Abstract
OX40 is a potent co-stimulatory receptor that can potentiate T cell receptor signaling on the surface of T lymphocytes, leading to their activation by a specifically recognized antigen. In particular, OX40 engagement by ligands present on dendritic cells dramatically increases the proliferation, effector function and survival of T cells. Preclinical studies have shown that OX40 agonists increase anti-tumor immunity and improve tumor-free survival. In this study, we performed a Phase I clinical trial using a mouse monoclonal antibody (mAb) that agonizes human OX40 signaling in patients with advanced cancer. Patients treated with one course of the anti-OX40 mAb showed an acceptable toxicity profile and regression of at least one metastatic lesion in 12/30 patients. Mechanistically, this treatment increased T and B cell responses to reporter antigen immunizations, led to preferential upregulation of OX40 on CD4+ FoxP3+ regulatory T cells in tumor-infiltrating lymphocytes and increased the anti-tumor reactivity of T and B cells in patients with melanoma. Our findings clinically validate OX40 as a potent immune-stimulating target for treatment in cancer patients, providing a generalizable tool to favorably influence the antitumor properties of circulating T cells, B cells and intratumoral regulatory T cells.
Keywords: Immunotherapy, T lymphocytes, immune biomarkers, T cell co-stimulation, tumor specific T cell response
Introduction
Antibodies (Abs), that target T cell surface proteins, have been shown to restore and enhance the function of tumor-reactive T cells in vivo in tumor-bearing hosts (1-5). The antagonists, anti-CTLA-4 and anti-PD-1, block negative signals to the T cells, while the agonists, anti-4-1BB and anti-OX40, enhance T cell function by increasing costimulation (6). A phase III clinical trial in patients with metastatic melanoma demonstrated enhanced survival in patients receiving anti-CTLA-4 and these results led to the recent FDA approval of this antibody (7). Abs directed to PD-1 or PD-1 ligand have produced complete and partial responses as well as durable stable disease in patients with cancer (8, 9). The strategy of blocking inhibitory T cell pathways has shown clinical activity and there is ample preclinical evidence that T-cell co-stimulation via 4-1BB (10, 11) or OX40 can induce anti-tumor effects (12-15).
We completed a translational research study to determine the potential value of immunostimulatory antibody against OX40. OX40 is a TNF-receptor family member that is expressed primarily on activated CD4+ and CD8+ T cells (16-18). Preclinical cancer models have shown that anti-OX40 has potent anti-tumor activity against multiple tumor types, which is dependent on both CD4+ and CD8+ T cells (12-15). Immunization models have shown that anti-OX40 increased T cell proliferation, effector cytokine production, cytotoxicity, and decreased activation-induced cell death leading to an increase in memory T cells (19-23). This report describes the clinical, immunological and anti-tumor effects of an agonist antibody to OX40 in patients with advanced cancer.
Materials and Methods
Clinical trial ID#NCT01644968
Clinical trial was designed and performed as described in Supplemental Materials and Methods.
Anti-OX40 mAb (9B12)
9B12 is a murine IgG1, anti-OX40 mAb directed against the extracellular domain of human OX40 (CD134). The mAb was selected as described in Supplemental Material and Methods.
ELISA Assays for Tetanus and KLH
Recombinant tetanus toxoid C fragment, (Roche), at 2ug/ml, or KLH (Biosyn Corp), at 10ug/ml, was absorbed on the surface of 96-well plates (Fisher). Serum samples were then incubated for 1 hour, followed by peroxidase-conjugated goat anti-human IgG, (Jackson Immuno Research Lab). TMB substrate solution (SureBlue TMB, KPL Inc) was added, followed by a stopping solution (85% O-Phosphoric Acid, Fisher). Spectrophotmetry measurements were made at 450nm (Wallac Victor2 spectrophotometer, Perkin Elmer).
ELISA Assay for Measurement of Anti-OX40 (CD134) in Human Serum
Titers in human serum were measured as indicated in Supplemental Material and Methods.
Flow Cytometry
Peripheral blood mononuclear cells (PBMC) were obtained from patients and cryopreserved samples were used for flow cytometry studies. The fluorochrome-labeled antibodies to CD3, CD4, CD8, CD95, HLA-DR, CD45RA, CCR7 and Ki-67 were purchased from BD Pharmingen, Foxp3 and CXCR5 from eBioscience, CD28 from Beckman Coulter, CD25 from Miltenyi Biotech and CD38, OX40 from BioLegend and Streptavidin AF-700 from Invitrogen. Intracellular staining was performed using the Fix/Perm kit from eBioscience according to the manufacturer's instructions. To prevent the interference of HAMA with staining, cells were preincubated with the mAb anti-OX40 (9B12). Detection of anti-OX40 binding was performed on fresh PBMCs using an anti-mouse IgG and FITC-labeled anti-rat IgG (Invitrogen). Stained cells were analyzed on an LSRII or the FACS Aria (BD Biosciences). Data analysis was performed using either Winlist (Verity Software House) or FACSDiva (Becton Dickinson) software.
