Abstract
A large number of studies suggest that calcium triggers and accelerates vesicle endocytosis at many synapses and non-neuronal secretory cells. However, many studies show that prolonging the duration of the stimulation train, which induces more calcium influx, slows down endocytosis; and several studies suggest that instead of triggering endocytosis, calcium actually inhibits endocytosis. Here we addressed this apparent conflict at a large nerve terminal, the calyx of Held in rat brainstem, in which recent studies suggest that transient calcium increase up to tens of micromolar concentration at the micro/nano domain triggers endocytosis. By dialyzing 0–1 μm calcium into the calyx via a whole-cell pipette, we found that slow endocytosis was inhibited by calcium dialysis in a concentration-dependent manner. Thus, prolonged, small, and global calcium increase inhibits endocytosis, whereas transient and large calcium increase at the micro/nano domain triggers endocytosis and facilitates endocytosis. This yin and yang effect of calcium may reconcile apparent conflicts regarding whether calcium accelerates or inhibits endocytosis. Whether endocytosis is fast or slow depends on the net outcome between the yin and yang effect of calcium.
Keywords: calcium, endocytosis
Introduction
Exocytosis releases neurotransmitters to mediate synaptic transmission, whereas endocytosis recycles exocytosed vesicles to maintain synaptic transmission. Accumulated studies show that buffering calcium with EGTA or reducing the extracellular calcium concentration slows down endocytosis at many synapses and endocrine cells, suggesting that calcium speeds up endocytosis (Ceccarelli and Hurlbut, 1980; Artalejo et al., 1995; Henkel and Betz, 1995; Cousin and Robinson, 1998; Marks and McMahon, 1998; Moser and Beutner, 2000; Neves et al., 2001; Sankaranarayanan and Ryan, 2001; Wu et al., 2005; Balaji et al., 2008). Recent studies at calyces and hippocampal synapses show that calcium may speed up slow, rapid, and bulk endocytosis by tens of folds, suggesting that calcium triggers endocytosis (Hosoi et al., 2009; Wu et al., 2009; Sun et al., 2010). Consequently, how calcium triggers endocytosis has been actively studied (Sun et al., 2010; Yamashita et al., 2010; J. Yao et al., 2011; Yao and Sakaba, 2012; L.H. Yao et al., 2012).
In contrast to the above conclusion, prolonging the stimulation train, which increases calcium influx, prolongs endocytosis at many preparations, such as goldfish ribbon-type synapses (von Gersdorff and Matthews, 1994), frog neuromuscular junctions (Wu and Betz, 1996), hippocampal synapses (Sankaranarayanan and Ryan, 2000; Balaji et al., 2008; Sun et al., 2010), calyces (Sun et al., 2002; Wu et al., 2005; Yamashita et al., 2005; Renden and von Gersdorff, 2007), and chromaffin cells (Artalejo et al., 1995; Elhamdani et al., 2006). A pioneering study two decades ago shows that increasing the intracellular calcium concentration at goldfish ribbon-type synapses inhibits endocytosis, suggesting that calcium inhibits endocytosis (von Gersdorff and Matthews, 1994). A study of single-vesicle endocytosis at hippocampal synapses suggests that calcium inhibits endocytosis (Leitz and Kavalali, 2011). A recent study at hippocampal synapses shows that calcium speeds up endocytosis in the first few action potentials, but slows down endocytosis as action potentials continue (Armbruster et al., 2013).
Apparently, how calcium regulates endocytosis is rather controversial. We attempted to resolve this controversy at the calyx of Held nerve terminal, where studies suggest that large, transient calcium influx at >10 μm at the calcium micro/nano domain triggers slow endocytosis (Hosoi et al., 2009; Wu et al., 2009; Yamashita et al., 2010). This suggestion is based on three observations: (1) reducing calcium influx during brief depolarization by reducing the extracellular calcium concentration or the voltage driving force abolishes slow endocytosis (Wu et al., 2009); (2) the fast calcium buffer BAPTA (1–10 mm), but not the slow buffer EGTA, abolishes slow endocytosis induced by brief depolarization, suggesting that the transient calcium micro/nano domain triggers endocytosis (Hosoi et al., 2009; Wu et al., 2009; Yamashita et al., 2010); and (3) >10 μm calcium is needed to induce slow endocytosis (Hosoi et al., 2009). Here we determined whether endocytosis is inhibited by prolonged, global, but small calcium increase to ∼0.5–1 μm, which has been observed in seconds to minutes after repetitive stimulation at nerve terminals (Kamiya and Zucker, 1994; von Gersdorff and Matthews, 1994; Wu and Betz, 1996; Wu and Borst, 1999; Zucker and Regehr, 2002; Habets and Borst, 2005; Wadel et al., 2007; Xue et al., 2012). We found that prolonged, small, global calcium increase inhibits slow endocytosis, contrasting to the transient, large calcium increase that triggers endocytosis. This opposite effect may reconcile most conflicts regarding calcium's effect on endocytosis.
Materials and Methods
Animal care and use were performed in accordance with NIH guidelines and approved by the NIH Animal Care and Use Committee. Parasagittal brainstem slices (200 μm thick) containing the medial nucleus of the trapezoid body were prepared from 7- to 10-d-old male or female rats using a vibratome (Sun and Wu, 2001; Sun et al., 2004). Whole-cell capacitance measurements were made with the EPC-9 amplifier together with the software lock-in amplifier (PULSE, HEKA) that implements Lindau-Neher's technique (Sun and Wu, 2001; Sun et al., 2004). The frequency of the sinusoidal stimulus was 1000 Hz and the peak-to-peak voltage of the sine wave was ≤60 mV. We pharmacologically isolated presynaptic Ca2+ currents with a bath solution (∼22–24°C) containing the following (in mm): 105 NaCl, 20 TEA-Cl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 0.4 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate, 0.001 tetrodotoxin, 0.1 3,4-diaminopyridine, pH 7.4 when bubbled with 95% O2 and 5% CO2. The control presynaptic pipette solution contained the following (in mm): 125 Cs-gluconate, 20 CsCl, 4 MgATP, 10 Na2-phosphocreatine, 0.3 GTP, 10 HEPES, 0.05 BAPTA, pH 7.2, adjusted with CsOH. To control the free calcium concentration in the pipette, we replaced BAPTA with different concentrations of EGTA and CaCl2, as described in Results.
