Abstract
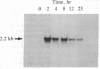
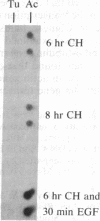
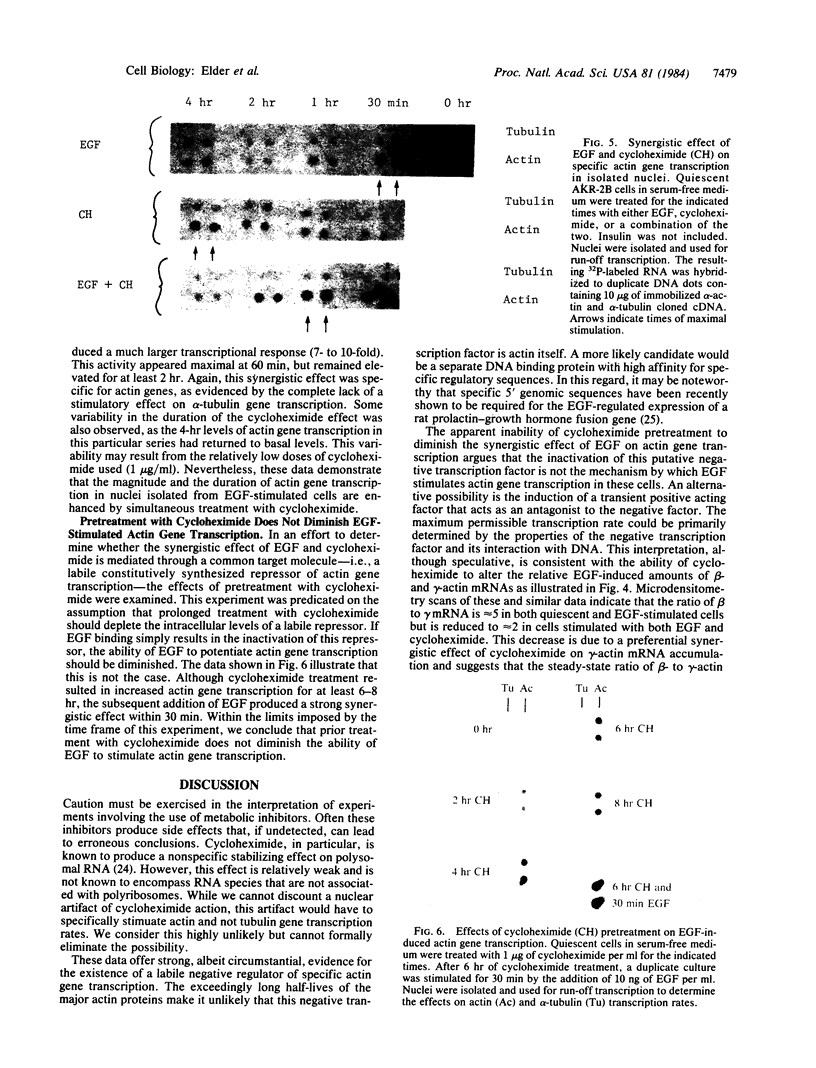
Stimulation of quiescent AKR-2B mouse embryo cells with epidermal growth factor (EGF) results in a rapid and specific induction of actin mRNA sequences. These mRNAs include those coding for both beta- and gamma-cytoskeletal, but not alpha-skeletal muscle, actin isotypes. Elongation of nascent RNA chains in isolated nuclei (run-off transcription) demonstrates that the mRNA accumulation is preceded by an increase in actin gene transcription. This increase is transient, however, and is followed by a rapid attenuation of transcriptional activity. An inhibitor of protein synthesis, cycloheximide, was also found to induce beta- and gamma-actin mRNA accumulation. Furthermore, the simultaneous addition of EGF and cycloheximide produced a synergistic effect on actin sequences in both steady-state nuclear and polysomal RNA. Run-off transcription experiments demonstrate that this synergistic effect results from an increase in the magnitude and duration of actin gene transcription. It is also specific in that alpha-tubulin gene transcription is not similarly affected. These data suggest the existence of a specific labile repressor of actin gene transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz E. W., Jr, Getz M. J., Wells D. J., Moses H. L. Nuclear RNA polymerase activities and poly(A)-containing mRNA accumulation in cultured AKR mouse embryo cells stimulated to proliferate. Exp Cell Res. 1977 Aug;108(1):157–165. [PubMed] [Google Scholar]
- Bitter G. A., Roeder R. G. Transcription of viral genes by RNA polymerase II in nuclei isolated from adenovirus 2 transformed cells. Biochemistry. 1978 May 30;17(11):2198–2205. doi: 10.1021/bi00604a028. [DOI] [PubMed] [Google Scholar]
- Boschek C. B., Jockusch B. M., Friis R. R., Back R., Grundmann E., Bauer H. Early changes in the distribution and organization of microfilament proteins during cell transformation. Cell. 1981 Apr;24(1):175–184. doi: 10.1016/0092-8674(81)90513-4. [DOI] [PubMed] [Google Scholar]
- Bushman F. D., Crain W. R., Jr Conserved pattern of embryonic actin gene expression in several sea urchins and a sand dollar. Dev Biol. 1983 Aug;98(2):429–436. doi: 10.1016/0012-1606(83)90372-x. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Linsley P. S., Wrann M. Receptor remodeling and regulation in the action of epidermal growth factor. Fed Proc. 1982 Nov;41(13):2988–2995. [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Getz M. J., Elder P. K., Benz E. W., Jr, Stephens R. E., Moses H. L. Effect of cell proliferation on levels and diversity of poly(A)-containing mRNA. Cell. 1976 Feb;7(2):255–65. doi: 10.1016/0092-8674(76)90025-8. [DOI] [PubMed] [Google Scholar]
- Giebelhaus D. H., Heikkila J. J., Schultz G. A. Changes in the quantity of histone and actin messenger RNA during the development of preimplantation mouse embryos. Dev Biol. 1983 Jul;98(1):148–154. doi: 10.1016/0012-1606(83)90343-3. [DOI] [PubMed] [Google Scholar]
- Hodgson C. P., Elder P. K., Ono T., Foster D. N., Getz M. J. Structure and expression of mouse VL30 genes. Mol Cell Biol. 1983 Dec;3(12):2221–2231. doi: 10.1128/mcb.3.12.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Leavitt A., Attallah A. M. Dissimilar modes of expression of beta- and gamma-actin in normal and leukemic human T lymphocytes. J Biol Chem. 1980 Jun 10;255(11):4984–4987. [PubMed] [Google Scholar]