Tumor-Specific T cell Assays
Tumor-specific reactivity before and after anti-OX40 administration was assessed in PBMC from 3 melanoma patients. Autologous or HLA-matched melanoma cells were co-cultured with PBMC at a PBMC:tumor ratio of 8:1 for five days. IFN-γ in the supernatant was quantified using an IFN-γ ELISA kit (BD Biosciences). Western blot: FEMX (ATCC) or HEK 293 (ATCC) cells cell lysate were heated to 100°C in gel sample buffer with SDS for 5 minutes and 15 ug of protein were separated by electrophoresis on a 10% SDS PAGE gel. Proteins were transferred to nitrocellulose. The blocked membrane was incubated with patient sera, then with a peroxidase-conjugated secondary Ab, and exposed with ECL Western Blotting substrate (Pierce).
T cell Proliferation Assay
Cryopreserved PBMC (12 Arm A samples and 11 Arm B samples), 1×105 PBMC per well, were stimulated with 0.5μg/ml of anti-CD28/anti-CD49d (BD Biosciences) and with tetanus toxoid antigen (EMD Calbiochem) at 1ug/ml for 4 days. Cells were then labeled with [3H]Thymidine (MP Biomedicals) for 18hrs, then harvested and counted on a Wallac Trilux Microbeta scintillation counter. Tetanus-specific proliferation was measured by subtracting proliferation in the absence of Ag from the tetanus toxoid stimulated cultures. Fold-increases were calculated by dividing the proliferation measured on day 43 (Arm A) or day 15 (Arm B) from the proliferation observed pre anti-OX40 administration.
Statistical Analyses
Ki-67 Analyses
For each cell population the mean differences between treatment cohorts at study days 8 and 15 were compared using one-way analysis of variance (ANOVA) on the log10 fold-change from baseline. The log10 transformation was performed to satisfy statistical model assumptions. Comparisons between cohorts were not adjusted for multiple comparisons due to the exploratory nature of the analyses. Mean differences in fold-change between responders and non-responders at each study day were analyzed using one-way ANOVA. Analyses were performed using JMP version 9 (SAS Institute).
Tetanus and KLH-specific ELISA
For all statistical tests of serum antibody response to KLH and tetanus, a cut-off optical density (OD) value was established that would determine the dilution at which the sera would be considered negative (endpoint dilution). A best-fit curve generated by a 1-phase decay model was used to determine the OD values for the bottom plateau for each ELISA plate. The mean for all of the plateaus on the plate + 4 standard deviations was used as the cut-off OD. The dilution at which each curve intersected the cutoff is the endpoint dilution (titer) for that curve. The two arms from all three cohorts were compared using a two-tailed Mann–Whitney (Wilcoxon rank-sum test) analysis to determine whether the mean fold-change increased significantly from baseline between the two arms.
T-cell Proliferation Assay
A two-tailed Mann-Whitney (Wilcoxon rank-sum test) was used to determine whether the mean thymidine incorporation was significantly increased when comparing the pre-treatment levels to either day 43 for Arm A or day 15 for Arm B.
Tumor-specificity Assay
IFNγ secretion from supernatants containing either pre-treatment or post-treatment PBMC co-cultured with either autologous, HLA-mismatched cell line, or flu, were compared using a one-tailed unpaired t-test to determine statistical significance.
Results
Clinical Results
Patients with metastatic solid malignancies refractory to conventional therapy were enrolled in a phase 1 dose-escalation study using the 9B12 murine agonistic anti-human OX40 mAb, Trial ID#NCT01644968 (see Supplemental Figure 1 and Supplemental Materials and Methods). Consenting participants received a single cycle of anti-OX40 given intravenously (IV) on days 1, 3 and 5. The study was designed with three cohorts of ten patients each. Patients in cohort 1 received one cycle of 0.1 mg/kg, cohort 2, 0.4mg/kg and cohort 3, 2mg/kg of the mAb. The main objectives of the clinical trial were to determine the maximum tolerated dose, toxicity, immunologic activity, and potential clinical activity following one cycle of anti-OX40.
Demographics
Supplemental Table 1 provides the clinical characteristics and details of prior treatments. Prior therapy included surgery, radiation, chemotherapy, targeted therapy and immunotherapy. The median number of prior cancer treatments was 2 (range 0 – 8) and all had progression of their cancer at the time of enrollment. All patients had performance status ECOG 0 or 1.
Toxicity Assessment
Twenty-eight of 30 patients received all three planned anti-OX40 doses at the assigned dose level. Table 1 summarizes the toxicities related to anti-OX40. Most toxicity was grade 1 or 2 with the exception of lymphopenia where grade 3 and 4 events were observed. Lymphopenia, fatigue, rash and flu-like symptoms with fever and chills were the most common toxicities; they usually started after the last anti-OX40 dose and generally resolved 72 hours later. Lymphopenia was transient (Supplemental Figure 2), and resolved by day 15 in cohort 1 and day 28 in cohort 2. Anti-OX40 was well tolerated and the MTD was not reached.
