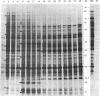
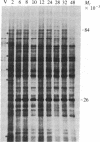
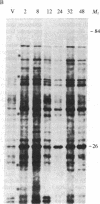
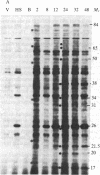
Abstract
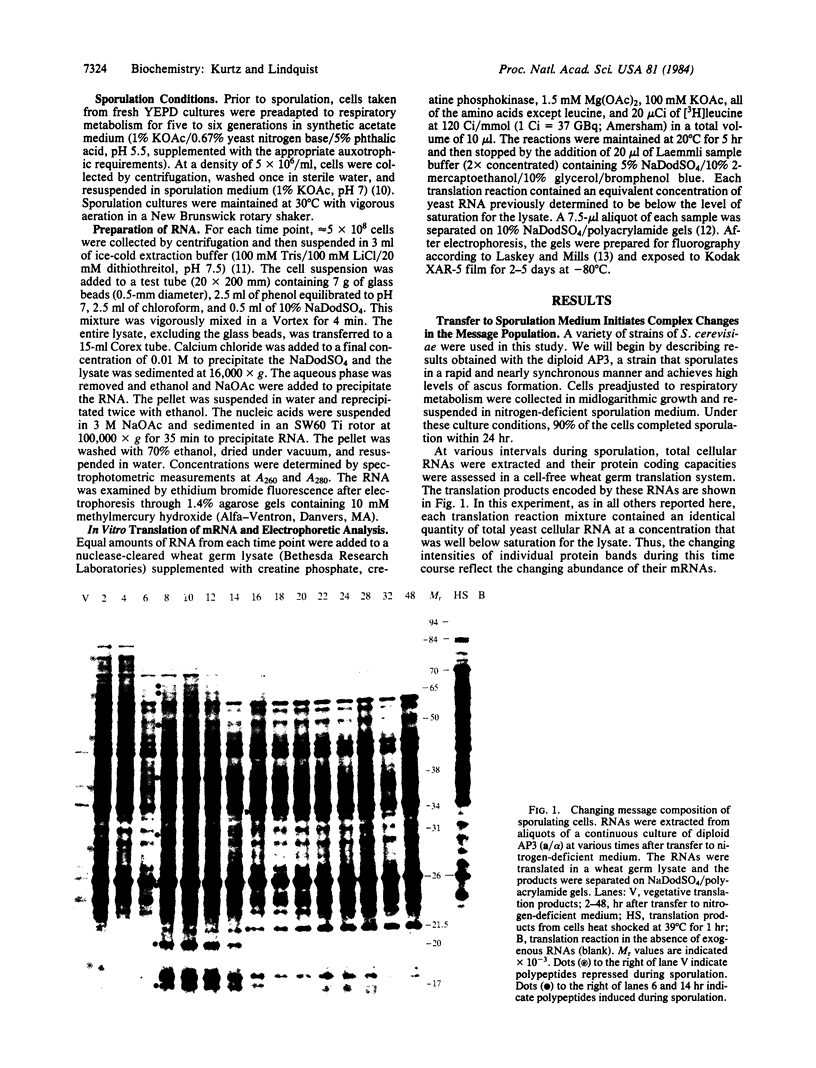
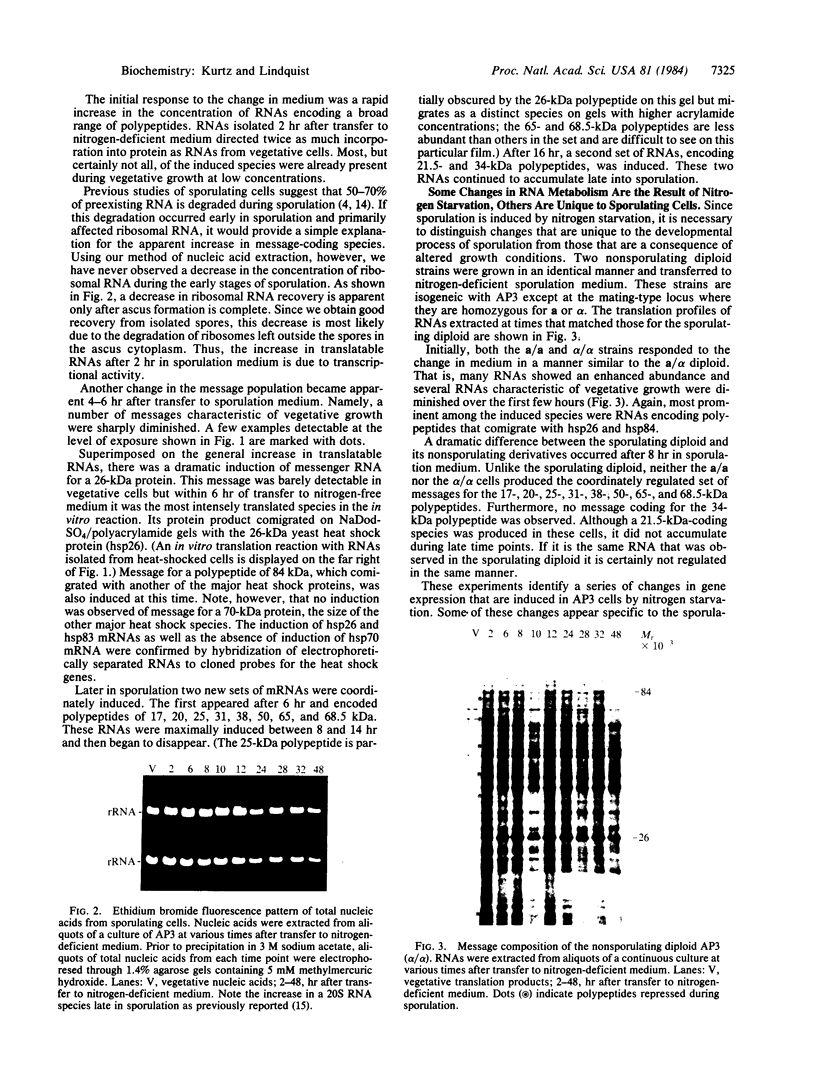
Analysis of RNAs isolated from the yeast Saccharomyces cerevisiae reveals a dramatic series of changes in protein coding sequences during sporulation. Shortly after transfer to sporulation medium, mRNAs for certain proteins are repressed while a broad array of mRNAs for other proteins is induced. Superimposed on this general increase in transcriptional activity is the very strong induction of a particular subset of heat shock mRNAs, the same subset that is induced during the normal course of oogenesis in Drosophila. At distinct times later in sporulation, two sets of abundant mRNAs are coordinately induced. Unlike the earlier changes in the message complement, these changes are unique to sporulating cells. As asci mature, one set of sporulation-specific RNAs is selectively degraded. The second set, as well as the broad array of mRNAs induced earlier in development, is retained in a highly stable and fully translatable form.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chia L. L., McLaughlin C. The half-life of mRNA in Saccharomyces cerevisiae. Mol Gen Genet. 1979 Feb 26;170(2):137–144. doi: 10.1007/BF00337788. [DOI] [PubMed] [Google Scholar]
- Clancy M. J., Buten-Magee B., Straight D. J., Kennedy A. L., Partridge R. M., Magee P. T. Isolation of genes expressed preferentially during sporulation in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 May;80(10):3000–3004. doi: 10.1073/pnas.80.10.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast D. Sporulation synchrony of Saccharomyces cerevisiae grown in various carbon sources. J Bacteriol. 1973 Nov;116(2):925–930. doi: 10.1128/jb.116.2.925-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Clancy M. J., Magee P. T. Properties of polyadenylate-associated ribonucleic acid from Saccharomyces cerevisiae ascospores. J Bacteriol. 