Abstract
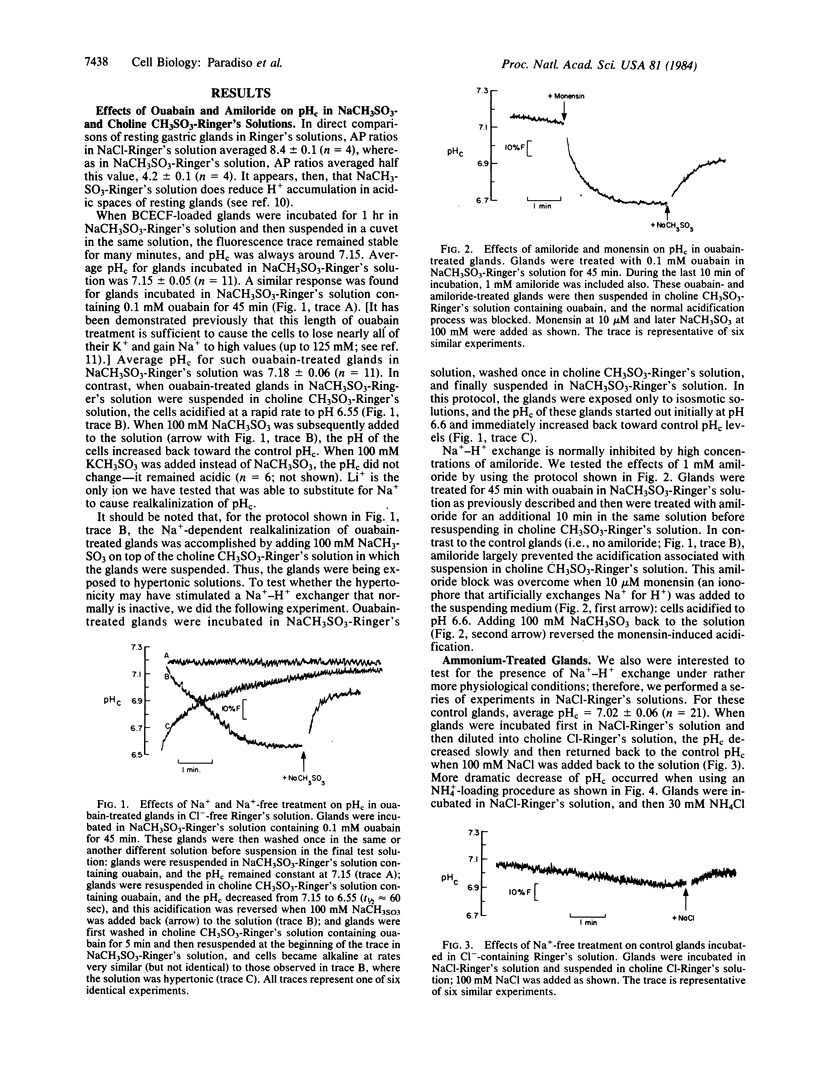
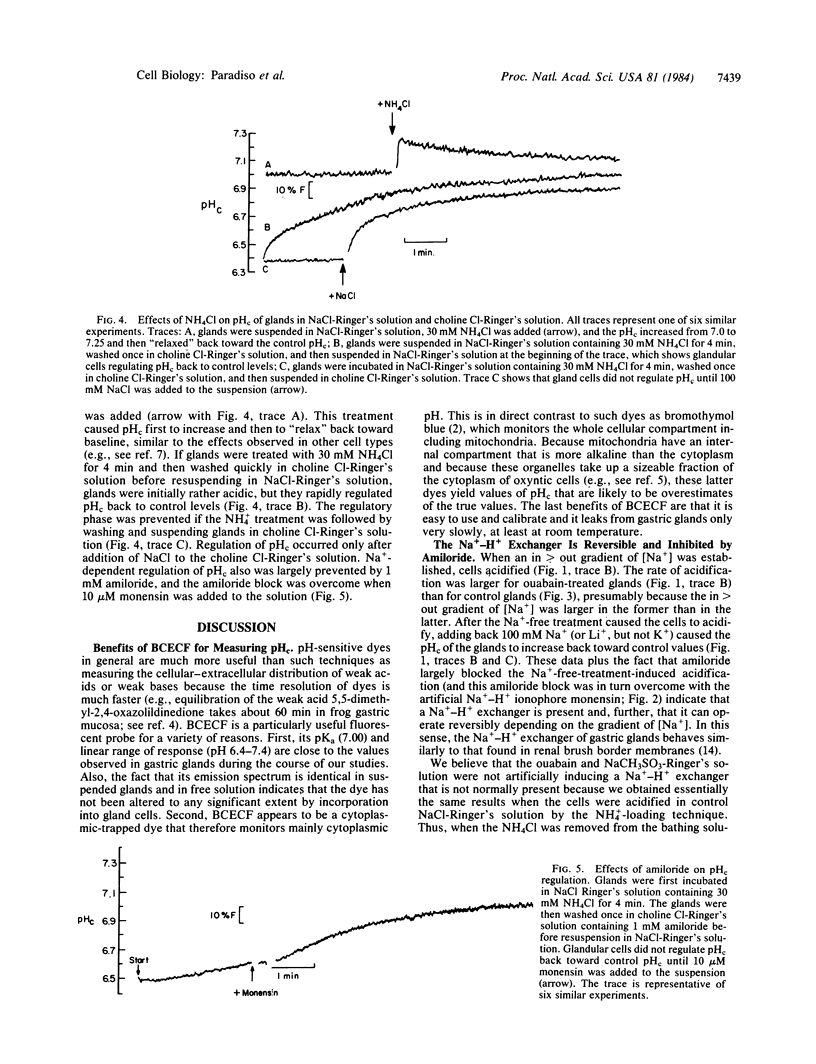
We have used the pH-sensitive, fluorescent, cytoplasmic-trapped dye 2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein (BCECF) to identify Na+-H+ exchange in gastric glands isolated from rabbit stomachs by high-pressure perfusion and collagenase digestion. The fluorescence of BCECF-loaded glands was calibrated in terms of cytosolic pH (pHc) by permeabilizing the cell membranes and titrating the extracellular solution to different pH values. In one set of experiments in Cl--free solutions, glands were treated with 0.1 mM ouabain for 45 min to increase cellular cytosolic molar sodium ion concentration [( Na+]c) to high levels. Subsequent suspension of these cells in a Na+-free Ringer's solution (to generate [Na+]c greater than [Na+]o) caused cells to acidify rapidly (t1/2 approximately equal to 60 sec) from pHc approximately equal to 7.15 to pHc approximately equal to 6.55. Subsequent addition of 100 mM Na+ or Li+, but not K+, caused cells rapidly to increase pHc (t1/2 approximately equal to 30 sec) toward the control value. These changes of pHc were blocked when ouabain-treated glands had been preequilibrated for 10 min with 1 mM amiloride, and this block was overcome by adding 10 microM monensin (an ionophore that artificially exchanges Na+ for H+). In another set of experiments in Cl--containing Ringer's solution, glands were acid-loaded by treatment with 30 mM NH4Cl for 4 min, followed by washing the NH4Cl from the solutions. Under these conditions, pHc decreased from 7.02 to approximately equal to 6.5; subsequent alkalinization of cells back to control pHc was stimulated by Na+ (t1/2 approximately equal to 60 sec), but not K+, and was inhibited by 1 mM amiloride. This amiloride block also was overcome by further addition of 10 microM monensin. We conclude that gastric glands contain a Na+-H+ exchanger that appears independent of Cl-, not activated by K+, and blocked by 1 mM amiloride. This exchanger is likely localized to the serosal membrane of gland cells. Na+-H+ exchange may play an important role in regulation of pHc in oxyntic and chief cells exposed to high luminal acidity, where back diffusion of H+ into cells may occur at rapid rates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berglindh T. Absolute dependence on chloride for acid secretion in isolated gastric glands. Gastroenterology. 1977 Oct;73(4 Pt 2):874–880. [PubMed] [Google Scholar]
