiration-related discharge patterns have been reported in anesthetized or decerebrate cats (Hwang et al. 1983; Mitra and Cherniack 1983; Withington-Wray et al. 1988), but as in rodents and human subjects, the great majority of the motoneurons studied show pure phasic inspiratory discharge, although a small fraction of units commence discharge late in expiration and/or continue to fire throughout the early expiratory period. It is important to emphasize that in these studies in cats the recordings were made from axons in the hypoglossal nerves or from unidentified neurons in the hypoglossal motor nucleus. As a result, the muscular targets of the axons and motor neurons are unknown.
In summary, available data demonstrate that the respiration-related activity of hypoglossal motoneurons innervating both extrinsic and intrinsic tongue muscles in mammals, including humans, is strongly inspiratory modulated. These data are consistent with the major respiratory-related function of the tongue muscles, which is dilating and stiffening the pharynx during inspiration when pharyngeal transmural pressure is negative. We note, however, that in the present study we were likely biased toward sampling low-threshold units, as our absolute levels of tongue retraction force never exceeded about 30% of the value obtained under conditions of maximal respiration-related drive in this model (6–7 g; Fuller et al. 1998). Thus we cannot say with certainty that all respiration-related HG motor units have a phasic, inspiratory discharge pattern.
The majority of the motor units studied here showed rate coding as force spontaneously increased, with the average mean discharge frequency increasing from 41 to 59 Hz (Table 1). However, five of the units studied had increases in firing rate that exceeded 2 SD of the average increase. If these five units are excluded from the analysis, the mean firing rate averages 42 Hz under low-force conditions and 53 Hz in the high-force condition. In our laboratory's previous study of genioglossus muscle motor units, the mean firing rate increased from 55 to about 85 Hz from baseline to maximal hypercapnic stimulation (John et al. 2005), although we did not measure tongue muscle force output in that study. Instead, we used the average whole muscle EMG as an index of muscle force, and estimated that increases in motor unit firing rate accounted for 15–20% of the change in whole muscle EMG activity, consistent with the results reported here (Fig. 4). These observations are consistent with data in the diaphragm muscle of cats (Bishop et al. 1981; Iscoe et al. 1976) and rabbits (Road et al. 1995), where recruitment was shown to be the dominant mechanism for increasing force within a normal breath cycle, and also when animals were challenged with chemoreceptor stimulation (Iscoe et al. 1976) or changes in transpulmonary pressure (Bishop et al. 1981; Road et al. 1995). Recent studies in the human genioglossus show either no rate coding with sleep (Bailey et al. 2007) or hypercapnia (Richardson and Bailey 2010), or minimal changes in firing rate (Saboisky et al. 2010) (1–2 Hz) even under conditions where breathing was stimulated by increasing the alveolar CO2 by as much as 10 mmHg. Interestingly, work in rat and cat hindlimb muscles also show that modulation of firing rate is less important for grading weak contractions than strong ones (Bakels and Kernell 1994), and a recent study in mouse hindlimb motor units showed that force modulation is due largely to the number of motor units recruited (Manuel and Heckman 2011).
Our experimental design, focused on systems output in a behaving animal model, did not allow us to determine why the tongue muscles appear to favor recruitment over rate coding when grading respiration-related force output. Of interest in this regard are data suggesting that the nervous system can use different strategies for different muscles (De Luca et al. 1982; Kukulka and Clamann 1981; Oya et al. 2009). Although the reason for this is unknown, the tongue muscles may indeed employ different strategies than limb muscles because of their unique anatomy and functional requirements. For example, the tongue muscles do not cross a joint, have very few muscle spindles (O'Reilly and FitzGerald 1990), and are unattached on one end (extrinsic muscles) or both ends (intrinsic muscles), allowing movements in multiple planes. Moreover, previous work in both animal models (Bailey and Fregosi 2004; Bailey et al. 2005; Fuller et al. 1998; Janssen et al. 2000) and human subjects (Mateika et al. 1999) shows that multiple tongue muscles are coactivated in phase with inspiration, suggesting that drive to the hypoglossal motor nucleus is distributed broadly, both within and across muscle motoneuron pools. Such “cross muscle strategies” allow motor units from several muscles to be activated simultaneously to produce the intended movement (Sokoloff 2004), consistent with the muscular hydrostat hypothesis of tongue motor control (Kier and Smith 1985). This would allow several muscles to share the load, with each muscle's contribution composed of only its small, low-threshold units, allowing crucial behaviors, such as breathing and swallowing, to occur with relatively low-energy expenditure. Support for this idea comes from a recent study showing that the incidence of correlated firing in pairs of tongue muscle motor units is significantly lower than that in the diaphragm and external intercostal muscles, consistent with broadly distributed premotor input to the hypoglossal motoneuron pool (Rice et al. 2011). However, an important caveat is that in the present study, and in the majority of studies done in anesthetized animal models, the upper airway is bypassed by tracheotomy. Under these conditions, potent excitatory input from mucosal mechanoreceptors in the upper airway is lost, and this may reduce the magnitude of rate coding by removing an important source of excitation.