As in many previous studies (Wu et al., 2009; Sun et al., 2010; Xue et al., 2012), we measured the initial rate of the capacitance decay after the capacitance jump (Ratedecay) as the slope within 4 s after a 20 ms depolarization (from −80 to +10 mV, depol20ms) that induced slow endocytosis, and within 1–1.5 s after 10 pulses of 20 ms depolarization at 10 Hz (depol20ms×10).
Results
Calyces were whole-cell voltage-clamped with pipettes containing different concentrations of free calcium. We varied the concentration of the calcium buffer (EGTA or BAPTA) and Ca2+ (CaCl2) in the pipette solution in five different combinations to control free calcium concentrations at five different values: (1) 0 μm free calcium achieved with 0.05 mm BAPTA and 0 Ca2+, which mimicked the endogenous calcium buffer concentration (Borst et al., 1995; Helmchen et al., 1997) and served as the control; (2) 0.1 μm free Ca2+ (2 mm EGTA and 0.8 mm Ca2+ in the pipette); (3) 0.2 μm free Ca2+ (2 mm EGTA and 1.15 mm Ca2+ in the pipette); (4) 0.5 μm free Ca2+ (2 mm EGTA and 1.55 mm Ca2+ in the pipette); and (5) 1 μm free Ca2+ (2 mm EGTA and 1.75 mm Ca2+). The free calcium concentration was calculated based on the Max-Chelator program (Stanford University, Stanford, CA) in which the calcium dissociation constant of EGTA is 0.15 μm (Augustine and Neher, 1992; Elhamdani et al., 2006). We had previously confirmed this calculation with fluorescence intracellular calcium measurements (Wu et al., 2009).
To determine how these five different calcium concentrations affect endocytosis, we induced endocytosis with two stimulation protocols, a 20 ms depolarization (depol20ms) from −80 mV (holding potential) to +10 mV (applies if not mentioned), and 10 pulses of 20 ms depolarization at 10 Hz (depol20ms×10). These two stimuli induce two most widely observed forms of endocytosis, slow and rapid endocytosis, in the control condition with 0.05 mm BAPTA in the pipette (W. Wu et al., 2005; X.S. Wu et al., 2009). They are equivalent to 10–50 and 200 action potentials at 100–300 Hz, respectively, yet easier for manipulation and measurements of calcium currents (ICa) and endocytosis (W. Wu et al., 2005; X.S. Wu et al., 2009).
Prolonged calcium concentration increase inhibits slow endocytosis
With 0.05 mm BAPTA in the pipette, depol20ms induced an ICa of 2.3 ± 0.1 nA, a capacitance jump (ΔCm) of 458 ± 24 fF, followed by a slow monoexponential decay with a τ of 17.2 ± 3.1 s and an initial decay rate (Ratedecay, see Materials and Methods for measurement) of 29 ± 3 fF/s (n = 12, Figs. 1A, 2A). Although both τ and Ratedecay reflect endocytosis, we used Ratedecay for statistics (W. Wu et al., 2005; X.S. Wu et al., 2009; Sun et al., 2010), because τ was often too slow to estimate at higher intracellular calcium concentrations as shown later.
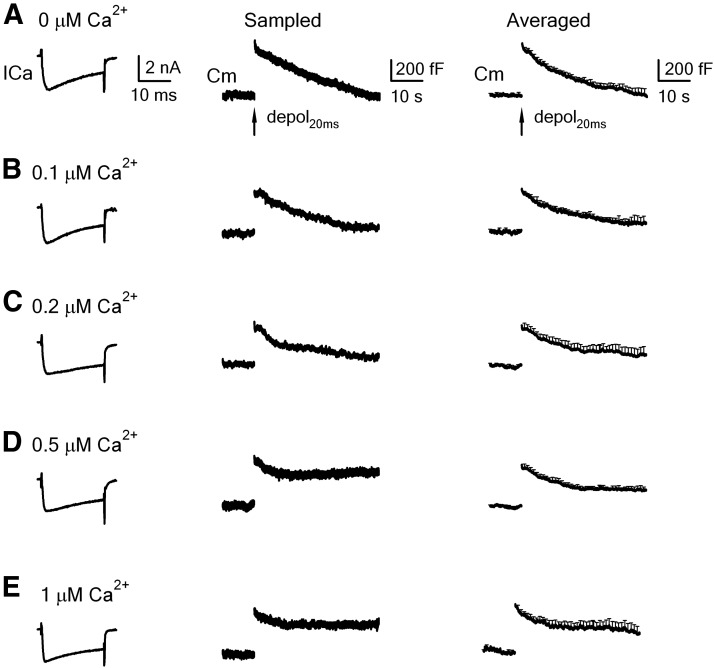
Figure 1.
Slow endocytosis at calyces being dialyzed with various concentrations of free calcium. A, Left, Sampled ICa and the capacitance change (Cm) induced by depol20ms in control (pipette solution: 0 mm calcium, 0.05 mm BAPTA in the pipette). Right, Mean Cm change (mean + SEM, n = 12 calyces) induced by a 20 ms depolarization in control (0.05 mm BAPTA). B–E, Similar to A, except that the pipette contained free calcium at 0.1 μm (B, n = 7), 0.2 μm (C, n = 9), 0.5 μm (D, n = 11), and 1 μm (E, n = 12).
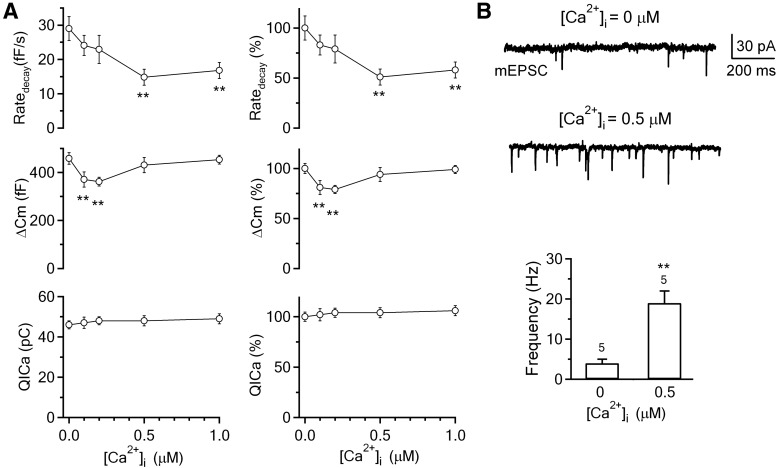
Figure 2.
