Abstract
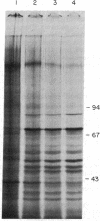
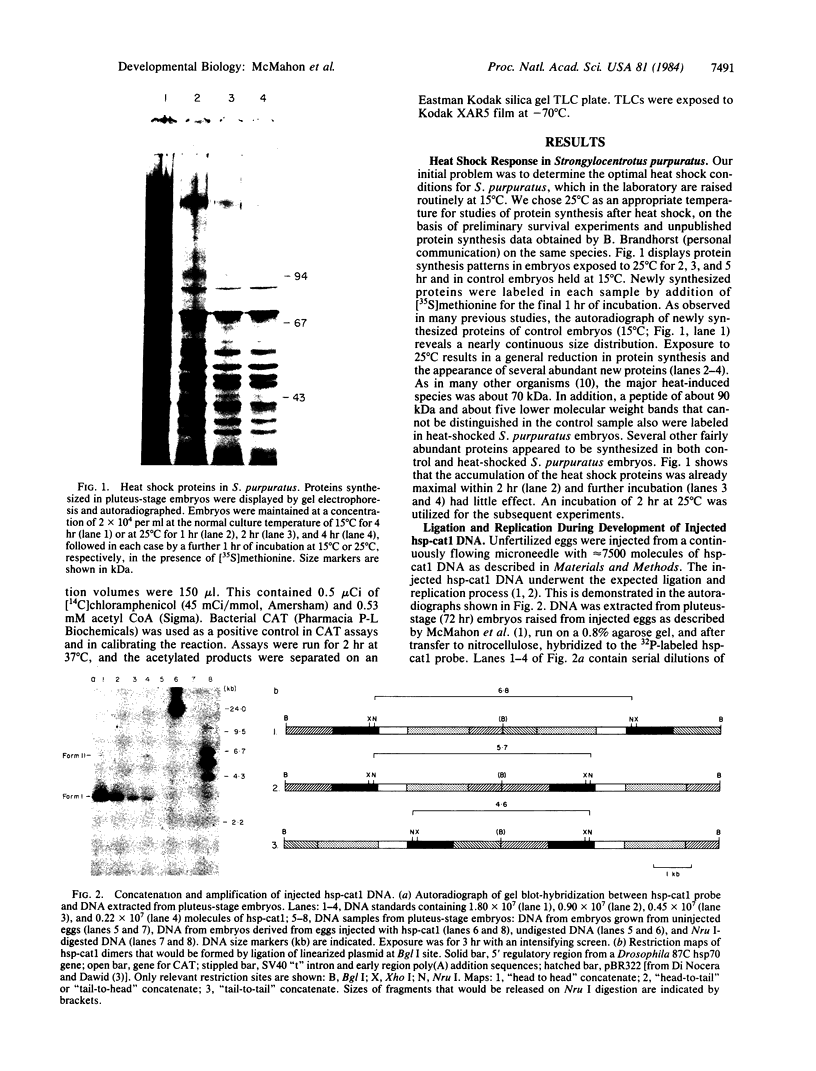
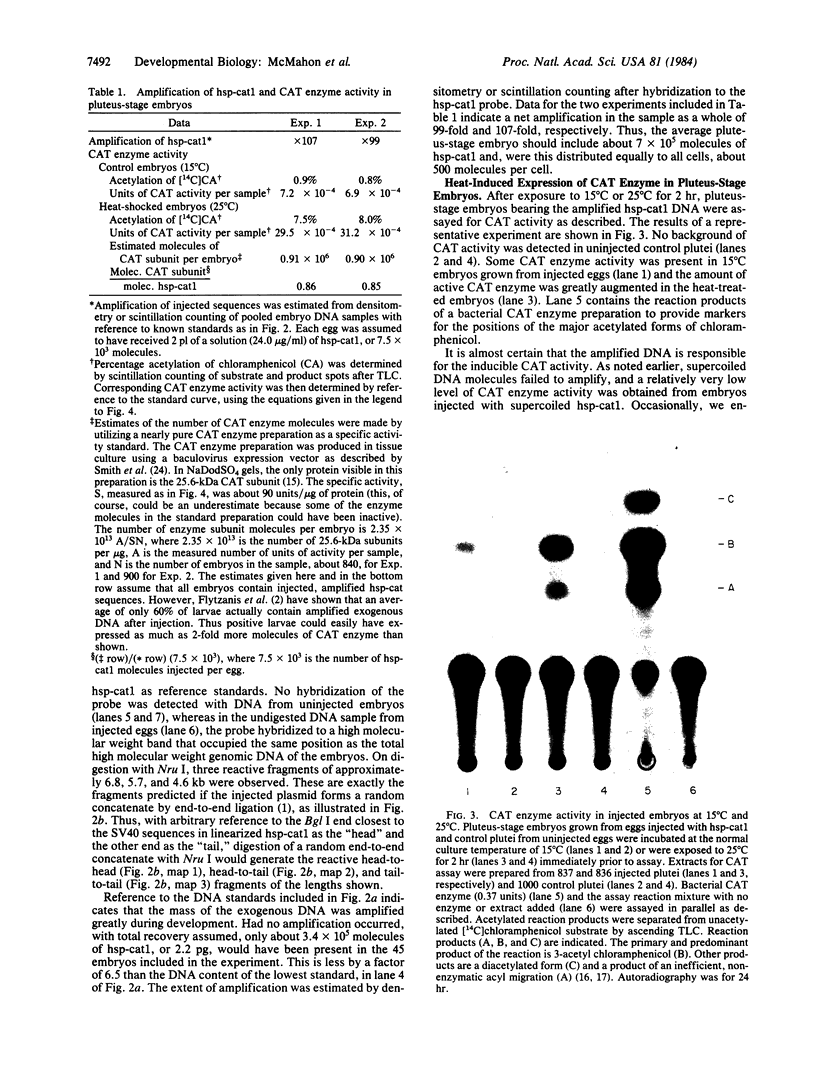
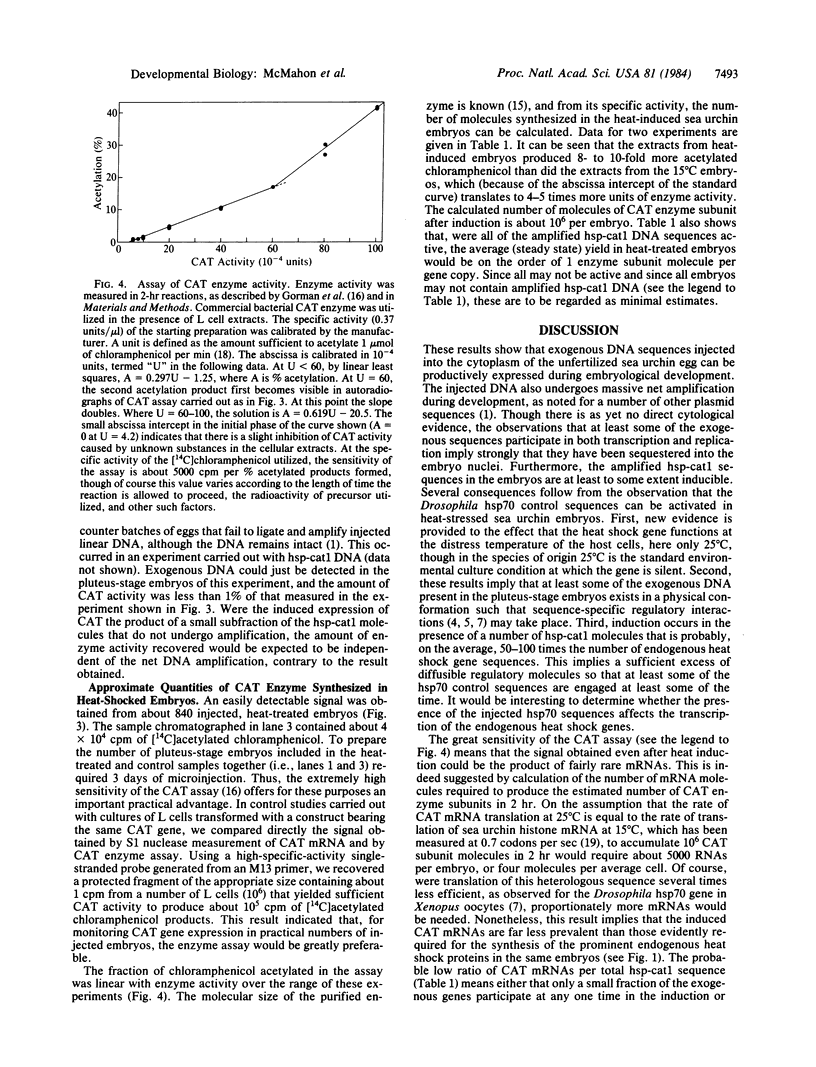
A fusion gene construct, in which the coding sequence for bacterial chloramphenicol acetyltransferase (CAT; acetyl-CoA: chloramphenicol 3-O-acetyltransferase, EC 2.3.1.28) was placed under the control of the regulatory region of the Drosophila gene encoding the 70-kilodalton heat shock protein [Di Nocera, P.P. & Dawid, I.B. (1983) Proc. Natl. Acad. Sci. USA 80, 7095-7098], was microinjected into the cytoplasm of unfertilized sea urchin eggs. Pluteus-stage embryos developing from the injected eggs were exposed to high temperature conditions that we found would elicit an endogenous sea urchin heat shock response. These embryos express the gene for CAT and, after heat treatment, display 8-10 times more CAT enzyme activity than do extracts from control embryos cultured at normal temperatures. The injected DNA is present in high molecular weight concatenates and, during development, is amplified about 100-fold. Amplified sequences are responsible for all or most of the induced CAT