Abstract
Precursors to two periplasmic proteins and one outer membrane protein were synthesized in a membrane-free extract from Escherichia coli programmed with plasmid DNA. In the presence of inverted plasma membrane vesicles from E. coli up to 25% of the precursor molecules were converted into their mature forms. Using externally added proteinase K as a probe, we found the processed proteins segregated within the membrane vesicles. By the same criteria, a small amount of each precursor also proved to be translocated, indicating that translocation and signal sequence cleavage are not necessarily coupled processes. Furthermore, we present conclusive evidence that the translocation step can occur post-translationally even as late as 60 min after the beginning of translation.
Full text
PDF
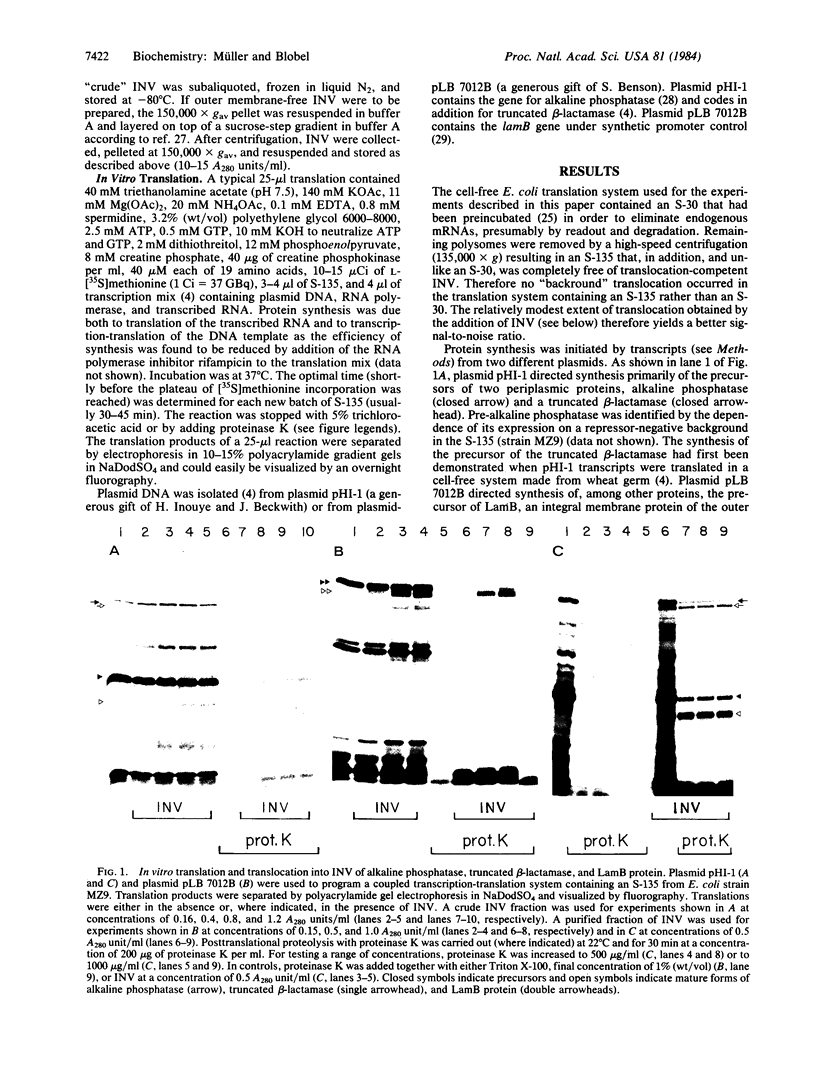
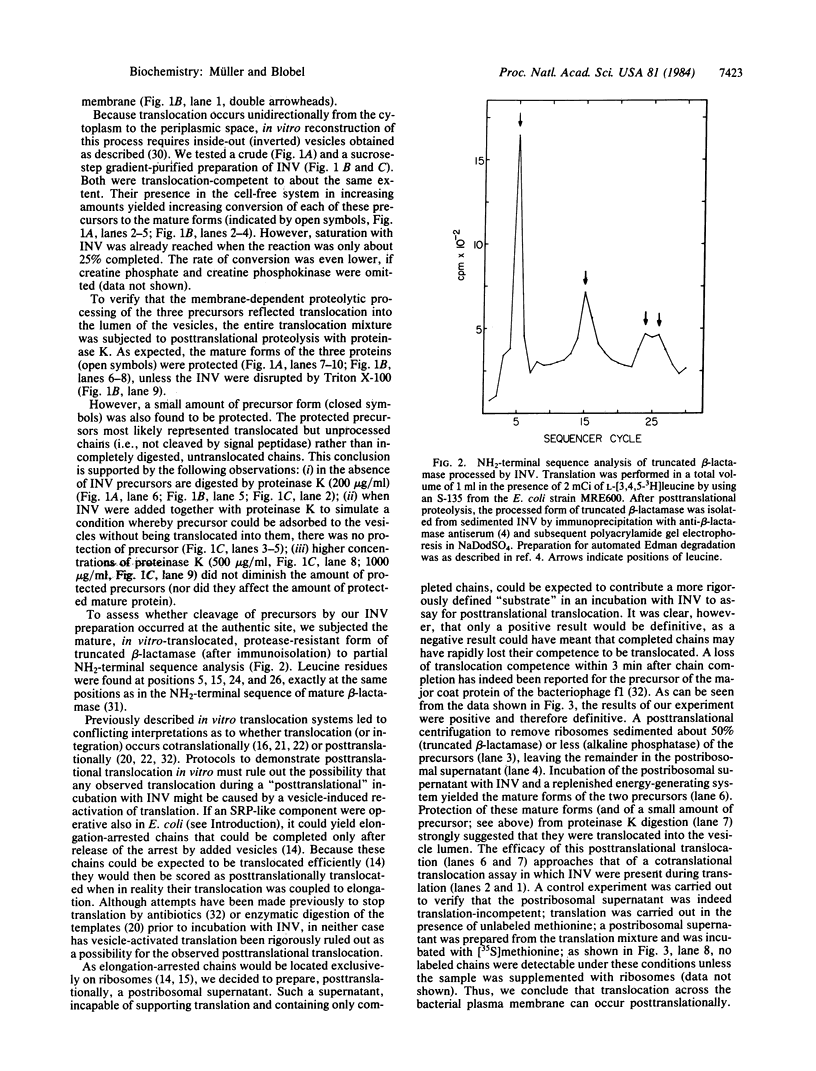


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Inouye H., Model P., Beckwith J. Processing of alkaline phosphatase precursor to the mature enzyme by an Escherichia coli inner membrane preparation. J Bacteriol. 1980 May;142(2):726–728. doi: 10.1128/jb.142.2.726-728.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Model P., Blobel G. Membrane biogenesis: cotranslational integration of the bacteriophage f1 coat protein into an Escherichia coli membrane fraction. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1251–1255. doi: 10.1073/pnas.76.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowlesmith I., Gamon K., Henning U. Precursor proteins are intermediates in vivo in the synthesis of two major outer membrane proteins, the OmpA and OmpF proteins, of Escherichia coli K12. Eur J Biochem. 1981 Jan;113(2):375–380. doi: 10.1111/j.1432-1033.1981.tb05076.x. [DOI] [PubMed] [Google Scholar]
- Decker K. P., Brusilow W. S., Gunsalus R. P., Simoni R. D. In vitro membrane association of the F0 polypeptides of the Escherichia coli proton translocating ATPase. J Bacteriol. 1982 Nov;152(2):815–821. doi: 10.1128/jb.152.2.815-821.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- Fraser T. H., Bruce B. J. Chicken ovalbumin is synthesized and secreted by Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5936–5940. doi: 10.1073/pnas.75.12.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G. Transient involvement of signal recognition particle and its receptor in the microsomal membrane prior to protein translocation. Cell. 1983 Dec;35(3 Pt 2):677–685. doi: 10.1016/0092-8674(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. M., Watts C., Wickner W. Membrane assembly: posttranslational insertion of M13 procoat protein into E. coli membranes and its proteolytic conversion to coat protein in vitro. Cell. 1981 May;24(2):437–441. doi: 10.1016/0092-8674(81)90334-2. