Abstract
Background
WHO recommends initiating combination antiretroviral treatment (ART) at the minimal threshold of 350 CD4 cells/mm3. In sub-Saharan Africa, the time for a recently infected patient to reach this threshold is unclear.
Method
We estimated the probability of reaching different CD4 thresholds over time in the ANRS 1220 cohort of HIV-1 seroconverters in Côte d’Ivoire. CD4 slopes were estimated using a mixed linear model. Probabilities of crossing the 350 and 500 CD4 cells/mm3 thresholds were estimated by the Kaplan-Meier method.
Results
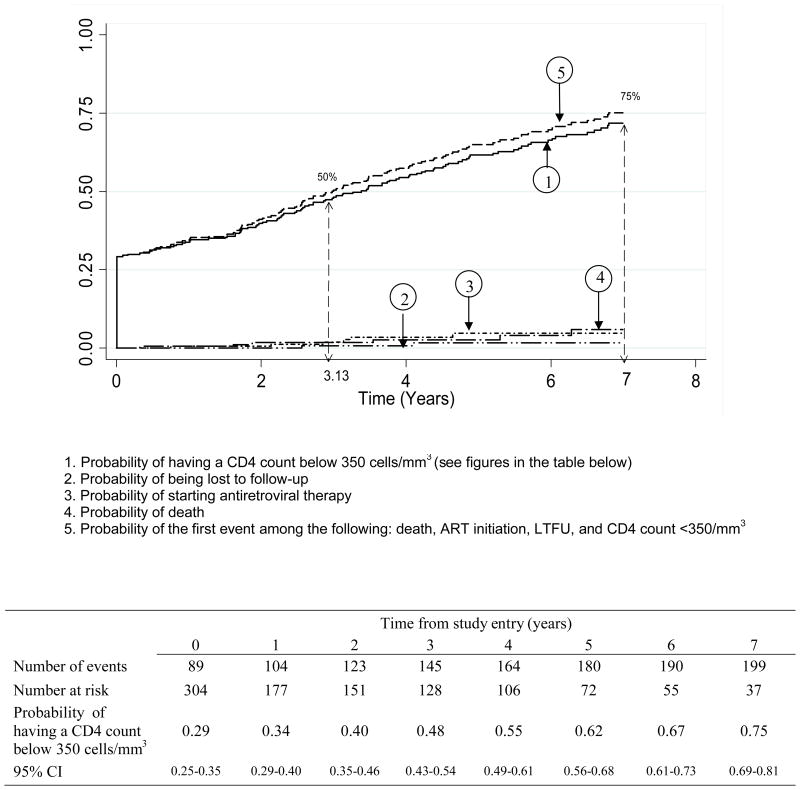
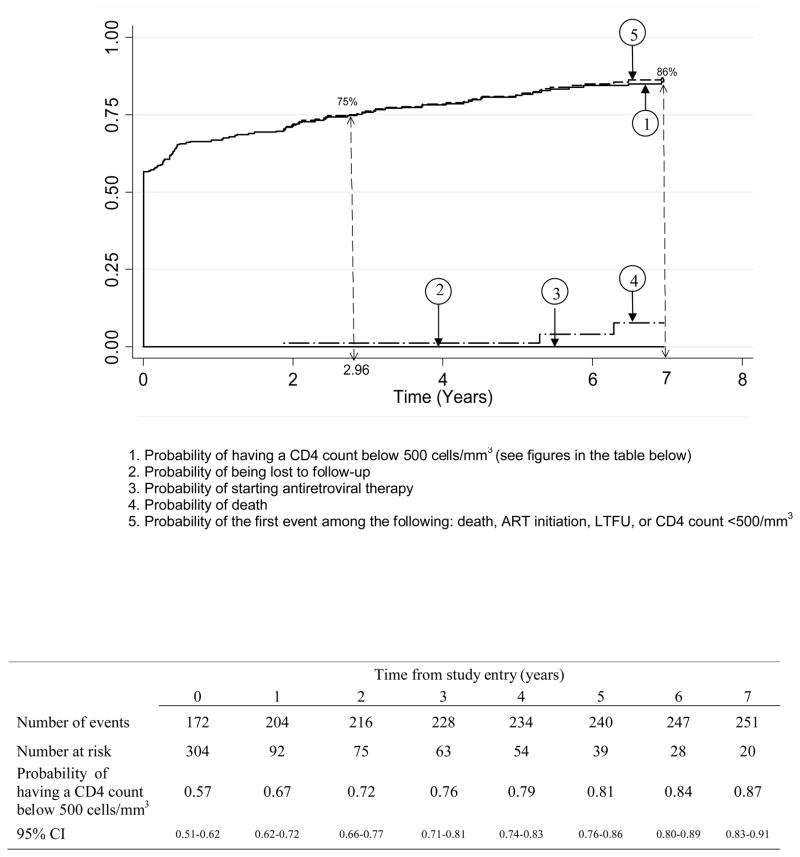
Between 1997 and 2009, 304 recent seroconverters have been enrolled in the Primo-CI cohort (62% men, median baseline age 29 years, median time since the estimated date of seroconversion 9 months). The probability of having a first CD4 count below 500/mm3 was 0.57, 0.72, 0.79 and 0.84 at study entry, 2, 4 and 6 years, respectively. For a first CD4 count below 350/mm3, these figures were 0.29, 0.40, 0.55 and 0.67. The time for 75% of patients to reach the threshold was 3.0 years for 500 CD4/mm3 and 7.0 years for 350 CD4/mm3.