Calcium dialysis into calyces inhibits slow endocytosis in a concentration-dependent manner. A, Left, The Ratedecay, ΔCm, and QICa induced by a 20 ms depolarization with a whole-cell pipette containing free calcium at 0 μm (0.05 mm BAPTA, n = 12, control), 0.1 μm (n = 7), 0.2 μm (n = 9), 0.5 μm (n = 11), and 1 μm (n = 12). Right, Same as in left, but with all data normalized to the mean of the control group (0 μm free calcium). Data are expressed as mean ± SEM. **p < 0.01. B, Sampled postsynaptic recordings of the mEPSCs and bar graphs showing the mEPSC frequency differences in calyces dialyzed with 0 (n = 5 calyces) and 0.5 μm (n = 5 calyces) free calcium. **p < 0.01.
With 0.1 μm free calcium in the pipette, depol20ms induced an ICa and Ratedecay similar to those of control (0.05 mm BAPTA, p = 0.17), but a ΔCm ∼81% of control (n = 7, p < 0.01; Figs. 1B, 2A). With 0.2 μm free calcium in the pipette, depol20ms induced an ICa and Ratedecay similar to control (p = 0.25), but a ΔCm 79% of control (n = 9, p < 0.01; Figs. 1C, 2A). Similar to control (Fig. 1A), the capacitance decay at 0.1–0.2 μm free calcium almost returned to the baseline within 40 s after stimulation (Fig. 1B,C). With 0.5 μm free calcium in the pipette, depol20ms induced an ICa and ΔCm similar to the control (p > 0.06–0.27), but a Ratedecay ∼51% of control (n = 11, p < 0.01), and the capacitance decay did not return to the baseline at 40 s after stimulation (Figs. 1D, 2A). Endocytosis inhibition at 1 μm free calcium was similar to that observed at 0.5 μm free calcium (Figs. 1E, 2A). These results show inhibition of slow endocytosis by the global calcium level in a concentration-dependent manner (Figs. 1, 2A).
The ∼20% decrease in the ΔCm at 0.1–0.2 μm free calcium (Fig. 2A) was likely due to the increase of the free EGTA concentration. The pipette in control contained only 0.05 mm BAPTA, whereas at 0.1 or 0.2 μm free calcium, the free EGTA concentration was ∼1.2 mm (2 mm EGTA and 0.8 mm Ca2+ in the pipette) or 0.85 mm (2 mm EGTA and 1.15 mm Ca2+ in the pipette), respectively. At 0.5 or 1 μm free calcium, the free EGTA concentration was reduced to ∼0.45 mm (2 mm EGTA and 1.55 mm Ca2+ in the pipette) or 0.25 mm (2 mm EGTA and 1.75 mm Ca2+ in the pipette), respectively, which may explain why the ΔCm was not decreased significantly at 0.5–1 μm free calcium compared with control (Fig. 2).
At 0.5 μm free calcium, the mEPSC frequency was increased to ∼10–20 Hz (X.S. Wu et al., 2007). To confirm this result, we recorded mEPSCs at the postsynaptic neuron at the whole-cell voltage-clamp configuration while dialyzing 0 or 0.5 μm calcium into the corresponding calyx. The mEPSC frequency was 4 ± 1 Hz (n = 5 synapses) and 19 ± 3 Hz (n = 5) at 0 and 0.5 μm free calcium, respectively (Fig. 2B), which was in the same range as previously reported (X.S. Wu et al., 2007). This mEPSC frequency increase caused a baseline capacitance increase (∼1 fF/s) (X.S. Wu et al., 2007) too small to affect our measurement of the Ratedecay, which was ∼29 fF/s in control. The capacitance change reflects the net outcome of exocytosis and endocytosis. Would the slower capacitance decay in higher free calcium concentrations (Figs. 1, 2) reflect an increase of asynchronous release after depol20ms rather than a slowdown of endocytosis? This possibility is highly unlikely for two reasons. First, the free EGTA concentration (∼0.45–0.25 mm) was actually higher than the control BAPTA concentration (0.05 mm), which should more effectively prevent residual calcium from generating enormous amounts of asynchronous release to offset the capacitance decay. Second, if asynchronous release induced by depol20ms was responsible for the slower capacitance decay, more asynchronous release induced by a stronger stimulus, depol20ms×10, should cause slower capacitance decay. This prediction was incorrect. As described below, the capacitance increase after depol20ms×10 returned to the baseline with a similar time course in various free calcium concentrations ranging from 0 to 1 μm (see Figs. 3, 4 for detail).
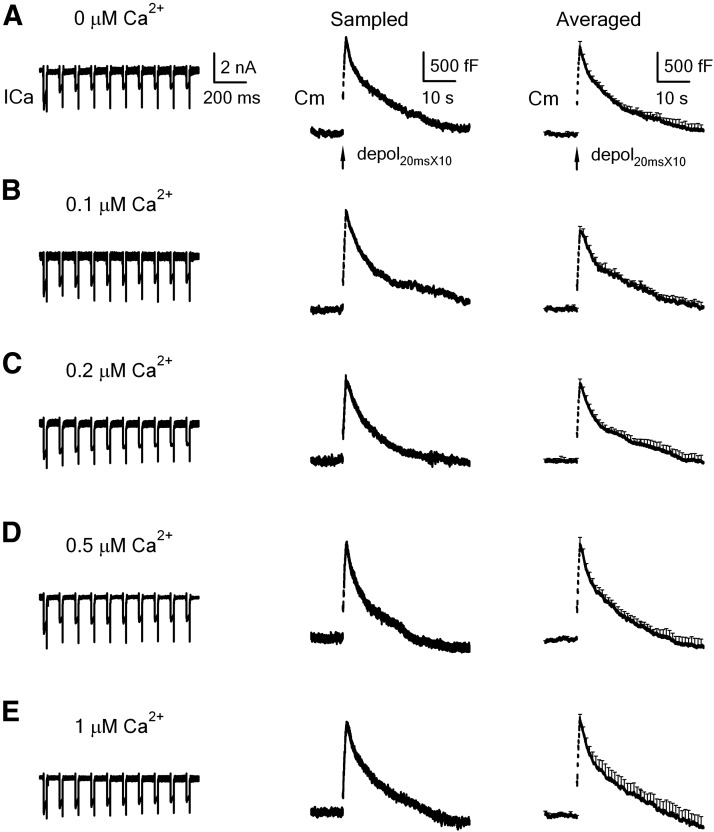
Figure 3.