Table 1. Summary of maximum toxicities related to anti-OX40 at all dose levels.
Patients with multiple instances of the same toxicity were tabulated once with the highest grade for that toxicity.
| Adverse events | ||||
|---|---|---|---|---|
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Lymphopenia | 3 | 10 | 6 | 1 |
| Fatigue | 7 | 12 | ||
| Rash/Skin Changes | 4 | 6 | ||
| Pruritis | 5 | 1 | ||
| Fever/Chills | 11 | 2 | ||
| Splenomegaly | 7 | |||
| Arthralgias/Myalgias | 5 | 5 | ||
| Nausea/Vomiting | 4 | 3 | ||
| Increased AST, ALT or alkaline phosphatase | 2 | 1 | ||
| Anemia | 1 | 8 | ||
Pharmacokinetics and Human Anti-mouse Ab (HAMA) Production
There was a dose-dependent increase in peak serum anti-OX40 levels after the first and third doses (serum was not assessed following the second dose), and decreased thereafter (Supplemental Figure 3). Peak anti-OX40 levels were observed 2 hours after the third dose. Anti-OX40 Ab was detected on the surface of PBMC when examined ex vivo by flow cytometry, using an anti-mouse specific Ab. Between 9.9 to 26.9% of CD4 T cells and 0.6 to 7.8% of CD8 T cells were positive using this technique (Supplemental Figure 4). All but one patient tested had high serum HAMA levels on day 28 (data not shown). There was no increased toxicity or HAMA titers as the anti-OX40 dose increased.
Tumor Regression
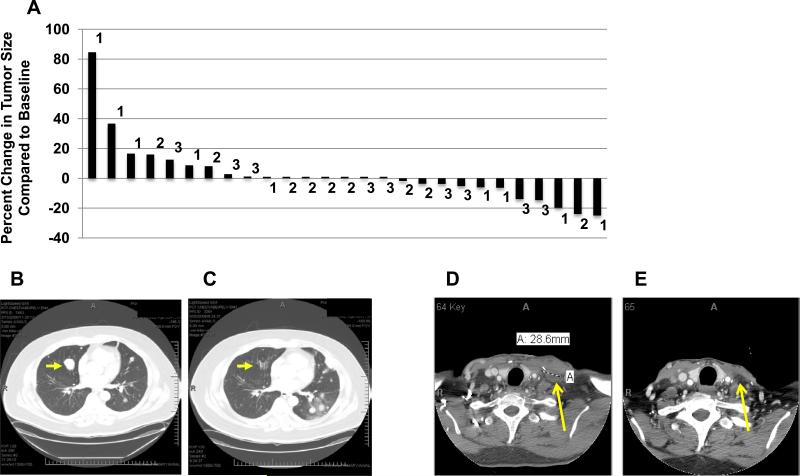
Figure 1A shows a waterfall plot for best response by RECIST after anti-OX40. No patient achieved a partial response by RECIST (>30% overall tumor shrinkage). However, at least one tumor nodule regressed in 12 patients and no change in the measurement of target lesions was observed in 6 additional individuals during the 57-day observation period. Regression and SD were observed in patients with melanoma, renal cancer, squamous cell carcinoma of the urethra, prostate cancer and cholangiocarcinoma. The longest interval of stable disease lasted 470 days in a patient with renal cancer, who received no other therapy during that time. Some individuals had a decrease in measurable lesions at day 29, but had progression by day 57. Figure 1 B-E and Supplemental Figure 5 A-H show examples of tumor regression in three patients following anti-OX40. Mixed responses (e.g.: simultaneous regression of at least one tumor deposit and progression at other sites) were observed in two patients with melanoma and two patients with renal cancer.
Figure 1. Clinical observations.
(A) Waterfall plot showing percent change in the sum of the diameters of tumor target lesions above or below baseline using RECIST criteria. The numbers on the graph indicate the cohort in which the patients were enrolled. Patients in cohort 1 received one cycle of 0.1mg/kg, cohort 2 0.4mg/kg and cohort 3 2mg/kg of anti-OX40. Patients 10, 18 and 23 did not have follow-up scans due to clinical progression and are PD. (B-C) Regression of a pulmonary nodule in a patient (Cohort 1) with metastatic melanoma, and progression of other nodules. Panel B, imaging before anti-OX40. Panel C, 5 months after anti-OX40 administration. (D-E) Shrinkage of a lymph node in a melanoma patient (cohort 3). Imaging in panel E was obtained 28 days after anti-OX40.