Conclusion
Almost one third of recent seroconverters had a CD4 count below the current ART eligibility threshold at first contact, about 6% more crossed it each subsequent year, and 25% remained above this threshold after 7 years. If the threshold was raised to 500 cells/mm3, 57% of recent seroconverters would immediately be eligible, while 14% would remain above the threshold at 7 years. These results should help modelers and treatment providers anticipate the need in antiretroviral drugs.
Introduction
In sub-Saharan Africa, 3,910,000 adults have started combination antiretroviral therapy (ART) within the past five years (1). Despite this unprecedented scaling-up, patients started ART with a mean CD4 count between 100 and 150 cells/mm3 (2–5). In 2010, the World Health Organization (WHO) guidelines recommended initiating ART when the CD4 count drops below 350/mm3 (6). Little is known about the annual number of HIV-infected individuals who reach the CD4 threshold for ART initiation. Estimates of the probability of reaching this threshold in individuals who were diagnosed early would help modelers and drug providers anticipate the need for antiretroviral drugs. Moreover, estimates of the time spent by patients who, with a CD4 above the threshold, are thus unlikely to receive ART, may contribute to the knowledge of the risk of HIV transmission (4).
We estimated the probability of reaching different CD4 thresholds over time in a cohort of HIV-1 adults who recently seroconverted in Abidjan, Côte d’Ivoire.
Methods
Study Population
The ANRS 1220 Primo-CI cohort of seroconverters has been previously described (7). Since 1997, blood donors at the National Blood Bank (centre national de transfusion sanguine, CNTS) in Abidjan, Cote d’Ivoire who were tested HIV-1 positive were offered to be included in the cohort if the time since their estimated date of seroconversion was < 36 months. The estimated date of seroconversion was the midpoint between the last negative and the first positive HIV-1 test. Once included in the cohort, people benefited from a standardized follow-up, which includes a CD4 count every six months (FACScan then FACSCalibur, Becton Dickinson, Aalst-Erembodegem, Belgium). ART was given to patients who fulfilled ART initiation criteria according to WHO guidelines.