Rapid endocytosis in calyces being dialyzed with various concentrations of calcium. A, Left, Sampled ICa and the capacitance change (Cm) induced by depol20ms×10 in control (pipette solution: 0 mm calcium, 0.05 mm BAPTA in the pipette). Right, Mean Cm change (mean + SEM, n = 12 calyces) induced by depol20ms×10 in control (0.05 mm BAPTA). B–E, Similar to A, except that the pipette contained free calcium at 0.1 μm (B, n = 7), 0.2 μm (C, n = 9), 0.5 μm (D, n = 11), and 1 μm (E, n = 12).
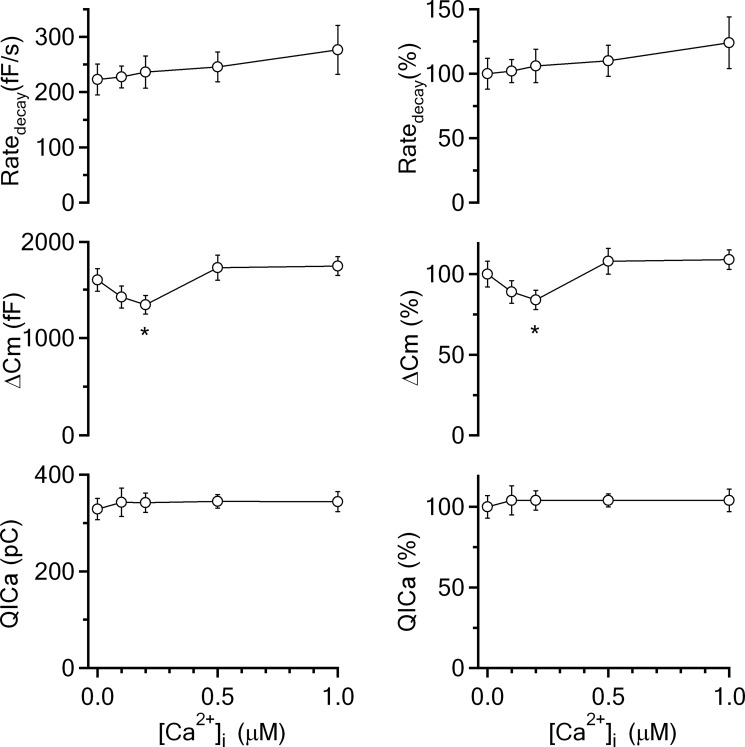
Figure 4.
Calcium dialysis into calyces does not inhibit rapid endocytosis. Left, The Ratedecay, ΔCm, and QICa induced by depol20ms×10 with a pipette containing free calcium at 0 μm (0.05 mm BAPTA, n = 12), 0.1 μm (n = 7), 0.2 μm (n = 9), 0.5 μm (n = 11), and 1 μm (n = 12). Right, Same as in left, but with all data normalized to the mean of the control group (0 μm free calcium). Data are expressed as mean ± SEM. *p < 0.05.
Prolonged calcium concentration increase does not inhibit endocytosis induced by strong stimulation
Next, we studied how calcium dialysis affects endocytosis induced by a more intense stimulation, the depol20ms×10. In control, depol20ms×10 induced a ΔCm of 1598 ± 117 fF, followed by a biexponential decay with τ of 2.4 ± 0.4 s (weight, 29 ± 3%) and 18 ± 3.5 s (n = 12, Fig. 3A). Ratedecay after depol20ms×10 was 223 ± 28 fF/s (n = 12, Figs. 3A, 4), which reflected mostly (>80%) the rapid component of endocytosis (W. Wu et al., 2005; X.S. Wu et al., 2009; Sun et al., 2010). When the pipette free calcium concentration was 0.1 μm (Fig. 3B), 0.2 μm (Fig. 3C), 0.5 μm (Fig. 3D), and 1 μm (Fig. 3E), ΔCm and Ratedecay did not change significantly in most conditions (p > 0.05; Figs. 3, 4), except that ΔCm was slightly reduced at 0.2 μm free calcium (p < 0.05; Fig. 4), likely due to the relatively high free EGTA concentration. The slow component of endocytosis induced by depol20ms×10 was not affected either (Fig. 3). These results suggest that prolonged calcium increase up to 1 μm does not affect either rapid or slow endocytosis induced by stronger intensity of stimulation. Inhibition of endocytosis by prolonged, small, global calcium increase as observed in Figures 1 and 2 is therefore limited to intermediate stimulation intensities.