Immunologic Results
Anti-OX40 increases proliferation of CD4 FoxP3neg and CD8 T lymphocytes
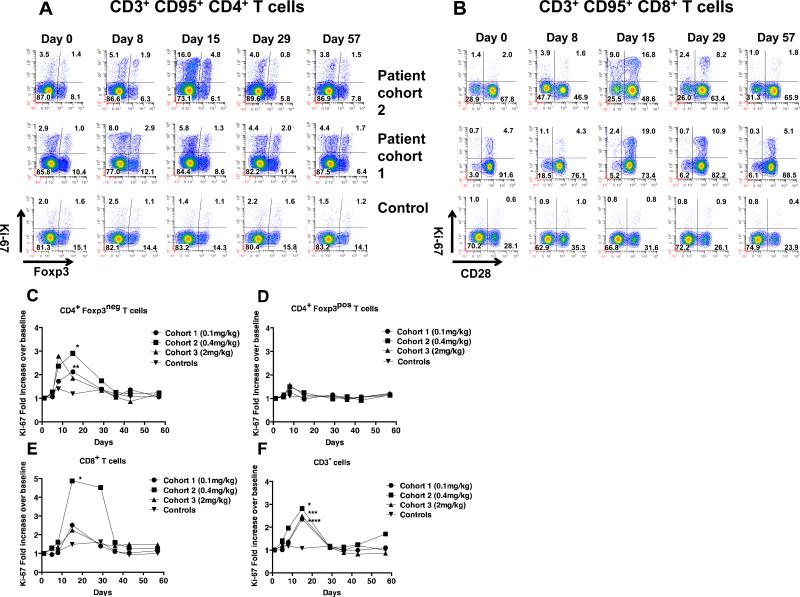
To determine the effects of anti-OX40 on T cells, we performed multi-color flow cytometry with antibodies that recognized CD3, CD4, CD8, CD95, CD25, FoxP3, CD28, and Ki-67. Changes in T-cell subpopulation frequencies were determined using FCOM analysis tool in Winlist (24) and statistically significant changes were assessed using exhaustive expansion analysis (25). Ki-67 expression, which is present only within proliferating cells, was measured since OX40 agonists are known to increase T-cell proliferation and this was the only marker that was associated with significant changes in the exhaustive expansion analysis referenced above. Changes in Ki-67 expression for both CD4+ and CD8+ T cells after anti-OX40 administration and from a normal individual immunized with tetanus were analyzed (Figure 2A-B and Supplemental Figure 6A-B). PBMC were gated on CD3, CD95, and CD4; these markers are reported to identify Ag-experienced/memory T cells (26), and further analyzed for FoxP3 (Treg) and Ki-67 (Figure 2A). PBMC from the same patients were also gated on CD3, CD95, and CD8 and analyzed for CD28 and Ki-67 (Figure 2B). At baseline the percentage of proliferating/Ki-67+ CD4+ and CD8+ T cells ranged between 0.5-6.0% in patients and normal controls (Figure 2 and data not shown). The percentage of Ki-67+ CD4+ T cells started to increase early (day 8) and the Ki-67+ CD8+ T cells later (day 15) after anti-OX40 (summarized in Figure 2 C-F); the percentage of proliferating cells in both populations usually returned to pre-treatment percentages by day 57. Typically, proliferating CD8+ T cells were observed in both the CD28 positive and negative populations on day 15; however, the majority of proliferating CD8+ T cells on day 29 were CD28+. No significant changes in the percentage of Ki-67+ PBL were detected among the normal donors that were immunized with tetanus toxoid for any of these populations at any time points (Figure 2 C-F). There was a significant increase in Ki-67+ lymphocytes in anti-OX40 treated patients compared to controls. Significant increases were observed for CD4+/FoxP3- T cells, CD8+ T cells and CD3-/NK cells (Figure 2 C, E and F). The proliferation of CD4+ FoxP3+ Treg was unaffected in anti-OX40 treated patients at any dose when compared to controls (Figure 2D). At day 15, the proliferation of CD4+ FoxP3- cells showed a statistically significant increase in cohorts 1 and 2 compared to the control group (p<0.02). The augmentation of CD4+ FoxP3- T-cell proliferation in patients within the third cohort peaked on day 8 and declined thereafter, which was different pattern of expression than the first two cohorts. The increase in proliferation of CD8+ T cells peaked on day 15 for all three cohorts; however, a significant increase in proliferation was only observed in the second cohort when compared to the control group (p=0.001). A sustained increase in Ki-67+ CD8+ T cells was found predominantly in patients from the second cohort (through day 29). The average increase in proliferation of the CD8+ T cells in cohort 3 was less than that observed in cohort 2, despite the fact that patients received a five-fold higher dose of anti-OX40. The increase in Ki-67 expression within the CD3-/CD56+ cells was statistically significant in all cohorts compared to normal donors and peaked 15 days after mAb treatment. The largest average fold-increase in the percentage of cycling cells for all three cell populations (CD4+/FoxP3-, CD8+, and NK cells) was observed in cohort 2 (Figure 2 C, E and F). In a post-hoc analysis patients were designated as: “non-progressors”: initial decrease or stabilization of the disease, and “progressors”: tumor progression. Ki-67+ T cells among the CD4+/FoxP3- population in non-progressors on day 8 and among CD8+ T lymphocytes on day 5, 8 and 15 was significantly greater compared to progressors (Supplemental Figure 7A-B). No significant differences were observed in Ki-67 expression in CD4+ FoxP3+ T cells or CD3- lymphocytes (Supplemental Figure 7C-D).