Statistical Analysis
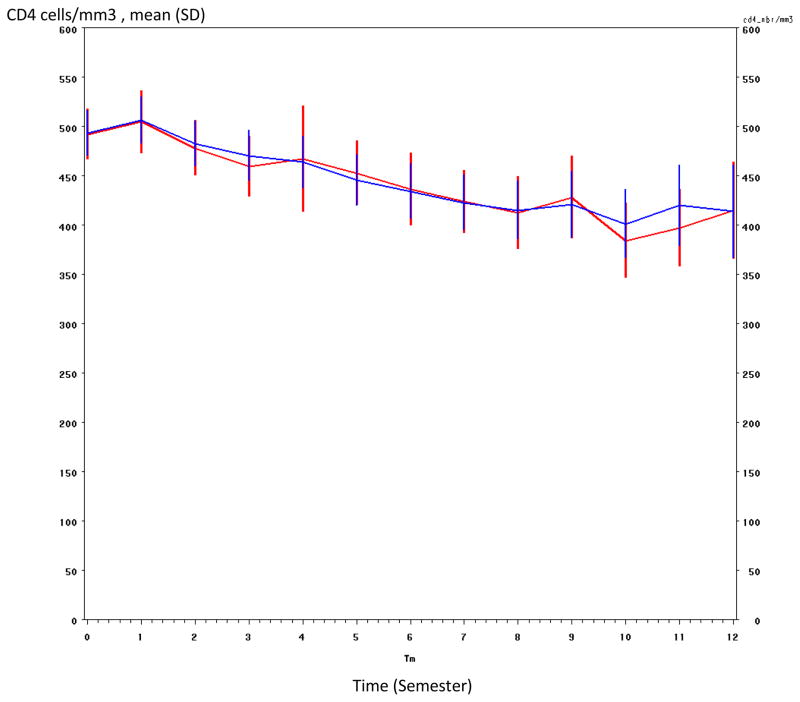
We modelled the CD4 decline on the individual level using a linear mixed model with two slopes (before and after 6 months), adjusted for the delay since the estimated date of seroconversion (<6 months, 6–12 months and >12 months), the baseline plasma HIV-1 RNA level (≤4.5 vs >4.5 log10 copies/ml), the baseline CD4 count (<350, 350–500, >500/mm3), mortality and ART initiation during follow-up. The model was validated by confirming that the simulated CD4 evolution fit the CD4 evolution in the cohort (Figure 1). The probabilities over time of having a CD4 count below 500 cells/mm3 and below 350 CD4 cells/mm3 were estimated by the Kaplan-Meier method. The robustness of the results was studied in sensitivity analysis by estimating the probability of the first event among the following: death, loss to follow-up, ART initiation or first CD4 count below the threshold.
Figure 1. CD4 cell model validation.
Blue line : predicted by the model
Red line : values actually recorded in the cohort
Results
From June 1st 1997 to December 31st 2009, 304 HIV-1 infected patients were enrolled in the cohort, of whom 62% were men. The median time between last negative and first positive HIV-1 test was 7.8 months. At study entry, the median time since the estimated date of seroconversion was 9 months (interquartile range [IQR]: 5–18); the median age was 29 years (IQR: 25–34); the median CD4 count was 463 cells/mm3 (IQR: 327–631); 42% of patients had ≥500 CD4/mm3, 28% had 350 to 499 CD4 cells/mm3, 24% had 200 to 349 cells/mm3, and 6% had <200 CD4 cells/mm3. The median plasma HIV-1 RNA level was 4.5 log10 copies/ml (IQR: 3.9–5.0); only 11 patients (4%) had a plasma HIV-1 RNA level below the detectability threshold (300 copies/ml), all of whom had CD4 >500/mm3. All but two of the participants were classified as infected through heterosexual contact. At enrolment, 289 patients (95%) were classified at clinical stage A according to 1993 CDC classification, 13 at CDC stage B and two at CDC stage C. There was a significant difference between men and women in terms of age (men: median 31 years, IQR: 27–37, women: median 27 years, IQR: 24–32, = 0.002), time since seroconversion (men: median 8 months, IQR: 4–19, women: median 11 months, IQR: 6–19, p = 0.001), plasma HIV-1 RNA (men: median 4.70 log10 copies/ml, IQR: 4.00–5.10, women: 4.25 log10 copies/ml, IQR: 3.80–4.82, p = 0.02). Baseline CD4 count was not significantly different between genders.
The median follow-up time in the cohort was 5.4 years (IQR: 4.0–8.6). The probability of having a CD4 count <350 cells/mm3 was 0.29, 0.40, 0.55 and 0.67 at study entry, 2, 4 and 6 years, respectively (Figure 2A). The probability of having a CD4 count <500 cells/mm3 was 0.57, 0.72, 0.79 and 0.84 at study entry, 2, 4 and 6 years, respectively (Figure 2B). These findings did not change significantly when considering the outcomes of death, loss to follow-up, initiation of ART or first CD4 count below the threshold (Figures 2A and 2B). The time for 75% of patients to reach the threshold was 3 years for 500 CD4 cells/mm3 and 7 years for 350 CD4 cells/mm3.
Figure 2.