The duration of the motor unit spike train increased by an average of 63% between low- and high-force conditions. This is consistent with longer lasting, suprathreshold depolarizing input to hypoglossal motoneurons in the high- compared with the low-force condition. The longer train duration, coupled with the increase in mean firing rate in the high-force condition, resulted in a doubling of the number of spikes generated per respiratory cycle (Table 1). This is consistent with previous data in the genioglossus muscle (John et al. 2005), showing that the number of spikes per respiratory cycle rose from 8 under baseline conditions to 11 under conditions of maximal respiration-related drive to the muscle. In our model, the central pattern generator dictates the duration of the inspiratory phase and thus the time available to provide respiration-related depolarizing input to the hypoglossal motoneuron pool. Within this time constraint, the system can increase the intensity of excitatory synaptic input without changing its duration, or instead could keep the intensity of the synaptic input constant while prolonging its duration. It appears that both mechanisms, together with the recruitment of inactive motor units, play a role in increasing the force produced by the HG muscle during spontaneous breathing.
GRANTS
These studies were supported by the National Institute of Deafness and Other Communications Disorders Grant DC-007692.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.L.P., A.R., and S.J.B.-C. performed experiments; G.L.P., A.R., S.J.B.-C., and R.F.F. analyzed data; G.L.P. and R.F.F. interpreted results of experiments; G.L.P. and R.F.F. edited and revised manuscript; G.L.P. and R.F.F. approved final version of manuscript; R.F.F. conception and design of research; R.F.F. prepared figures.
REFERENCES
- Bailey EF. Activities of human genioglossus motor units. Respir Physiol Neurobiol 179: 14–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 96: 440–449, 2004 [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol 98: 3284–3291, 2007 [DOI] [PubMed] [Google Scholar]
- Bailey EF, Janssen PL, Fregosi RF. Po2-dependent changes in intrinsic and extrinsic tongue muscle activities in the rat. Am J Respir Crit Care Med 171: 1403–1407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 525–534, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakels R, Kernell D. Threshold-spacing in motoneurone pools of rat and cat: possible relevance for manner of force gradation. Exp Brain Res 102: 69–74, 1994 [DOI] [PubMed] [Google Scholar]
- Bishop B, Settle S, Hirsch J. Single motor unit activity in the diaphragm of cat during pressure breathing. J Appl Physiol 50: 348–357, 1981 [DOI] [PubMed] [Google Scholar]
- Bodine SC, Roy RR, Eldred E, Edgerton VR. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. J Neurophysiol 57: 1730–1745, 1987 [DOI] [PubMed] [Google Scholar]
- Butler JE, Gandevia SC. The output from human inspiratory motoneurone pools. J Physiol 586: 1257–1264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Discharge properties and recruitment of human diaphragmatic motor units during voluntary inspiratory tasks. J Physiol 518: 907–920, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy EA, Morin EL, Merletti R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J Electromyogr Kinesiol 12: 1–16, 2002 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Rankin LL, Stuart DG, Volz KA. Fatigability of rat hindlimb muscle: associations between electromyogram and force during a fatigue test. J Physiol 408: 251–270, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF. Respiratory related control of hypoglossal motoneurons–knowing what we do not know. Respir Physiol Neurobiol 179: 43–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol 110: 295–306, 1997 [DOI] [PubMed] [Google Scholar]
- Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 507: 265–276, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Fregosi RF. Fatiguing contractions of tongue protrudor and retractor muscles: influence of systemic hypoxia. J Appl Physiol 88: 2123–2130, 2000 [DOI] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 519: 601–613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaige TA, Benner T, Wang R, Wedeen VJ, Gilbert RJ. Three dimensional myoarchitecture of the human tongue determined in vivo by diffusion tensor imaging with tractography. J Magn Reson Imaging 26: 654–661, 2007 [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Gorman RB, McKenzie DK, De Troyer A. Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med 160: 1598–1603, 1999 [DOI] [PubMed] [Google Scholar]
- Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol 74: 547–555, 1995 [DOI] [PubMed] [Google Scholar]