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Schwartz M. Evidence for a coupling of synthesis and export of an outer membrane protein in Escherichia coli. EMBO J. 1983;2(1):15–19. doi: 10.1002/j.1460-2075.1983.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Michaelis S., Wright A., Beckwith J. Cloning and restriction mapping of the alkaline phosphatase structural gene (phoA) of Escherichia coli and generation of deletion mutants in vitro. J Bacteriol. 1981 May;146(2):668–675. doi: 10.1128/jb.146.2.668-675.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Soberon X., Franceschini T., Nakamura K., Itakura K., Inouye M. Role of positive charge on the amino-terminal region of the signal peptide in protein secretion across the membrane. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3438–3441. doi: 10.1073/pnas.79.11.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Mandel G., Wickner W. Soluble precursor of an integral membrane protein: synthesis of procoat protein in Escherichia coli infected with bacteriophage M13. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1199–1203. doi: 10.1073/pnas.76.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Evidence for posttranslational translocation of beta-lactamase across the bacterial inner membrane. Cell. 1982 Oct;30(3):893–902. doi: 10.1016/0092-8674(82)90294-x. [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M., Fennick B. J., Vasicek T. J. Transport and cleavage of bacterial pre-beta-lactamase by mammalian microsomes. J Cell Biol. 1983 Apr;96(4):1117–1119. doi: 10.1083/jcb.96.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Oliver D. B., Beckwith J. Signal sequence mutations disrupt feedback between secretion of an exported protein and its synthesis in E. coli. 1984 Apr 26-May 2Nature. 308(5962):863–864. doi: 10.1038/308863a0. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Müller M., Blobel G. Protein export in Escherichia coli requires a soluble activity. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7737–7741. doi: 10.1073/pnas.81.24.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Ibrahimi I., Chang C. N., Walter P., Blobel G. A bacterial secretory protein requires signal recognition particle for translocation across mammalian endoplasmic reticulum. J Biol Chem. 1982 Oct 25;257(20):11860–11863. [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Daniels C. J., Landick R., Gunsalus R. P., Zurawski G., Yanofsky C. Amino-terminal sequence and processing of the precursor of the leucine-specific binding protein, and evidence for conformational differences between the precursor and the mature form. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2005–2009. doi: 10.1073/pnas.77.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J. M., Holland I. B., Spratt B. G. Precursor forms of penicillin-binding proteins 5 and 6 of E. coli cytoplasmic membrane. Nature. 1981 Sep 24;293(5830):307–309. doi: 10.1038/293307a0. [DOI] [PubMed] [Google Scholar]
- Randall L. L. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell. 1983 May;33(1):231–240. doi: 10.1016/0092-8674(83)90352-5. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Tai P. C., Davis B. D. Energy-requiring translocation of the OmpA protein and alkaline phosphatase of Escherichia coli into inner membrane vesicles. J Bacteriol. 1984 Jul;159(1):63–70. doi: 10.1128/jb.159.1.63-70.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenkamp R., Kustermann-Kuhn B., Hollenberg C. P. Expression and processing of bacterial beta-lactamase in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4466–4470. doi: 10.1073/pnas.78.7.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. J., Soberon X., Marumoto Y., McMahon J., Itakura K. Biological expression of an Escherichia coli consensus sequence promoter and some mutant derivatives. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3203–3207. doi: 10.1073/pnas.80.11.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982 Jan;28(1):177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P. Cotranslational secretion of diphtheria toxin and alkaline phosphatase in vitro: involvement of membrane protein(s). J Bacteriol. 1980 Mar;141(3):1142–1147. doi: 10.1128/jb.141.3.1142-1147.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Stahl S., Gilbert W. Eukaryotic signal sequence transports insulin antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3369–3373. doi: 10.1073/pnas.77.6.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C., Silver P., Wickner W. Membrane assembly from purified components. II. Assembly of M13 procoat into liposomes reconstituted with purified leader peptidase. Cell. 1981 Aug;25(2):347–353. doi: 10.1016/0092-8674(81)90053-2. [DOI] [PubMed] [Google Scholar]
- Wickner W., Mandel G., Zwizinski C., Bates M., Killick T. Synthesis of phage M13 coat protein and its assembly into membranes in vitro. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1754–1758. doi: 10.1073/pnas.75.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel-Dreasen O., Zamir A. Secretion and processing of an immunoglobulin light chain in Escherichia coli. Gene. 1984 Mar;27(3):315–322. doi: 10.1016/0378-1119(84)90076-3. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]