Figure 2A: Cumulative probability of having a CD4 count below 350 cells/mm3 in recently HIV-1 infected adults, ANRS 1220 PRIMO-CI cohort 1997–2009
Figure 2B : Cumulative probability of having a CD4 count below 500 cells/mm3 in recently HIV-1 infected adults, ANRS 1220 PRIMO-CI cohort 1997–2009
Discussion
In this cohort, almost one third of recent seroconverters had a CD4 count below the current ART eligibility threshold at first contact, about 6% more crossed it each year, and 25% still remained above this threshold until 7 years. If the threshold was raised to 500 cells/mm3, 57% patients would be immediately eligible while 14% would remain above the threshold at 7 years.
In our study, the median time between the last negative and first positive HIV test was 8 months, and the median delay between seroconversion and enrolment was 9 months. In a study in Thailand, 17% of injection drug users had a CD4 count < 350/mm3 after a similar time since seroconversion (8), while 65% of blood donors enrolled in another study within 2 years after seroconversion had a baseline CD4 count <500 cells/mm3 (9). In the Cascade cohort of seroconverters from high income countries, 27% reached the 350 CD4 cells/mm3 threshold one year after seroconversion and 55% after five years (10). Thus, the time to first CD4 count < 350/mm3 seems shorter in our population compared to Europe and compared to in injection drug users in Thailand, but close to that reported from blood donors in Thailand. Our population of blood donors includes a high proportion of men and may not be entirely representative of the overall population of HIV-infected adults in Côte d’Ivoire who are predominantly women (11).
Different methods have been proposed to estimate the date of seroconversion. In this cohort, we used the mid point between last negative and first positive test, a method commonly used by others (12–16). Alternative methods, including Weibull analysis, lead to similar estimates (17). In this study, however, we used the date of first contact with care as time zero, rather than the date of seroconversion. Contrary to the latter, the date of entry into care is accurately known and can been used as a landmark by policy makers. We wanted entry into care to be as close as possible to seroconversion, while also being something that could be reached in the real life. Enrolment in a seroconverter cohort represents the earliest entry into care that could be reached in programs offering HIV testing on a pluri-annual basis.
Our findings have two practical implications.
First, the high proportion of individuals whose CD4 count was already below the current threshold for ART strongly suggests that efforts should be made to diagnose HIV-infection as soon as possible after seroconversion. This requires implementation of HIV testing in the general population much more frequently than it is currently done (2), and even more so if guidelines continue to move toward higher CD4 thresholds for ART initiation (18).
Second, even if the CD4 threshold for treatment was raised to 500 cells/mm3, a minority, but still significant proportion of patients, would remain untreated for many years. Knowing who these patients are and how they participate in the HIV transmission would be critical in the current debate considering ART as a prevention tool (4).
Acknowledgments
Financial support: This study was funded by the French National Agency for research on AIDS and Viral Hepatitis (ANRS, France) ANRS 1220; and the Côte d’Ivoire Ministry of Public Health.
ANRS 1220 Primo-CI Study Group
Cohort Team
Principal Investigators: Albert Minga, Charlotte Lewden
Clinical Investigators and Event Documentation Committee: Yao Abo, Lambert Dohoun, Albert Minga
Cohort Management: Emma Anini Yah Drowa, Isidore Bohouo Bades, Tatiana Kouamé, Marie Julie N’Dri, Minata Ouattara, Abdelh Sidibé
Biology: Roseline Afi, Arlette Emieme, André Inwoley, Toni Thomas d’Aquin
Data-management and statistics: Ali Coulibaly, Germain Bomisso, Mamadou Konaté
Steering Committee
Xavier Anglaret, Brigitte Bazin, Francois Dabis, Christine Danel, Christiane Deveau, Cécile Goujard, André Inwoley, Laurence Quinty, Laurence Meyer, Roger Salamon, Rodolphe Thiébaut, Marie-Laure Chaix, Christine Rouzioux, Martine Sinet
Support
Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS) - French Agency on Aids and Viral Hepatitis
Footnotes
Authors’ contributions
Albert Minga, Xavier Anglaret and Charlotte Lewden developed the analysis plan. Albert Minga, Germain Bomisso and Delphine Gabillard performed statistical analyses. All authors contributed to interpretation of the data. Albert Minga, Charlotte Lewden and Xavier Anglaret wrote the report, to which all authors then contributed. Albert Minga and Germain Bomisso have full access to all data. Albert Minga, Charlotte Lewden and Xavier Anglaret had final responsibility for the decision to submit for publication.