Figure 2. Changes in Ki-67 expression within CD4+ and CD8+ T cell subsets examined over time after anti-OX40.
PBMC were analyzed using a multi-color flow cytometry. (A) Cells gated on CD3+, CD95+, CD4+ analyzed for FoxP3 and Ki-67. (B) Cells gated on CD3+ CD95+, CD8+ T cells analyzed for CD28 and Ki-67. (C-F) Average-fold increase in Ki-67 expression for the four lymphocyte sub-types analyzed. The fold increase was calculated by using Ki-67 percentages on various days following anti-OX40 and dividing it by the percentage of Ki-67+ cells on Day 0 (baseline). Statistical analyses were performed as described in material and methods. * p=0.001, ** p=0.013, *** p=0.004, **** p=0.007.
The generation of HAMA in all patients led us to consider the possibility that the increase in Ki-67+ cells could be attributed to an anti-mouse specific immune response or tetanus immunization rather than enhancing the endogenous T cell repertoire. Since this could not be directly tested in humans, we performed a study in non-human primates to determine whether the infusion of a control mouse IgG with tetanus vaccination would elicit a similar increase in Ki-67+ expression by CD4+ and CD8+ T cells as was observed in the clinical trial. Two groups of monkeys received tetanus on day 1 and an IV injection of either 1 mg/kg of anti-OX40 mAb or mouse IgG on days 1, 3 and 5. PBMC were analyzed by flow cytometry with a panel similar to the one described for human PBL. The mouse anti-human OX40 antibody increased Ki-67 expression in both CD4+ and CD8+ T cells (Supplemental Figure 8A-B). However, no significant increases in Ki-67 expression were observed in either CD4+ or CD8+ lymphocytes isolated from monkeys that had received tetanus and mouse IgG. These data show that increased Ki-67 expression was induced by anti-OX40 and not by a de novo immune response elicited to a foreign mouse protein or tetanus immunization. To definitively establish that the increased proliferation of CD4+ and CD8+ T cells was induced by the engagement of OX40, we injected four monkeys with an agonistic monkey OX40L:Ig fusion protein, which increased the mean percent of Ki-67+ CD4+ and CD8+ T cells with kinetics similar to the anti-OX40 mAb (Supplemental Figure 8C-D).
CD4+ T cell analysis
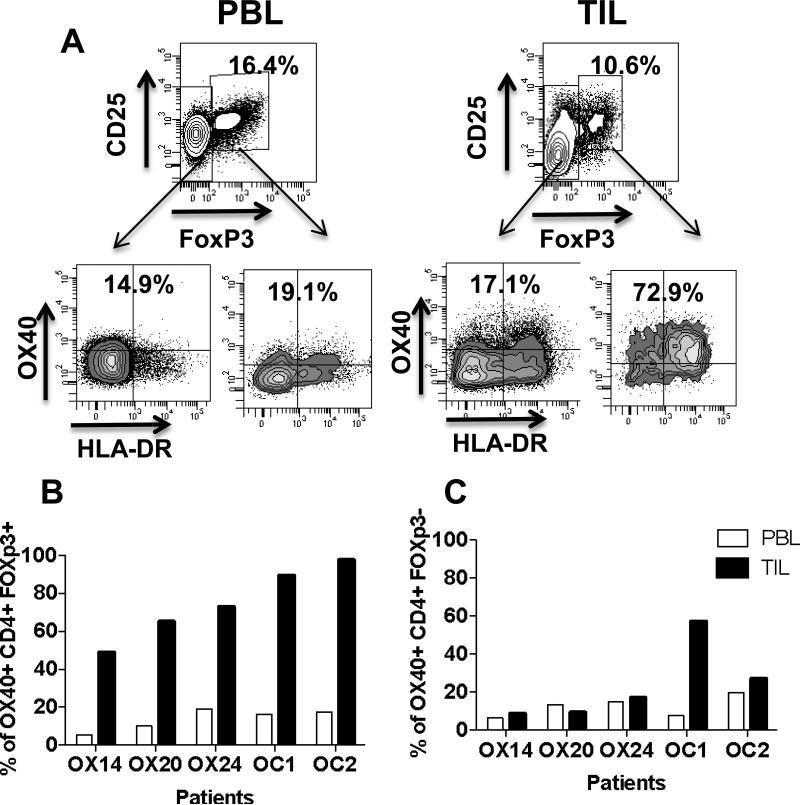
We sought to explain why proliferation of Treg in PBL was less than other T-cell subsets. Therefore, we examined OX40 expression on Treg and CD4+/FoxP3- T cells in tumors (Figure 3A). We also compared surface OX40 expression on Treg and CD4+/FoxP3- T cells in PBL and TIL in 3 melanoma patients enrolled in this study and 2 untreated ovarian cancer (OC) patients. OX40 surface expression on non-Treg and Treg in the PBL was less than 20%, while more than 50% of Treg from TIL expressed OX40 (Figure 3B-C). Moreover, the MFI for OX40 was up to 16 fold higher in TIL compared to PBL Treg, suggesting that the anti-OX40 may modulate Treg function in the tumor.
