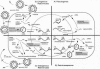
Abstract
Human endogenous retroviruses (HERVs) are very likely footprints of ancient germ-cell infections. HERV sequences encompass about 1% of the human genome. HERVs have retained the potential of other retroelements to retrotranspose and thus to change genomic structure and function. The genomes of almost all HERV families are highly defective. Recent progress has allowed the identification of the biologically most active family, HTDV/HERV-K, which codes for viral proteins and particles and is highly expressed in germ-cell tumors. The demonstrable and potential roles of HTDV/HERV-K as well as of other human elements in disease and in maintaining genome plasticity are illustrated.
Full text
PDF
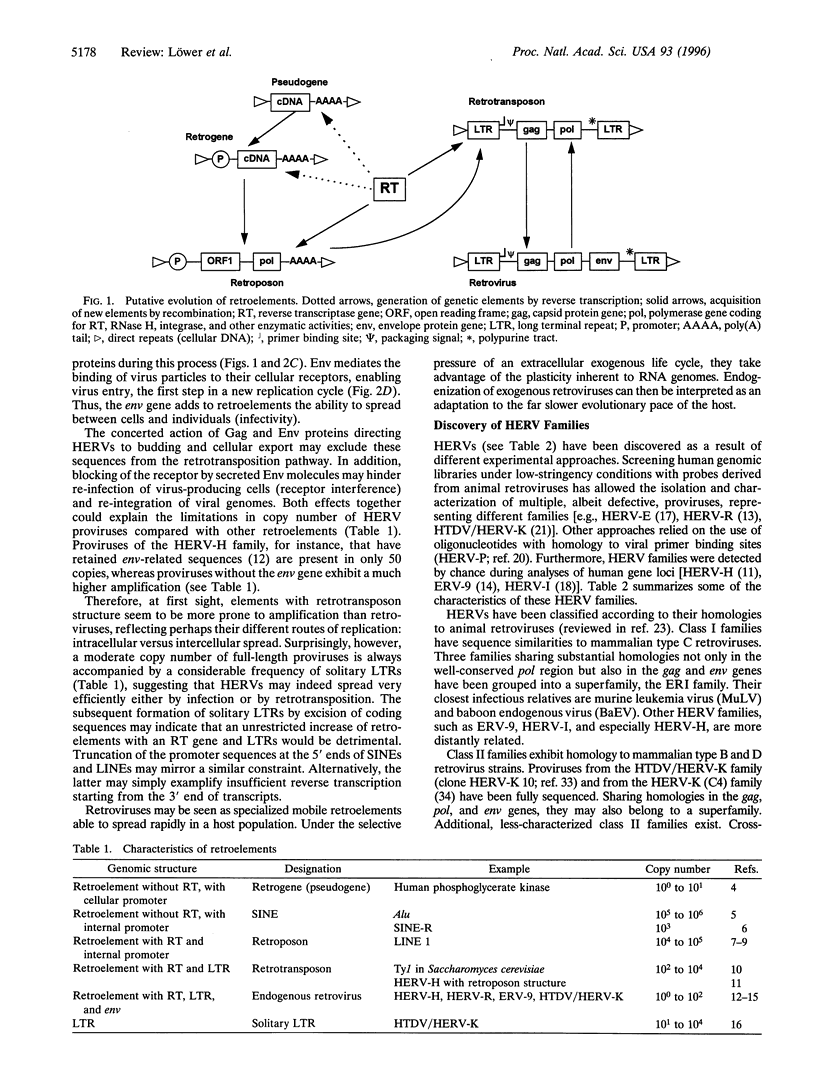
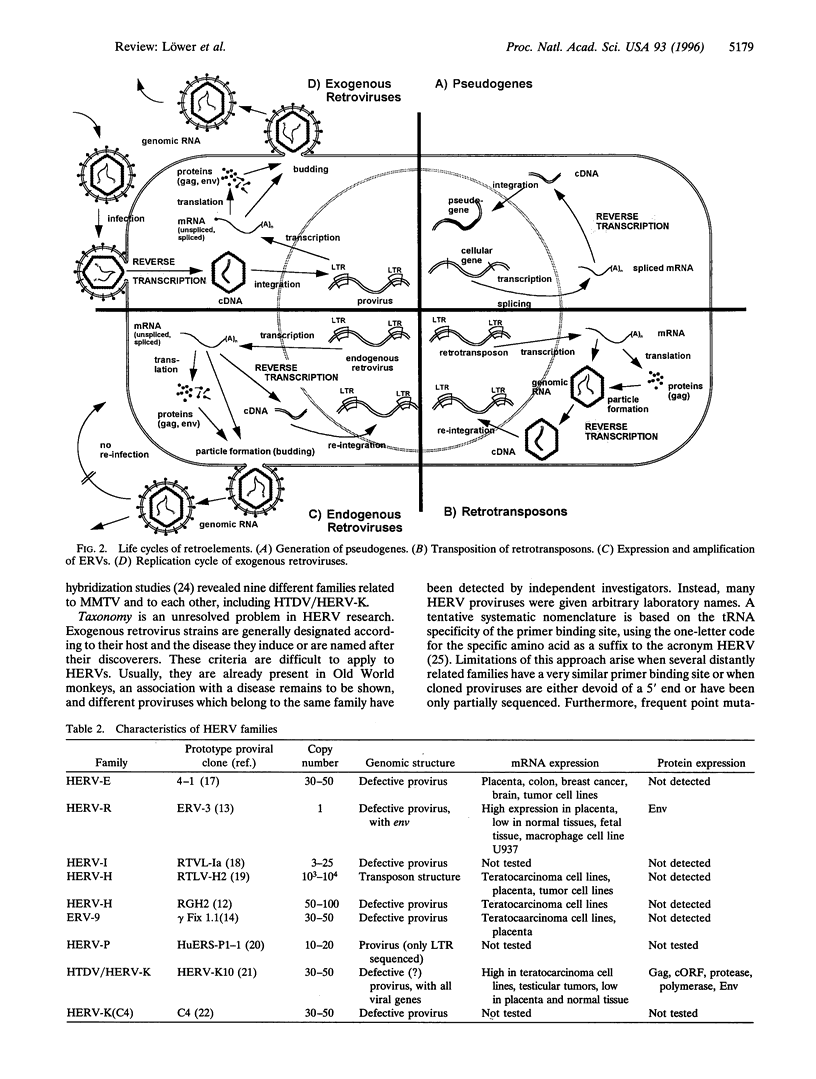





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Adey N. B., Schichman S. A., Graham D. K., Peterson S. N., Edgell M. H., Hutchison C. A., 3rd Rodent L1 evolution has been driven by a single dominant lineage that has repeatedly acquired new transcriptional regulatory sequences. Mol Biol Evol. 1994 Sep;11(5):778–789. doi: 10.1093/oxfordjournals.molbev.a040158. [DOI] [PubMed] [Google Scholar]
- Arvidsson A. K., Svensson A. C., Widmark E., Andersson G., Rask L., Larhammar D. Characterization of three separated exons in the HLA class II DR region of the human major histocompatibility complex. Hum Immunol. 1995 Mar;42(3):254–264. doi: 10.1016/0198-8859(94)00102-v. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Retroviruses and retrotransposons: the role of reverse transcription in shaping the eukaryotic genome. Cell. 1985 Mar;40(3):481–482. doi: 10.1016/0092-8674(85)90190-4. [DOI] [PubMed] [Google Scholar]
- Boller K., Frank H., Löwer J., Löwer R., Kurth R. Structural organization of unique retrovirus-like particles budding from human teratocarcinoma cell lines. J Gen Virol. 1983 Dec;64(Pt 12):2549–2559. doi: 10.1099/0022-1317-64-12-2549. [DOI] [PubMed] [Google Scholar]
- Boller K., König H., Sauter M., Mueller-Lantzsch N., Löwer R., Löwer J., Kurth R. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology. 1993 Sep;196(1):349–353. doi: 10.1006/viro.1993.1487. [DOI] [PubMed] [Google Scholar]
- Boyd M. T., Bax C. M., Bax B. E., Bloxam D. L., Weiss R. A. The human endogenous retrovirus ERV-3 is upregulated in differentiating placental trophoblast cells. Virology. 1993 Oct;196(2):905–909. doi: 10.1006/viro.1993.1556. [DOI] [PubMed] [Google Scholar]