A train of action potential-like stimuli inhibits slow, but not fast endocytosis
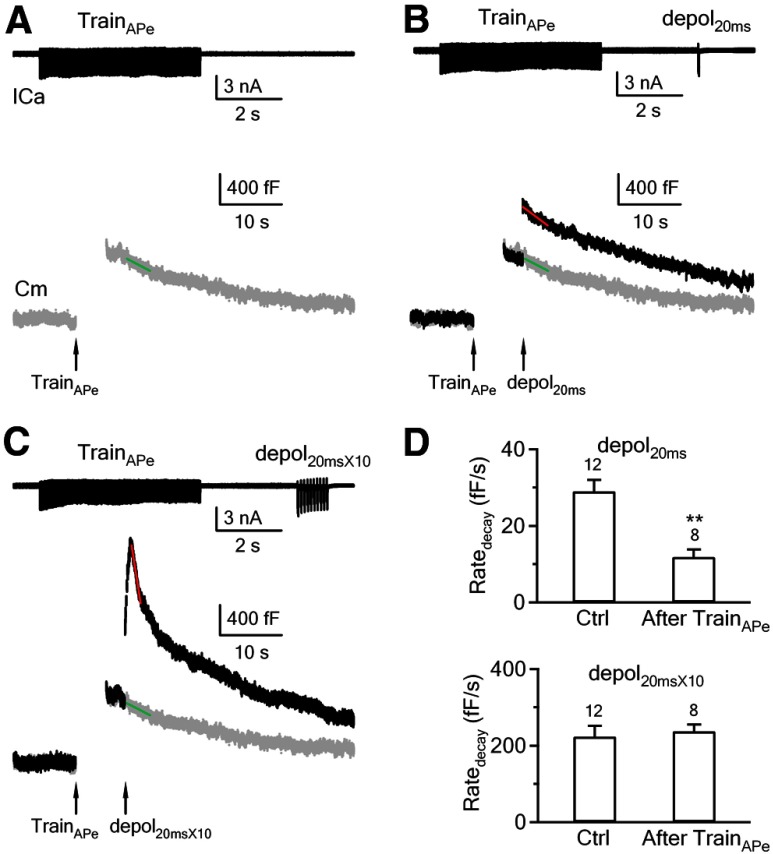
We showed that persistent calcium concentration increase inhibits slow, but not fast endocytosis. To mimic prolonged calcium concentration increase in physiological conditions, we applied 100 action potential-equivalent (APe) stimuli at 20 Hz using a control pipette containing 0 μm calcium (0.05 mm BAPTA; Fig. 5A). APe was mimicked as a 1 ms depolarization from −80 to +7 mV, which induced a presynaptic ICa and a postsynaptic EPSC similar to an action potential (Sun et al., 2002; Xu and Wu, 2005). At 3 s after applying this APe train, we applied depol20ms (Fig. 5B) or depol20ms×10 (Fig. 5C) to determine whether Ratedecay is reduced compared with the control without the preceding train. The control traces are shown in Figures 1A and 3A, and their mean Ratedecay values are shown in Figures 2A and 4. The Ratedecay after depol20ms (with preceding APe train) was calculated as the rate of decay within 4 s after depol20ms (Fig. 5B, red line) subtracted by the rate of decay within 3–7 s after 100 APe at 20 Hz was applied alone at the same cell (Fig. 5A,B, green line). This subtraction was necessarily because the 100 APe alone induced a non-negligible capacitance decay of 20 ± 4 fF/s (n = 8) at 3–7 s after the stimulation, the same time window in which we measured the Ratedecay if a depol20ms was applied at 3 s after the APe train. Similar subtraction was applied to measure the Ratedecay within 1–1.5 s after depol20ms×10 (Fig. 5C). With these calculation methods, we found that following the APe train, the Ratedecay induced by depol20ms (12 ± 2 fF/s, n = 8) was much smaller than that in control without the preceding APe train (29 ± 3 fF/s, n = 12, p < 0.01), whereas the Ratedecay after depol20ms×10 (237 ± 18 fF/s, n = 8) was similar to the control (223 ± 28 fF/s, n = 12, p = 0.42; see bar graphs in Fig. 5D). Thus, APe train mimicked calcium dialysis in inhibiting slow, but not fast endocytosis, which suggests that physiological stimulus may inhibit slow endocytosis by increasing the global calcium level.
Figure 5.
An APe train causes inhibition of slow, but not rapid endocytosis. A, Sampled ICa and Cm trace (gray trace) in response to 100 APe at 20 Hz (TrainAPe). The pipette contained 0 μm calcium (0.05 mm BAPTA, applies to A–C). The green line is the linear fit of the capacitance decay within 3–7 s after TrainAPe. B, Sampled ICa and Cm traces in response to a depol20ms applied 3 s after the TrainAPe. The red line is the linear fit of the capacitance decay within 4 s after depol20ms. The gray trace (and the green line) is the Cm trace caused by TrainAPe alone (same as in A). The difference between the slope of the red and the green line is the Ratedecay induced by a depol20ms applied 3 s after a TrainAPe. C, Sampled ICa and Cm traces in response to a depol20ms×10 applied 3 s after TrainAPe (100 APe at 20 Hz). The red line is the linear fit of the capacitance decay within 1.5 s after depol20ms×10. The gray trace (and the green line) is the Cm trace caused by TrainAPe alone (same as in A). The difference between the slope of the red and the green line is the Ratedecay induced by a depol20ms×10 applied 3 s after the TrainAPe. A–C were from the same calyx. D, Ratedecay induced by depol20ms (top) and depol20ms×10 (bottom) in control (Ctrl, no preceding APe train) and at 3 s after 100 APe train at 20 Hz (TrainAPe). The calyx numbers are labeled in the bar graph. The control data are taken from Figures 1A and 3A. **p < 0.01.
Relation between endocytosis time constant and the step depolarization duration
We showed that prolonged APe trains inhibit slow endocytosis (Fig. 5), likely by increasing the global calcium concentration, which is different from acceleration of endocytosis by large, local calcium increase at calyces (Hosoi et al., 2009; Wu et al., 2009; Yamashita et al., 2010). Thus, it seems obvious that the net outcome between inhibition and acceleration of endocytosis may determine the endocytosis rate. In support of this possibility, we found, as described below, that as the depolarization duration increased from 2 to 200 ms, slow endocytosis was slowed down, whereas rapid endocytosis was activated as the depolarization duration was prolonged to 200 ms.
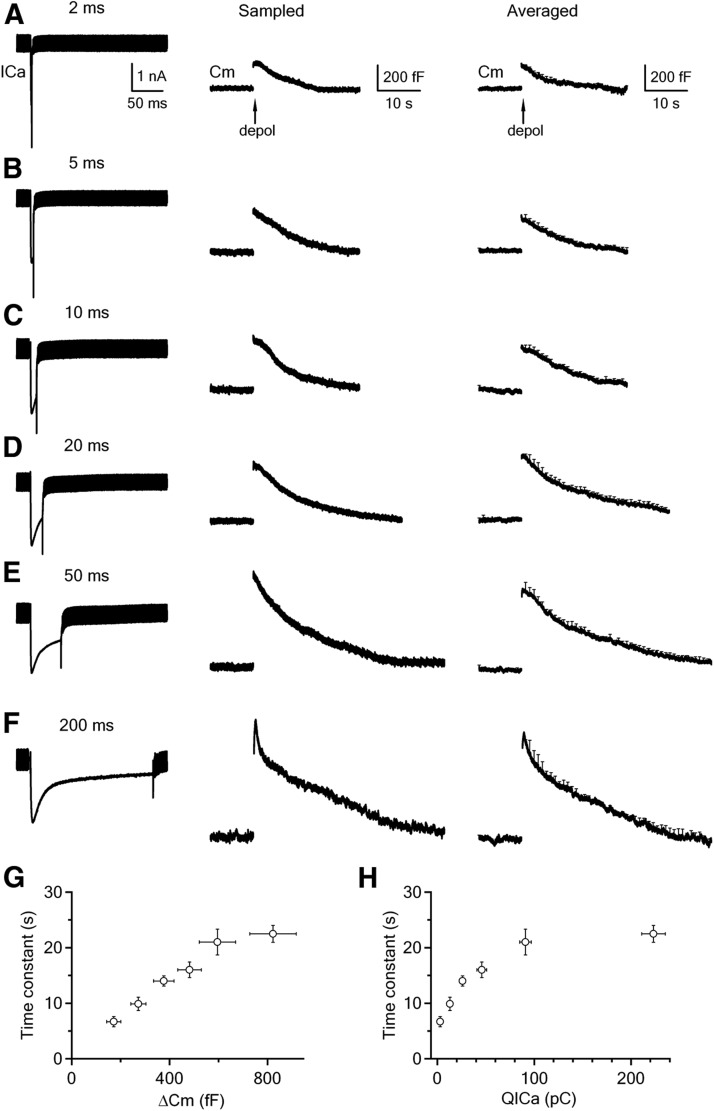
We applied 2, 5, 10, 20, 50, or 200 ms depolarization from −80 to +10 mV to the calyx via control pipettes containing 0 μm free calcium. As the depolarization duration increased, the QICa increased, the ΔCm increased, and the τ of the capacitance decay increased (Fig. 6A–F, summarized in Fig. 6G,H). Notice here that we used τ instead of the Ratedecay for analysis, because the ΔCm differed by severalfold (Fig. 6A–F), which made the comparison of the Ratedecay not meaningful.