enzyme activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienz M., Pelham H. R. Expression of a Drosophila heat-shock protein in Xenopus oocytes: conserved and divergent regulatory signals. EMBO J. 1982;1(12):1583–1588. doi: 10.1002/j.1460-2075.1982.tb01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Corces V., Pellicer A., Axel R., Meselson M. Integration, transcription, and control of a Drosophila heat shock gene in mouse cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7038–7042. doi: 10.1073/pnas.78.11.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findly R. C., Pederson T. Regulated transcription of the genes for actin and heat-shock proteins in cultured Drosophila cells. J Cell Biol. 1981 Feb;88(2):323–328. doi: 10.1083/jcb.88.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Wilt F. H. Direct measurement of histone peptide elongation rate in cleaving sea urchin embryos. Biochim Biophys Acta. 1982 Oct 29;699(1):22–27. doi: 10.1016/0167-4781(82)90167-1. [DOI] [PubMed] [Google Scholar]
- Hubbard B. D., Lazarides E. Copurification of actin and desmin from chicken smooth muscle and their copolymerization in vitro to intermediate filaments. J Cell Biol. 1979 Jan;80(1):166–182. doi: 10.1083/jcb.80.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel P. M., Bautz E. K. Heat-shock proteins are associated with hnRNA in Drosophila melanogaster tissue culture cells. EMBO J. 1983;2(5):705–710. doi: 10.1002/j.1460-2075.1983.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Helmsing P. J., Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirault M. E., Southgate R., Delwart E. Regulation of heat-shock genes: a DNA sequence upstream of Drosophila hsp70 genes is essential for their induction in monkey cells. EMBO J. 1982;1(10):1279–1285. doi: 10.1002/j.1460-2075.1982.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Packman L. C., Burleigh B. D., Dell A., Morris H. R., Hartley B. S. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature. 1979 Dec 20;282(5741):870–872. doi: 10.1038/282870a0. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Rungger D. Transcription of a Drosophila heat shock gene is heat-induced in Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1776–1780. doi: 10.1073/pnas.79.6.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]