- Bronson D. L., Ritzi D. M., Fraley E. E., Dalton A. J. Morphologic evidence for retrovirus production by epithelial cells derived from a human testicular tumor metastasis. J Natl Cancer Inst. 1978 Jun;60(6):1305–1308. doi: 10.1093/jnci/60.6.1305. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Conrad B., Weidmann E., Trucco G., Rudert W. A., Behboo R., Ricordi C., Rodriquez-Rilo H., Finegold D., Trucco M. Evidence for superantigen involvement in insulin-dependent diabetes mellitus aetiology. Nature. 1994 Sep 22;371(6495):351–355. doi: 10.1038/371351a0. [DOI] [PubMed] [Google Scholar]
- Craig L. C., Pirtle I. L., Gracy R. W., Pirtle R. M. Characterization of the transcription unit and two processed pseudogenes of chimpanzee triosephosphate isomerase (TPI). Gene. 1991 Mar 15;99(2):217–227. doi: 10.1016/0378-1119(91)90130-4. [DOI] [PubMed] [Google Scholar]
- Dangel A. W., Mendoza A. R., Baker B. J., Daniel C. M., Carroll M. C., Wu L. C., Yu C. Y. The dichotomous size variation of human complement C4 genes is mediated by a novel family of endogenous retroviruses, which also establishes species-specific genomic patterns among Old World primates. Immunogenetics. 1994;40(6):425–436. doi: 10.1007/BF00177825. [DOI] [PubMed] [Google Scholar]
- Deragon J. M., Sinnett D., Labuda D. Reverse transcriptase activity from human embryonal carcinoma cells NTera2D1. EMBO J. 1990 Oct;9(10):3363–3368. doi: 10.1002/j.1460-2075.1990.tb07537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A., Strazzullo M., Longo L., La Mantia G. Characterization and genomic mapping of the ZNF80 locus: expression of this zinc-finger gene is driven by a solitary LTR of ERV9 endogenous retroviral family. Nucleic Acids Res. 1995 Aug 11;23(15):2823–2830. doi: 10.1093/nar/23.15.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombroski B. A., Mathias S. L., Nanthakumar E., Scott A. F., Kazazian H. H., Jr Isolation of an active human transposable element. Science. 1991 Dec 20;254(5039):1805–1808. doi: 10.1126/science.1662412. [DOI] [PubMed] [Google Scholar]
- Ellerbrok H., D'Auriol L., Vaquero C., Sitbon M. Functional tolerance of the human immunodeficiency virus type 1 envelope signal peptide to mutations in the amino-terminal and hydrophobic regions. J Virol. 1992 Aug;66(8):5114–5118. doi: 10.1128/jvi.66.8.5114-5118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin G. C., Chretien S., Hanson I. M., Rochefort H., May F. E., Westley B. R. Expression of human sequences related to those of mouse mammary tumor virus. J Virol. 1988 Apr;62(4):1203–1210. doi: 10.1128/jvi.62.4.1203-1210.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkina T. V., Chervonsky A., Dudley J. P., Ross S. R. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992 May 15;69(4):637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- Harada F., Tsukada N., Kato N. Isolation of three kinds of human endogenous retrovirus-like sequences using tRNA(Pro) as a probe. Nucleic Acids Res. 1987 Nov 25;15(22):9153–9162. doi: 10.1093/nar/15.22.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann O., Heidmann T. Retrotransposition of a mouse IAP sequence tagged with an indicator gene. Cell. 1991 Jan 11;64(1):159–170. doi: 10.1016/0092-8674(91)90217-m. [DOI] [PubMed] [Google Scholar]