- Berglindh T., Obrink K. J. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand. 1976 Feb;96(2):150–159. doi: 10.1111/j.1748-1716.1976.tb10184.x. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Machen T. E. Impedance analysis in epithelia and the problem of gastric acid secretion. J Membr Biol. 1983;72(1-2):17–41. doi: 10.1007/BF01870312. [DOI] [PubMed] [Google Scholar]
- Ekblad E. B. Increase of intracellular pH in secreting frog gastric mucosa. Biochim Biophys Acta. 1980 Oct 15;632(3):375–385. doi: 10.1016/0304-4165(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Forte J. G., Machen T. E. Transport and electrical phenomena in resting and secreting piglet gastric mucosa. J Physiol. 1975 Jan;244(1):33–51. doi: 10.1113/jphysiol.1975.sp010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey S. J. Intracellular pH measurements in gastric mucosa. Am J Physiol. 1979 Jul;237(1):E82–E89. doi: 10.1152/ajpendo.1979.237.1.E82. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Koelz H. R., Sachs G., Berglindh T. Cation effects on acid secretion in rabbit gastric glands. Am J Physiol. 1981 Nov;241(5):G431–G442. doi: 10.1152/ajpgi.1981.241.5.G431. [DOI] [PubMed] [Google Scholar]
- Machen T. E., Silen W., Forte J. G. Na+ transport by mammalian stomach. Am J Physiol. 1978 Mar;234(3):E228–E235. doi: 10.1152/ajpendo.1978.234.3.E228. [DOI] [PubMed] [Google Scholar]
- Machen T. E., Zeuthen T. Cl- transport by gastric mucosa: cellular Cl- activity and membrane permeability. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):559–573. doi: 10.1098/rstb.1982.0152. [DOI] [PubMed] [Google Scholar]
- Manning E. C., Machen T. E. Effects of bicarbonate and pH on chloride transport by gastric mucosa. Am J Physiol. 1982 Jul;243(1):G60–G68. doi: 10.1152/ajpgi.1982.243.1.G60. [DOI] [PubMed] [Google Scholar]
- Michelangeli F. Acid secretion and intracellular pH in isolated oxyntic cells. J Membr Biol. 1978 Jan 12;38(1-2):31–50. doi: 10.1007/BF01875161. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Nichols J. W., Deamer D. W. Net proton-hydroxyl permeability of large unilamellar liposomes measured by an acid-base titration technique. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2038–2042. doi: 10.1073/pnas.77.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]