1980 Aug;143(2):958–965. doi: 10.1128/jb.143.2.958-965.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel P. M., Bautz E. K. Heat-shock proteins are associated with hnRNA in Drosophila melanogaster tissue culture cells. EMBO J. 1983;2(5):705–710. doi: 10.1002/j.1460-2075.1983.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig E., Haber J. E. Messenger ribonucleic acid and protein metabolism during sporulation of Saccharomyces cerevisiae. J Bacteriol. 1980 Dec;144(3):1098–1112. doi: 10.1128/jb.144.3.1098-1112.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Linn T., Losick R. The program of protein synthesis during sporulation in Bacillus subtilis. Cell. 1976 May;8(1):103–114. doi: 10.1016/0092-8674(76)90191-4. [DOI] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Lynn R. R., Magee P. T. Development of the spore wall during ascospore formation in Saccharomyces cerevisiae. J Cell Biol. 1970 Mar;44(3):688–692. doi: 10.1083/jcb.44.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival-Smith A., Segall J. Isolation of DNA sequences preferentially expressed during sporulation in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):142–150. doi: 10.1128/mcb.4.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew B. J., Friesen J. D., Moens P. B. Two-dimensional protein patterns during growth and sporulation in Saccharomyces cerevisiae. J Bacteriol. 1979 Apr;138(1):60–69. doi: 10.1128/jb.138.1.60-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir-Thompson E. M., Dawes I. W. Developmental changes in translatable RNA species associated with meiosis and spore formation in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Apr;4(4):695–702. doi: 10.1128/mcb.4.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejksnora P. J., Haber J. E. Ribonucleoprotein particle appearing during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):246–260. doi: 10.1128/jb.134.1.246-260.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]