- Hirose Y., Takamatsu M., Harada F. Presence of env genes in members of the RTVL-H family of human endogenous retrovirus-like elements. Virology. 1993 Jan;192(1):52–61. doi: 10.1006/viro.1993.1007. [DOI] [PubMed] [Google Scholar]
- Holmes S. E., Dombroski B. A., Krebs C. M., Boehm C. D., Kazazian H. H., Jr A new retrotransposable human L1 element from the LRE2 locus on chromosome 1q produces a chimaeric insertion. Nat Genet. 1994 Jun;7(2):143–148. doi: 10.1038/ng0694-143. [DOI] [PubMed] [Google Scholar]
- Horn T. M., Huebner K., Croce C., Callahan R. Chromosomal locations of members of a family of novel endogenous human retroviral genomes. J Virol. 1986 Jun;58(3):955–959. doi: 10.1128/jvi.58.3.955-959.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügin A. W., Vacchio M. S., Morse H. C., 3rd A virus-encoded "superantigen" in a retrovirus-induced immunodeficiency syndrome of mice. Science. 1991 Apr 19;252(5004):424–427. doi: 10.1126/science.1850169. [DOI] [PubMed] [Google Scholar]
- Imai S., Okumoto M., Iwai M., Haga S., Mori N., Miyashita N., Moriwaki K., Hilgers J., Sarkar N. H. Distribution of mouse mammary tumor virus in Asian wild mice. J Virol. 1994 May;68(5):3437–3442. doi: 10.1128/jvi.68.5.3437-3442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambhu S., Falldorf P., Lee J. S. Endogenous retroviral long terminal repeats within the HLA-DQ locus. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4927–4931. doi: 10.1073/pnas.87.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Wong C., Youssoufian H., Scott A. F., Phillips D. G., Antonarakis S. E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature. 1988 Mar 10;332(6160):164–166. doi: 10.1038/332164a0. [DOI] [PubMed] [Google Scholar]
- La Mantia G., Maglione D., Pengue G., Di Cristofano A., Simeone A., Lanfrancone L., Lania L. Identification and characterization of novel human endogenous retroviral sequences prefentially expressed in undifferentiated embryonal carcinoma cells. Nucleic Acids Res. 1991 Apr 11;19(7):1513–1520. doi: 10.1093/nar/19.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E., Kato N., Cohen M. Human endogenous proviruses. Curr Top Microbiol Immunol. 1989;148:115–132. doi: 10.1007/978-3-642-74700-7_4. [DOI] [PubMed] [Google Scholar]