- Hwang JC, Bartlett D, Jr, St. John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol 55: 793–798, 1983 [DOI] [PubMed] [Google Scholar]
- Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol 26: 113–128, 1976 [DOI] [PubMed] [Google Scholar]
- Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol 89: 1345–1351, 2000 [DOI] [PubMed] [Google Scholar]
- Janssen PL, Williams JS, Fregosi RF. Consequences of periodic augmented breaths on tongue muscle activities in hypoxic rats. J Appl Physiol 88: 1915–1923, 2000 [DOI] [PubMed] [Google Scholar]
- John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med 172: 1331–1337, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K, Hashizume K. Factors causing difference in force output among motor units in the rat medial gastrocnemius muscle. J Physiol 448: 677–695, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D. Principles of force gradation in skeletal muscles. Neural Plast 10: 69–76, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier WM, Smith KK. Tongue tentacles and trunks: the biomechanics of movement in muscular hydrostats. Zool J Linn Soc 83: 307–324, 1985 [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res 219: 45–55, 1981 [DOI] [PubMed] [Google Scholar]
- Manuel M, Heckman CJ. Adult mouse motor units develop almost all of their force in the subprimary range: a new all-or-none strategy for force recruitment? J Neurosci 31: 15188–15194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 160: 1976–1982, 1999 [DOI] [PubMed] [Google Scholar]
- McDonald AC, Sanei K, Keir PJ. The effect of high pass filtering and nonlinear normalization on the EMG-force relationship during sub-maximal finger exertions. J Electromyogr Kinesiol 23: 564–571, 2013 [DOI] [PubMed] [Google Scholar]
- Mitra J, Cherniack NS. The effects of hypercapnia and hypoxia on single hypoglossal nerve fiber activity. Respir Physiol 54: 55–66, 1983 [DOI] [PubMed] [Google Scholar]
- Nelson RM, Soderberg GL, Urbscheit NL. Alteration of motor-unit discharge characteristics in aged humans. Phys Ther 64: 29–34, 1984 [DOI] [PubMed] [Google Scholar]
- Nelson RM, Soderberg GL, Urbscheit NL. Comparison of skeletal muscle motor unit discharge characteristics in young and aged humans. Arch Gerontol Geriatr 2: 255–264, 1983 [DOI] [PubMed] [Google Scholar]
- O'Reilly PM, FitzGerald MJ. Fibre composition of the hypoglossal nerve in the rat. J Anat 172: 227–243, 1990 [PMC free article] [PubMed] [Google Scholar]
- Oya T, Riek S, Cresswell AG. Recruitment and rate coding organisation for soleus motor units across entire range of voluntary isometric plantar flexions. J Physiol 587: 4737–4748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Greer JJ, Funk GD, Dickson CT. State-dependent modulation of breathing in urethane-anesthetized rats. J Neurosci 32: 11259–11270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A, Fuglevand AJ, Laine CM, Fregosi RF. Synchronization of presynaptic input to motor units of tongue, inspiratory intercostal, and diaphragm muscles. J Neurophysiol 105: 2330–2336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PA, Bailey EF. Tonically discharging genioglossus motor units show no evidence of rate coding with hypercapnia. J Neurophysiol 103: 1315–1321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Road J, Osborne S, Cairns A. Phrenic motoneuron firing rates during brief inspiratory resistive loads. J Appl Physiol 79: 1540–1545, 1995 [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 95: 2213–2221, 2006 [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Jordan AS, Eckert DJ, White DP, Trinder JA, Nicholas CL, Gautam S, Malhotra A. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol 109: 1939–1949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol 85: 316–335, 1984 [DOI] [PubMed] [Google Scholar]
- Smith JC, Goldberg SJ, Shall MS. Phenotype and contractile properties of mammalian tongue muscles innervated by the hypoglossal nerve. Respir Physiol Neurobiol 147: 253–262, 2005 [DOI] [PubMed] [Google Scholar]
- Sokoloff AJ. Activity of tongue muscles during respiration: it takes a village? J Appl Physiol 96: 438–439, 2004 [DOI] [PubMed] [Google Scholar]
- Staudenmann D, Roeleveld K, Stegeman DF, van Dieen JH. Methodological aspects of SEMG recordings for force estimation–a tutorial and review. J Electromyogr Kinesiol 20: 375–387, 2010 [DOI] [PubMed] [Google Scholar]
- Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol 65: 2124–2131, 1988 [DOI] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during arousal from sleep. Sleep 33: 379–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience 25: 1041–1051, 1988 [DOI] [PubMed] [Google Scholar]