References
- 1.WHO. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. Progress report. 2010 Available at: http://www.who.int/hiv/pub/2010progressreport/en/
- 2.Dodd P, Garnett G, Hallett T. Examining the promise of HIV elimination by ‘test and treat’ in hyperendemic settings. AIDS. 2010;24:729–35. doi: 10.1097/QAD.0b013e32833433fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walensky RP, Wolf LL, Wood R, Fofana MO, Freedberg KA, Martinson NA, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–66. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granich R, Gilks C, Dye C, De Cock K, Williams B. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 5.The ART-LINC Collaboration of International Databases to Evaluate AIDS (IeDEA) Keiser O, Anastos K, Schechter M, Balestre E, Boulle A, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–9. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents - recommendations for a Public Health Approach - 2010 revision. Available at: http://www.who.int/hiv/topics/treatment/en/index.html. [PubMed]
- 7.Minga A, Danel C, Abo Y, Dohoun L, Bonard D, Coulibaly A, et al. Progression to WHO criteria for antiretroviral therapy in a 7-year cohort of adult HIV-1 seroconverters in Abidjan, Côte d’Ivoire. Bull World Health Organ. 2007;85:116–123. doi: 10.2471/BLT.06.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchacz K, Hu D, Vanichseni S, Mock P, Chaowanachan T, Srisuwanvilai L, et al. Early Markers of HIV-1 Disease Progression in a Prospective Cohort of Seroconverters in Bangkok, Thailand: Implications for Vaccine Trials. J Acquir Immune Defic Syndr. 2004;36:853–860. doi: 10.1097/00126334-200407010-00013. [DOI] [PubMed] [Google Scholar]
- 9.Costello C, Nelson K, Suriyanon V, Sennun S, Tovanabutra S, Heilig C, et al. HIV-1 subtype E progression among northern Thai couples: traditional and non-traditional predictors of survival. Int J Epidemiol. 2005;34:577–84. doi: 10.1093/ije/dyi023. [DOI] [PubMed] [Google Scholar]
- 10.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiébaut R. Proportion of individuals likely to need treatment for CD4 thresholds <200, <350 and <500 cells/mm3. 17th Conference on Retroviruses and Opportunistic Infections; 2010; San Francisco, USA. 2010. [poster 525] [Google Scholar]
- 11.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Côte d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muga R, Ferreros I, Langohr K, de Olalla P, Del Romero J, Quintana M, et al. Changes in the incidence of tuberculosis in a cohort of HIV-seroconverters before and after the introduction of HAART. AIDS. 2007;21:2521–7. doi: 10.1097/QAD.0b013e3282f1c933. [DOI] [PubMed] [Google Scholar]
- 13.Wood E, Kerr T, Marshall B, Li K, Zhang R, Hogg R, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Todd J, Glynn J, Marston M, Lutalo T, Biraro S, Mwita W, et al. Time from HIV seroconversion to death: a collaborative analysis of eight studies in six low and middle-income countries before highly active antiretroviral therapy. AIDS. 2007;21 (Suppl 6):S55–63. doi: 10.1097/01.aids.0000299411.75269.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wandel S, Egger M, Rangsin R, Nelson K, Costello C, Lewden C, et al. Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex Transm Infect. 2008;84 (Suppl):i31–i36. doi: 10.1136/sti.2008.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolbers M, Babiker A, Sabin C, Young J, Dorrucci M, Chene G, et al. Pretreatment CD4 Cell Slope and Progression to AIDS or Death in HIV-Infected Patients Initiating Antiretroviral Therapy—The CASCADE Collaboration: A Collaboration of 23 Cohort Studies. PLoS Medicine. 2010;7:e1000239. doi: 10.1371/journal.pmed.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiarotti F, Palombi M, Schinaia N, Ghirardini A, Prospero L. Effects of different parametric estimates of seroconversion time on analysis of progression. Stat Med. 1992;11:591–601. doi: 10.1002/sim.4780110504. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan J, Benson C, Holmes K, Brooks J, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:58:1–207. quiz CE1–4. [PubMed] [Google Scholar]