- Leib-Mösch C., Haltmeier M., Werner T., Geigl E. M., Brack-Werner R., Francke U., Erfle V., Hehlmann R. Genomic distribution and transcription of solitary HERV-K LTRs. Genomics. 1993 Nov;18(2):261–269. doi: 10.1006/geno.1993.1464. [DOI] [PubMed] [Google Scholar]
- Löwer J., Wondrak E. M., Kurth R. Genome analysis and reverse transcriptase activity of human teratocarcinoma-derived retroviruses. J Gen Virol. 1987 Nov;68(Pt 11):2807–2815. doi: 10.1099/0022-1317-68-11-2807. [DOI] [PubMed] [Google Scholar]
- Löwer R., Boller K., Hasenmaier B., Korbmacher C., Müller-Lantzsch N., Löwer J., Kurth R. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4480–4484. doi: 10.1073/pnas.90.10.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwer R., Löwer J., Frank H., Harzmann R., Kurth R. Human teratocarcinomas cultured in vitro produce unique retrovirus-like viruses. J Gen Virol. 1984 May;65(Pt 5):887–898. doi: 10.1099/0022-1317-65-5-887. [DOI] [PubMed] [Google Scholar]
- Löwer R., Löwer J., Tondera-Koch C., Kurth R. A general method for the identification of transcribed retrovirus sequences (R-U5 PCR) reveals the expression of the human endogenous retrovirus loci HERV-H and HERV-K in teratocarcinoma cells. Virology. 1993 Feb;192(2):501–511. doi: 10.1006/viro.1993.1066. [DOI] [PubMed] [Google Scholar]
- Maeda N. Nucleotide sequence of the haptoglobin and haptoglobin-related gene pair. The haptoglobin-related gene contains a retrovirus-like element. J Biol Chem. 1985 Jun 10;260(11):6698–6709. [PubMed] [Google Scholar]
- Mager D. L., Freeman J. D. Human endogenous retroviruslike genome with type C pol sequences and gag sequences related to human T-cell lymphotropic viruses. J Virol. 1987 Dec;61(12):4060–4066. doi: 10.1128/jvi.61.12.4060-4066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. L. Ribonucleoprotein particles with LINE-1 RNA in mouse embryonal carcinoma cells. Mol Cell Biol. 1991 Sep;11(9):4804–4807. doi: 10.1128/mcb.11.9.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey J. R., Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987 Apr 2;326(6112):501–505. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- Meese E., Göttert E., Zang K. D., Sauter M., Schommer S., Mueller-Lantzsch N. Human endogenous retroviral element k10 (HERV-K10): chromosomal localization by somatic hybrid mapping and fluorescence in situ hybridization. Cytogenet Cell Genet. 1996;72(1):40–42. doi: 10.1159/000134157. [DOI] [PubMed] [Google Scholar]