Figure 3. OX40 expression on Treg in TIL and PBL and the percentage of follicular T helper cells in PBL after anti-OX40.
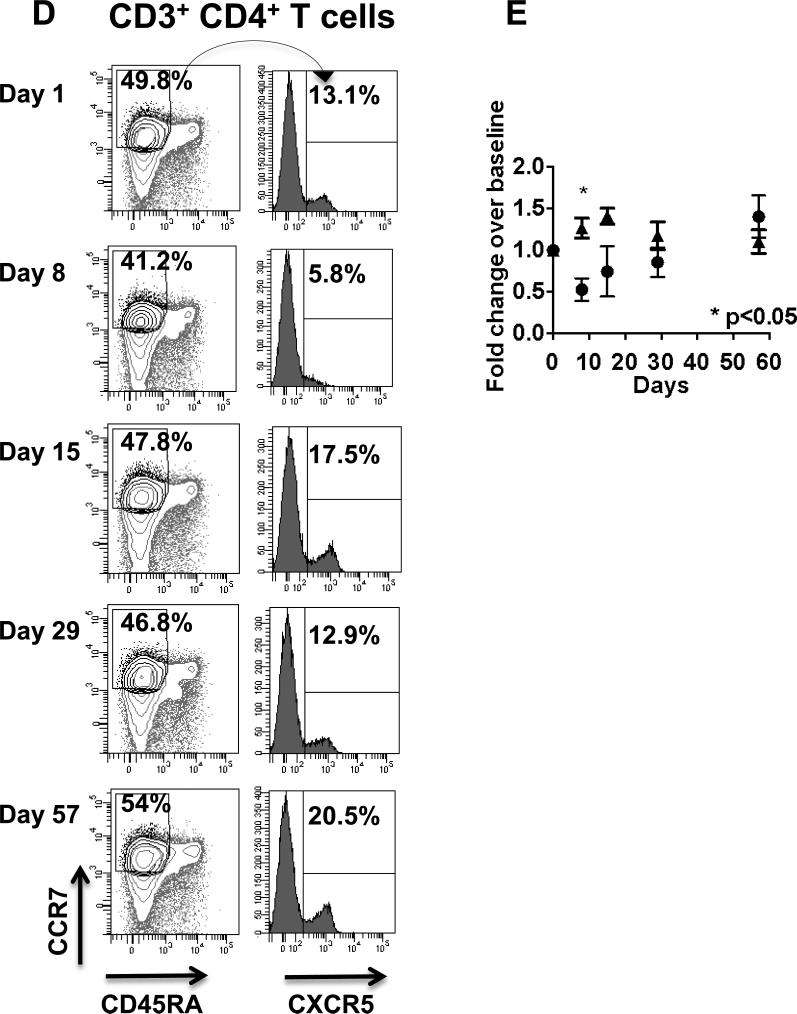
PBL and TIL gated on CD3+ CD4+ were analyzed for the expression of OX40 and HLADR on FoxP3+ Treg and CD4+/FoxP3- T cells. Percentage of OX40 positive Treg (B) and conventional CD4+ T cells (C) in PBL and TIL in 3 patients on the OX40 trial and 2 patients with untreated OC, not associated with this trial (open bars PBL, filled bars TIL). (E) PBL gated on CD3+ CCR7+ CD45RA- CD4+ T cells, central memory T cells (TCM) were assessed for expression of the follicular T helper cell marker CXCR5 at different times before and after anti-OX40. (B) Comparison of the mean percent of TCM that express CXCR5 in 6 anti-OX40 treated patients randomly selected from all three cohorts (black circles) and 6 normal donors (black triangles).
Anti-OX40 induces a transient decrease of follicular helper CD4+ T cells in the PBL
Anti-OX40 increased anti-TT and anti-KLH Abs, suggesting enhanced activity in T follicular helper cells. We analyzed the percentage of CD4+ CD45RA- CCR7+ CXCR5+ follicular helper T cells (27) in PBL from patients and controls before and after anti-OX40 Ab. Figure 3D shows that there is significant decrease of this cell subset at Day 8 in study patients compared to controls, who have received the TT vaccine on Day 1, but have not received anti-OX40 Ab (Figure 3E).