- Lin S., Riggs A. D. The general affinity of lac repressor for E. coli DNA: implications for gene regulation in procaryotes and eucaryotes. Cell. 1975 Feb;4(2):107–111. doi: 10.1016/0092-8674(75)90116-6. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F., Walsh R. C., Jr Dihydrocytochalasin B disorganizes actin cytoarchitecture and inhibits initiation of DNA synthesis in 3T3 cells. Cell. 1982 Aug;30(1):253–262. doi: 10.1016/0092-8674(82)90031-9. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Meister R. K., Hulman S. E., Johnson L. F. Rapid changes in poly (A)(+) mRNA content in growth stimulated fibroblasts following perturbations in protein synthesis. J Cell Physiol. 1979 Sep;100(3):531–538. doi: 10.1002/jcp.1041000315. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Guénet J. L., Buckingham M. E. Number and organization of actin-related sequences in the mouse genome. J Mol Biol. 1983 Jun 15;167(1):77–101. doi: 10.1016/s0022-2836(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Potter E., Nicolaisen A. K., Evans R. M., Rosenfeld M. G. Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature. 1982 Nov 11;300(5888):192–194. doi: 10.1038/300192a0. [DOI] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Nguyen-Huu M. C., Sippel A. A., Hynes N. E., Groner B., Schütz G. Preferential transcription of the ovalbumin gene in isolated hen oviduct nuclei by RNA polymerase B. Proc Natl Acad Sci U S A. 1978 Feb;75(2):686–690. doi: 10.1073/pnas.75.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle V. G., Dubrow R., Pardee A. B. Changes in the synthesis of actin and other cell proteins after stimulation of serum-arrested cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1298–1302. doi: 10.1073/pnas.76.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Dieckmann B., Vannice J. L., Trahey M., McCormick F. Inhibition of protein synthesis stimulates the transcription of human beta-interferon genes in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3964–3968. doi: 10.1073/pnas.81.13.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer U., Golden L., Hyman L. E., Colot H. V., Rosbash M. Some somatic sequences are absent or exceedingly rare in Xenopus oocyte RNA. Dev Biol. 1982 Nov;94(1):87–92. doi: 10.1016/0012-1606(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Childs C. B., Volkenant M. E., Moses H. L. Differential effects of epidermal growth factor, transforming growth factor, and insulin on DNA and protein synthesis and morphology in serum-free cultures of AKR-2B cells. Cancer Res. 1984 Feb;44(2):710–716. [PubMed] [Google Scholar]
- Sodja A., Arking R., Zafar R. S. Actin gene expression during embryogenesis of Drosophila melanogaster. Dev Biol. 1982 Apr;90(2):363–368. doi: 10.1016/0012-1606(82)90385-2. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Stallcup M. R., Ring J., Yamamoto K. R. Synthesis of mouse mammary tumor virus ribonucleic acid in isolated nuclei from cultured mammary tumor cells. Biochemistry. 1978 Apr 18;17(8):1515–1521. doi: 10.1021/bi00601a025. [DOI] [PubMed] [Google Scholar]
- Supowit S. C., Potter E., Evans R. M., Rosenfeld M. G. Polypeptide hormone regulation of gene transcription: specific 5' genomic sequences are required for epidermal growth factor and phorbol ester regulation of prolactin gene expression. Proc Natl Acad Sci U S A. 1984 May;81(10):2975–2979. doi: 10.1073/pnas.81.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin typing on total cellular extracts: a highly sensitive protein-chemical procedure able to distinguish different actins. Eur J Biochem. 1981 Jan;113(3):595–603. doi: 10.1111/j.1432-1033.1981.tb05104.x. [DOI] [PubMed] [Google Scholar]
- Wells D. J., Stoddard L. S., Getz M. J., Moses H. L. alpha-Amanitin and 5-fluorouridine inhibition of serum-stimulated DNA synthesis in quiescent AKR-2B mouse embryo cells. J Cell Physiol. 1979 Aug;100(2):199–214. doi: 10.1002/jcp.1041000202. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Penman S. The messenger RNA sequences in growing and resting mouse fibroblasts. Cell. 1975 Oct;6(2):197–206. doi: 10.1016/0092-8674(75)90010-0. [DOI] [PubMed] [Google Scholar]