Figure 6.
Endocytosis induced by 2–200 ms depolarization. A–F, Sampled ICa (left), sampled Cm trace (middle), and averaged Cm trace (right) induced by 2 (A, n = 9), 5 (B, n = 10), 10 (C, n = 9), 20 (D, n = 9), 50 (E, n = 8), and 200 ms (F, n = 8) depolarization from −80 to +10 mV. The scale bars in A apply to A–F. G, H, The capacitance decay (endocytosis) τ is plotted versus the ΔCm (G) and QICa (H) induced by 2, 5, 10, 20, 50, and 200 ms depolarization (data taken from those shown in A–F). For 200 ms depolarization, the τ of the slow, but not the fast component is included. Data are expressed as mean ± SEM. The number of calyces for each depolarization pulse is described in A–F.
The near-linear relation between the endocytic τ and the ΔCm (Fig. 6G) was consistent with previous findings that increasing the depolarization duration from 1 to 20 ms increases the ΔCm and the endocytosis τ at calyces (Sun et al., 2002; Wu et al., 2005; Yamashita et al., 2005; Renden and von Gersdorff, 2007). This observation was previously interpreted as reflecting saturation of the endocytic machinery (Sankaranarayanan and Ryan, 2000), but could now be reinterpreted as reflecting inhibition of endocytosis by global calcium increase, because the endocytic τ increased as QICa increased (Fig. 6H). Although our results do not allow us to fully exclude saturation as an interpretation, we prefer our interpretation of calcium-dependent inhibition, because there has been no direct evidence showing that the endocytic capacity can be saturated, whereas we showed directly that prolonged calcium increase inhibited slow endocytosis (Fig. 1).
Whereas the capacitance decay was monoexponential after 2–50 ms depolarization (Fig. 6A–E), it was biexponential with τ of 1.1 ± 0.2 s (weight, 20 ± 4%) and 23 ± 2 s (n = 8) after 200 ms depolarization (Fig. 6F). The τ of the rapid component (1.1 ± 0.2 s) was not included in the summary plot in Figure 6, G and H. The appearance of this rapid component is consistent with the previous finding that a larger increase of the calcium influx triggers rapid endocytosis (W. Wu et al., 2005; X.S. Wu et al., 2009). For example, as the pulse duration increases from 2 to 50 ms during 10 pulses of depolarization at 10 Hz, calcium influx is increased, rapid endocytosis is activated, and its τ decreases (Wu et al., 2009). Rapid endocytosis is more often observed when the intracellular calcium concentration increase induced by photolysis of a caged calcium compound is tens of micromolar or higher in hair cells (Beutner et al., 2001). Thus, 200 ms depolarization may increase the local calcium concentration above the threshold to activate rapid endocytosis. Local, transient saturation of the calcium buffer by 200 ms depolarization, but not by shorter duration of depolarization, might further contribute to the local calcium concentration increase (Felmy et al., 2003), and thus activation of rapid endocytosis.
In summary, as the depolarization duration and, thus, QICa increased, the τ of the slow endocytosis increased, and a rapid component of endocytosis occurred (Fig. 6). These results can be explained by the global calcium increase that inhibits slow endocytosis, and the local calcium increase that activates rapid endocytosis.
Discussion
At calyx nerve terminals, we found that prolonged calcium increase from 0 to 1 μm inhibited slow endocytosis in a concentration-dependent manner (Figs. 1, 2). APe trains also inhibited slow endocytosis (Fig. 5), suggesting that global calcium increase induced by physiological stimulation may inhibit slow endocytosis. These results are in contrast to the recent finding that the transient calcium concentration increase to more than ∼10 μm at the calcium micro/nano domain triggers slow endocytosis (Hosoi et al., 2009; Wu et al., 2009; Yamashita et al., 2010). Together, our results show that transient, large calcium influx at the micro/nano domain, as induced by action potentials, triggers slow endocytosis, whereas prolonged, small, and global calcium increase, as can be induced by action potential trains, inhibits slow endocytosis. Such a yin and yang effect of calcium on endocytosis is likely to have wide application beyond the large calyx nerve terminal, and may reconcile most conflicts in the literature regarding whether calcium facilitates or inhibits endocytosis.
Reconciling apparent conflicts at goldfish ribbon-type synapses
At the goldfish retinal bipolar nerve terminal, a pioneering study two decades ago found that calcium dialysis >1 μm abolishes rapid endocytosis, leading to the conclusion that calcium inhibits rapid endocytosis (von Gersdorff and Matthews, 1994). A subsequent study 7 years later showed that buffering calcium influx with EGTA blocks fast, but not slow endocytosis induced by brief depolarization, leading to the opposite conclusion that calcium selects rapid endocytosis (Neves et al., 2001). This apparent conflict can now be reconciled with our finding that prolonged, small, global calcium increase inhibits endocytosis, whereas large, transient calcium increase triggers endocytosis.