Anti-OX40 administration induces qualitative changes in cycling CD8+ T cells
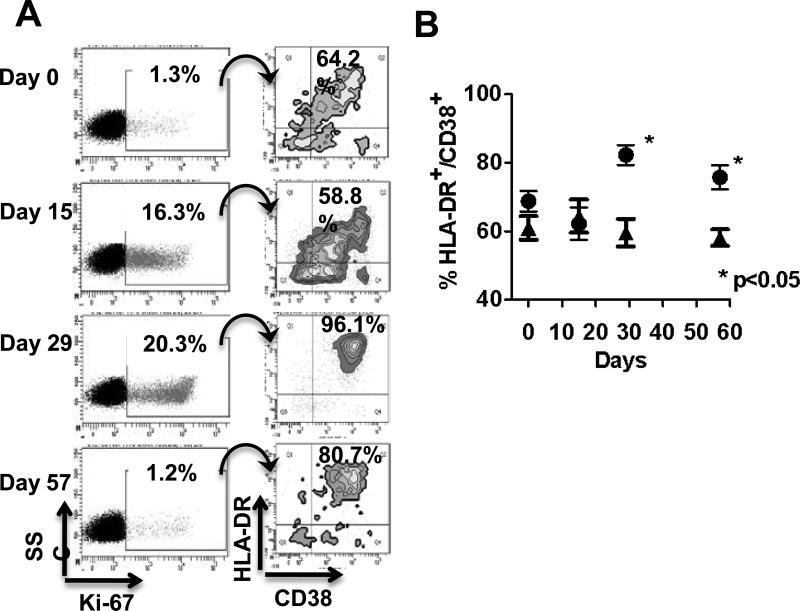
The effect of anti-OX40 on the activation phenotype of cycling CD8+ T cells was examined by assessing co-expression of CD38 and HLA-DR, which is a hallmark of proliferating, viral-specific human CD8+ T cells following vaccination (28). The expression of CD38 and HLA-DR on CD8+Ki-67+ T cells after anti-OX40 was analyzed. Figure 4A shows an individual patient, in whom 1.3% of the CD8+ T cells were Ki-67+ and 64.2% of them co-expressed CD38 and HLA-DR before treatment. The percentage of Ki-67+CD8+ T cells increased to 16.3 and 20.3% on days 15 and 29, respectively. On day 29 greater than 95% of cycling cells expressed both CD38 and HLA-DR (Figure 4A). A higher percentage of cycling CD8+ T cells expressed both activation markers 56 days after anti-OX40 even though the percentage of CD8+/Ki-67+ cells had returned to baseline. The mean percentage of CD38+/HLA-DR+/Ki-67+ CD8+ T cells in eleven anti-OX40 treated patients was compared with nine normal donors immunized with tetanus (Figure 4B). At Days 29 and 57, there was a statistically significant increase in the percentage of HLADR+CD38+Ki-67+ CD8+ T cells compared to controls, suggesting that engagement of OX40 increased proliferation and activation of CD8+ T cells.
Figure 4. Anti-OX40 increases HLA-DR and CD38 expression on cycling CD8+ T cells.
(A) PBL gated on CD3+ CD8+ T cells and analyzed for Ki-67 from a patient in cohort 2, and CD3+ CD8+ Ki-67+ were assessed for expression of the activation markers HLA-DR and CD38 at different times after anti-OX40 administration. (B) Comparison of the mean percent of cycling CD3+ CD8+ that co-express HLA-DR and CD38 T cells in 11 anti-OX40 treated patients randomly selected from all three cohorts (black circles) and 9 normal donors (black triangles).
Antibody and T cell responses to reporter antigens
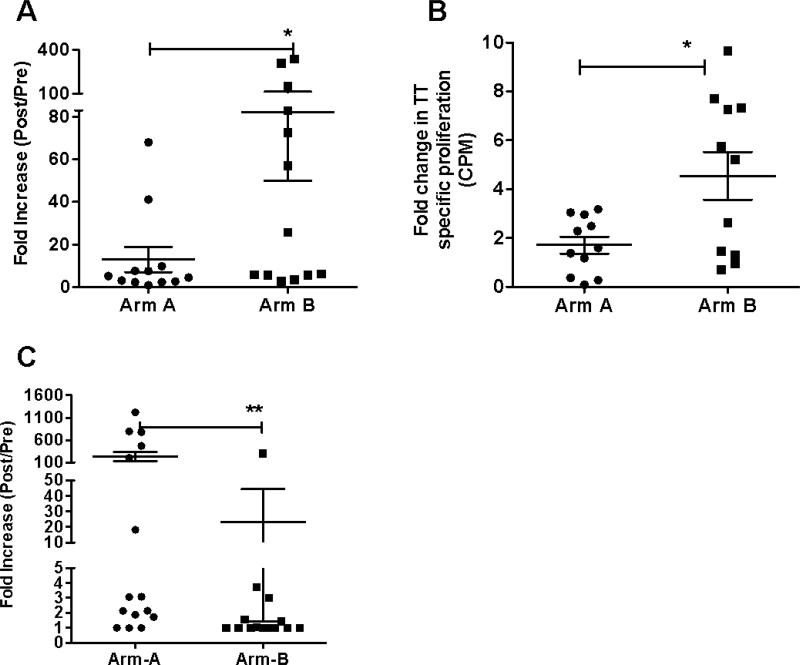
Patients at all dose levels were randomized into two groups; Arm A patients received KLH on day 1 (the same day anti-OX40 started) followed by a tetanus vaccine on day 29, and Arm B patients received tetanus on day 1 and KLH on day 29. Peak antibody levels to KLH and tetanus were assessed 15 days after immunization, which was either on day 15 or 43 (Arm A vs Arm B, respectively) after anti-OX40. Tetanus Ab titers were calculated both pre and post-anti-OX40 and fold-increases were calculated for all patients. A significant fold-increase in Ab response was found in patients who were immunized with tetanus or KLH on the same day as anti-OX40 compared to patients immunized 28 days later (Figure 5A-C and Supplemental Figure 9). Tetanus-specific T-cell proliferation was assessed from PBMCs obtained pre- and 15 days after immunization. There was a significant fold-increase in proliferation when the post-anti-OX40 samples from Arm B were compared to Arm A, p=0.0127 (Figure 5B). Patients in Arm A who had increases in their anti-KLH Ab response also had a significantly higher increase of Ki-67+ CD8+ T cells on day 15 as compared to low Ab responders (p=0.017, Satterthwaite's method).