- Miki Y., Nishisho I., Horii A., Miyoshi Y., Utsunomiya J., Kinzler K. W., Vogelstein B., Nakamura Y. Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Cancer Res. 1992 Feb 1;52(3):643–645. [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse B., Rotherg P. G., South V. J., Spandorfer J. M., Astrin S. M. Insertional mutagenesis of the myc locus by a LINE-1 sequence in a human breast carcinoma. Nature. 1988 May 5;333(6168):87–90. doi: 10.1038/333087a0. [DOI] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Sauter M., Weiskircher A., Kramer K., Best B., Buck M., Grässer F. Human endogenous retroviral element K10 (HERV-K10) encodes a full-length gag homologous 73-kDa protein and a functional protease. AIDS Res Hum Retroviruses. 1993 Apr;9(4):343–350. doi: 10.1089/aid.1993.9.343. [DOI] [PubMed] [Google Scholar]
- O'Connell C., O'Brien S., Nash W. G., Cohen M. ERV3, a full-length human endogenous provirus: chromosomal localization and evolutionary relationships. Virology. 1984 Oct 30;138(2):225–235. doi: 10.1016/0042-6822(84)90347-7. [DOI] [PubMed] [Google Scholar]
- Ono M., Kawakami M., Takezawa T. A novel human nonviral retroposon derived from an endogenous retrovirus. Nucleic Acids Res. 1987 Nov 11;15(21):8725–8737. doi: 10.1093/nar/15.21.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Kawakami M., Ushikubo H. Stimulation of expression of the human endogenous retrovirus genome by female steroid hormones in human breast cancer cell line T47D. J Virol. 1987 Jun;61(6):2059–2062. doi: 10.1128/jvi.61.6.2059-2062.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M. Molecular cloning and long terminal repeat sequences of human endogenous retrovirus genes related to types A and B retrovirus genes. J Virol. 1986 Jun;58(3):937–944. doi: 10.1128/jvi.58.3.937-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Yasunaga T., Miyata T., Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986 Nov;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., O'Neill R. R., Steele P. E., Martin M. A. Characterization and partial nucleotide sequence of endogenous type C retrovirus segments in human chromosomal DNA. Proc Natl Acad Sci U S A. 1983 Feb;80(3):678–682. doi: 10.1073/pnas.80.3.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltarelli M., Querat G., Konings D. A., Vigne R., Clements J. E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology. 1990 Nov;179(1):347–364. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- Samuelson L. C., Wiebauer K., Snow C. M., Meisler M. H. Retroviral and pseudogene insertion sites reveal the lineage of human salivary and pancreatic amylase genes from a single gene during primate evolution. Mol Cell Biol. 1990 Jun;10(6):2513–2520. doi: 10.1128/mcb.10.6.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M., Schommer S., Kremmer E., Remberger K., Dölken G., Lemm I., Buck M., Best B., Neumann-Haefelin D., Mueller-Lantzsch N. Human endogenous retrovirus K10: expression of Gag protein and detection of antibodies in patients with seminomas. J Virol. 