Comparing the present work with a study in goldfish retina
Although our observation that calcium dialysis inhibits slow endocytosis seems analogous to an early study in goldfish retina (von Gersdorff and Matthews, 1994), the present work is an advance over this early study in three aspects. First, conclusions are different. We suggest that only the prolonged, small, global calcium increase, but not all patterns of calcium increase as proposed in the early study, inhibits endocytosis. Second, we showed that slow endocytosis, the most commonly observed endocytic form, is inhibited by calcium dialysis at mammalian central synapses containing conventional active zones, whereas the early study shows that rapid endocytosis is inhibited by calcium dialysis at goldfish ribbon-type synapses. Third, we found that upon stronger stimulation (depol20ms×10), both rapid and slow forms of endocytosis were not inhibited by calcium dialysis (Figs. 3, 4). This result suggests that large, transient calcium influx at the micro/nano domain may overcome the inhibitory effect of prolonged, global calcium increase. Inhibition of endocytosis by calcium dialysis may therefore be limited to mild to intermediate levels of stimulation.
Reconciling apparent conflicts at conventional synapses
Reducing the extracellular calcium or buffering calcium influx slows down or abolishes endocytosis at hippocampal synapses and calyces (Sankaranarayanan and Ryan, 2001; Wu et al., 2009; Sun et al., 2010), whereas prolonging the stimulation train increases calcium influx, but prolongs endocytosis in many preparations, such as frog neuromuscular junctions (Wu and Betz, 1996), hippocampal synapses (Sankaranarayanan and Ryan, 2000; Balaji et al., 2008; Sun et al., 2010), calyces (Sun et al., 2002; Wu et al., 2005; Yamashita et al., 2005; Renden and von Gersdorff, 2007), and chromaffin cells (Artalejo et al., 1995; Elhamdani et al., 2006). These paradoxical results can be interpreted with the yin and yang effect of calcium: reducing extracellular calcium or buffering calcium with BAPTA may inhibit endocytosis by reducing the transient calcium increase at the micro/nano domain, whereas prolonging the stimulation train may prolong endocytosis by increasing the global calcium concentration. The transient calcium increase to tens of micromolar at the micro/nano domain dissipates mostly in ∼10 ms after an action potential (Bollmann and Sakmann, 2005; Neher and Sakaba, 2008), but may dissipate more slowly after prolonged depolarization. Rapid dissipation may lead to little calcium accumulation at the micro/nano domain during action potential trains at 30 Hz or less and during 20–50 ms depolarization trains at <1 Hz. However, these relatively low-frequency stimuli may induce global calcium increase to ∼0.2–1 μm for ∼30 s to minutes, as demonstrated at many nerve terminals (Kamiya and Zucker, 1994; Wu and Betz, 1996; Wu and Borst, 1999; Zucker and Regehr, 2002; Habets and Borst, 2005; Wadel et al., 2007; Xue et al., 2012), which may explain why endocytosis is prolonged after a longer train of stimulation. In contrast to lower-frequency stimulation, higher-frequency stimulation may induce substantial accumulation of calcium to tens of micromolar at the transient micro/nano domain, which may explain why action potential-equivalent stimuli at 100–300 Hz or 20–50 ms depolarization at 10 Hz speeds up endocytosis at calyces (W. Wu et al., 2005; X.S. Wu et al., 2009). Saturation of the calcium buffer because of massive calcium influx and insufficient time to pump out intracellular calcium may further contribute to the transient calcium increase at the micro/nano domain during higher-frequency stimulation (Felmy et al., 2003), which may further promote facilitation of endocytosis.
A recent study shows that endocytosis is speeded up in the first few action potentials, and then slowed down during prolonged lower-frequency (10 Hz) action potential trains (Armbruster et al., 2013). The speed-up of endocytosis could be accounted for by facilitation of endocytosis by the large calcium transient at the micro/nano domain, if facilitation of endocytosis continues for a short time after calcium microdomain dissipates. The slowdown of endocytosis could be due to prolonged, global calcium increase that inhibits endocytosis.
In summary, whether endocytosis is upregulated or downregulated may depend on the net outcome between facilitation by transient calcium micro/nano domain and inhibition by prolonged global calcium increase, which in turn depends on the stimulation frequency and duration of depolarization. These mechanisms may explain most apparent conflicts in the last two decades of studies regarding whether calcium upregulates or downregulates endocytosis. They may also provide an explanation for why the increase of the depolarization duration from 2 to 200 ms prolonged slow endocytosis, but activated a rapid component of endocytosis at calyces (Fig. 6).
What are the mechanisms underlying the opposite effects of calcium on endocytosis?
The present work raises the question as to what underlies the opposite effects of calcium on endocytosis. The yin and yang effect of the same factor has been reported in many systems. For example, long-term potentiation and depression of synaptic transmission are both induced by calcium, but at different patterns of stimulation (Malenka and Bear, 2004). Currently, we do not know how calcium exerts the opposite effects on endocytosis. Many potential mechanisms can be considered. Here we discuss two scenarios, which certainly would not exhaust all possibilities. First, prolonged calcium increase might interrupt the cycling of calcium-dependent phosphorylation and dephosphorylation of endocytic proteins. It has been suggested that calcium/calmodulin activates calcineurin to dephosphorylate endocytic proteins, including dynamin, which is needed to mediate and to facilitate slow endocytosis (Marks and McMahon, 1998; Cousin and Robinson, 2001; Wu et al., 2009; Sun et al., 2010; Xue et al., 2011; Armbruster et al., 2013). If prolonged calcium increase causes more calcium-dependent phosphorylation than dephosphorylation of endocytic proteins, it may explain why prolonged calcium increase inhibits endocytosis. Second, while large, transient calcium increase may trigger endocytosis, prolonged, small calcium increase may activate calcium-dependent chloride channels to increase intracellular chloride concentration, which has been shown to inhibit endocytosis at the goldfish retinal bipolar synapse (Hull and von Gersdorff, 2004). It would be of great interest to understand how prolonged, small calcium increase and transient, large calcium increase exert opposite effects in regulating endocytosis in the future.
Does yin and yang of calcium apply to all forms of endocytosis at the single-vesicle level?