1995 Jan;69(1):414–421. doi: 10.1128/jvi.69.1.414-421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982 Mar;28(3):433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Steinhuber S., Brack M., Hunsmann G., Schwelberger H., Dierich M. P., Vogetseder W. Distribution of human endogenous retrovirus HERV-K genomes in humans and different primates. Hum Genet. 1995 Aug;96(2):188–192. doi: 10.1007/BF00207377. [DOI] [PubMed] [Google Scholar]
- Svensson A. C., Setterblad N., Sigurdardóttir S., Rask L., Andersson G. Primate DRB genes from the DR3 and DR8 haplotypes contain ERV9 LTR elements at identical positions. Immunogenetics. 1995;41(2-3):74–82. doi: 10.1007/BF00182316. [DOI] [PubMed] [Google Scholar]
- Ullu E., Tschudi C. Alu sequences are processed 7SL RNA genes. Nature. 1984 Nov 8;312(5990):171–172. doi: 10.1038/312171a0. [DOI] [PubMed] [Google Scholar]
- Venables P. J., Brookes S. M., Griffiths D., Weiss R. A., Boyd M. T. Abundance of an endogenous retroviral envelope protein in placental trophoblasts suggests a biological function. Virology. 1995 Aug 20;211(2):589–592. doi: 10.1006/viro.1995.1442. [DOI] [PubMed] [Google Scholar]
- Vogetseder W., Dumfahrt A., Mayersbach P., Schönitzer D., Dierich M. P. Antibodies in human sera recognizing a recombinant outer membrane protein encoded by the envelope gene of the human endogenous retrovirus K. AIDS Res Hum Retroviruses. 1993 Jul;9(7):687–694. doi: 10.1089/aid.1993.9.687. [DOI] [PubMed] [Google Scholar]
- Wang C. T., Barklis E. Assembly, processing, and infectivity of human immunodeficiency virus type 1 gag mutants. J Virol. 1993 Jul;67(7):4264–4273. doi: 10.1128/jvi.67.7.4264-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York D. F., Vigne R., Verwoerd D. W., Querat G. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J Virol. 1992 Aug;66(8):4930–4939. doi: 10.1128/jvi.66.8.4930-4939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. B., Jian B., Volanakis J. E. Ancestry of SINE-R.C2 a human-specific retroposon. Hum Genet. 1994 May;93(5):545–551. doi: 10.1007/BF00202821. [DOI] [PubMed] [Google Scholar]
- Zybarth G., Kräusslich H. G., Partin K., Carter C. Proteolytic activity of novel human immunodeficiency virus type 1 proteinase proteins from a precursor with a blocking mutation at the N terminus of the PR domain. J Virol. 1994 Jan;68(1):240–250. doi: 10.1128/jvi.68.1.240-250.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kuyl A. C., Dekker J. T., Goudsmit J. Full-length proviruses of baboon endogenous virus (BaEV) and dispersed BaEV reverse transcriptase retroelements in the genome of baboon species. J Virol. 1995 Sep;69(9):5917–5924. doi: 10.1128/jvi.69.9.5917-5924.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]