At the single-vesicle level, there are three mechanistically different forms of endocytosis, classical clathrin-dependent endocytosis, kiss-and-run, and bulk endocytosis which forms a large endosome-like structure (L.G. Wu et al., 2007; Alabi and Tsien, 2013). Dialysis of blockers of clathrin-dependent endocytosis inhibits slow endocytosis at calyces, suggesting that slow endocytosis at calyces is clathrin dependent (Hosoi et al., 2009; Wu et al., 2009). Recent studies show that slow endocytosis is triggered by calcium influx at the micro/nano domain at calyces (Hosoi et al., 2009; Wu et al., 2009). Thus, the yin (inhibition by prolonged global calcium) and yang (trigger and facilitation by transient local calcium) of calcium on endocytosis may apply to clathrin-dependent endocytosis.
We recently found that transient calcium influx triggers all forms of endocytosis recorded at the whole-cell configuration, including slow endocytosis, bulk endocytosis, rapid endocytosis and endocytosis overshoot at calyces (Wu et al., 2009). It remains unclear whether transient local calcium influx triggers and facilitates kiss-and-run, and whether global calcium increase inhibits kiss-and-run and/or bulk endocytosis. Addressing these questions in the future may answer whether the yin and yang effect of calcium applies to kiss-and-run and bulk endocytosis.
Footnotes
This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program. We thank Drs. Fujun Luo, Jiansong Sheng, and Zhen Zhang for critical reading of this manuscript.
References
- Alabi AA, Tsien RW. Perspectives on kiss-and-run: role in exocytosis, endocytosis, and neurotransmission. Annu Rev Physiol. 2013;75:393–422. doi: 10.1146/annurev-physiol-020911-153305. [DOI] [PubMed] [Google Scholar]
- Armbruster M, Messa M, Ferguson SM, De Camilli P, Ryan TA. Dynamin phosphorylation controls optimization of endocytosis for brief action potential bursts. Elife. 2013;2:e00845. doi: 10.7554/eLife.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci U S A. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Armbruster M, Ryan TA. Calcium control of endocytic capacity at a CNS synapse. J Neurosci. 2008;28:6742–6749. doi: 10.1523/JNEUROSCI.1082-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/S0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B. Control of synaptic strength and timing by the release-site Ca2+ signal. Nat Neurosci. 2005;8:426–434. doi: 10.1038/nn1417. [DOI] [PubMed] [Google Scholar]
- Borst JGG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ba2+ does not support synaptic vesicle retrieval in rat cerebrocortical synaptosomes. Neurosci Lett. 1998;253:1–4. doi: 10.1016/S0304-3940(98)00610-7. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/S0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Azizi F, Artalejo CR. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci. 2006;26:3030–3036. doi: 10.1523/JNEUROSCI.5275-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmy F, Neher E, Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–811. doi: 10.1016/S0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Habets RL, Borst JG. Post-tetanic potentiation in the rat calyx of Held synapse. J Physiol. 2005;564:173–187. doi: 10.1113/jphysiol.2004.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Borst JGG, Sakmann B. Calcium dynamics associated with a single action potential in a CNS presynaptic terminal. Biophys J. 1997;72:1458–1471. doi: 10.1016/S0006-3495(97)78792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel AW, Betz WJ. Monitoring of black widow spider venom (BWSV) induced exo- and endocytosis in living frog motor nerve terminals with FM1-43. Neuropharmacology. 1995;34:1397–1406. doi: 10.1016/0028-3908(95)00126-Q. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Hull C, von Gersdorff H. Fast endocytosis is inhibited by GABA-mediated chloride influx at a presynaptic terminal. Neuron. 2004;44:469–482. doi: 10.1016/j.neuron.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- Leitz J, Kavalali ET. Ca(2) influx slows single synaptic vesicle endocytosis. J Neurosci. 2011;31:16318–16326. doi: 10.1523/JNEUROSCI.3358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/S0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Neves G, Gomis A, Lagnado L. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc Natl Acad Sci U S A. 2001;98:15282–15287. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renden R, von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J Neurophysiol. 2007;98:3349–3359. doi: 10.1152/jn.00898.2007. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynatpic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu W, Jin SX, Dondzillo A, Wu LG. Capacitance measurements at the calyx of Held in the medial nucleus of the trapezoid body. J Neurosci Methods. 2004;134:121–131. doi: 10.1016/j.jneumeth.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Sun T, Wu XS, Xu J, McNeil BD, Pang ZP, Yang W, Bai L, Qadri S, Molkentin JD, Yue DT, Wu LG. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 2010;30:11838–11847. doi: 10.1523/JNEUROSCI.1481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- Wadel K, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca(2+) channels determines fast neurotransmitter release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Wu LG, Betz WJ. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–779. doi: 10.1016/S0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Wu LG, Borst JGG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/S0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Wu LG, Ryan TA, Lagnado L. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J Neurosci. 2007;27:11793–11802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci. 2005;25:11676–11683. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Xue L, Mohan R, Paradiso K, Gillis KD, Wu LG. The origin of quantal size variation: vesicular glutamate concentration plays a significant role. J Neurosci. 2007;27:3046–3056. doi: 10.1523/JNEUROSCI.4415-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Xue J, Graham ME, Novelle AE, Sue N, Gray N, McNiven MA, Smillie KJ, Cousin MA, Robinson PJ. Calcineurin selectively docks with the dynamin Ixb splice variant to regulate activity-dependent bulk endocytosis. J Biol Chem. 2011;286:30295–30303. doi: 10.1074/jbc.M111.273110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Zhang Z, McNeil BD, Luo F, Wu XS, Sheng J, Shin W, Wu LG. Voltage-dependent calcium channels at the plasma membrane, but not vesicular channels, couple exocytosis to endocytosis. Cell Rep. 2012;1:632–638. doi: 10.1016/j.celrep.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307:124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Eguchi K, Saitoh N, von Gersdorff H, Takahashi T. Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+ Nat Neurosci. 2010;13:838–844. doi: 10.1038/nn.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kwon SE, Gaffaney JD, Dunning FM, Chapman ER. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat Neurosci. 2011;15:243–249. doi: 10.1038/nn.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Sakaba T. Activity-dependent modulation of endocytosis by calmodulin at a large central synapse. Proc Natl Acad Sci U S A. 2012;109:291–296. doi: 10.1073/pnas.1100608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LH, Rao Y, Varga K, Wang CY, Xiao P, Lindau M, Gong LW. Synaptotagmin 1 is necessary for the Ca2+ dependence of clathrin-mediated endocytosis. J Neurosci. 2012;32:3778–3785. doi: 10.1523/JNEUROSCI.3540-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]