Figure 5. Enhancement, by anti-OX40, of the immune response to reporter antigens.
(A) Anti-tetanus, (C) anti-KLH antibody titer fold increase as measured by ELISA on Day 15 and 43 (15 days after vaccination). Fold-increase in antibody titers were calculated by dividing post-anti-OX40 titers by pre-anti-OX40 titers. (B) Tetanus-specific T cell proliferation was measured on Day 43 for Arm A and Day 15 for Arm B, respectively 15 days after vaccination. Data are presented as fold changes compared to the proliferation prior to OX40 administration. * p< 0.05, ** p< 0.001.
In vitro assessment of anti-tumor reactivity
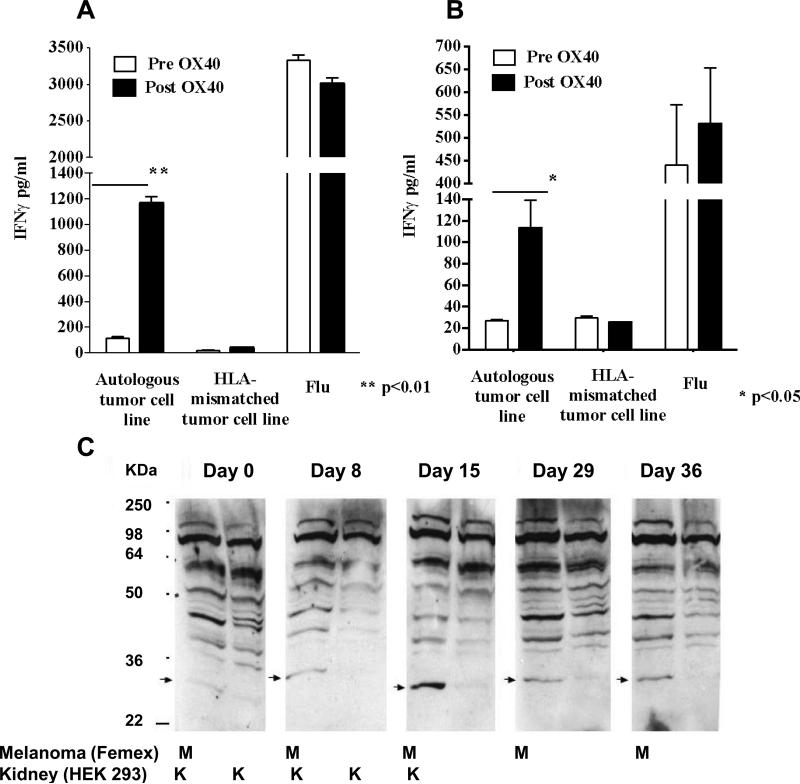
Autologous tumor cell lines from three melanoma patients enrolled in the clinical trial were available for testing. PBMC collected before and 57 days after anti-OX40 were co-cultured with autologous tumor, HLA-matched or HLA-mismatched melanoma cell lines for up to 5 days. Significant increases in IFN-γ were found in the PBMC/tumor supernatants after anti-OX40 in 2 out of 3 patients (Figure 6A and B). IFN-γ levels were increased in response to autologous tumor in two patients, but not to HLA-mismatched tumor lines. The increase in tumor-specific IFN-γ, following anti-OX40 was primarily elicited by CD8+ T cells, as the increase in IFN-γ production was blocked by anti-HLA-ABC antibody, but not by anti-HLA-DR antibody and it was selectively detected in CD8+ T cells by flow cytometry (data not shown). There were no significant differences in influenza-induced IFN-γ production by PBMC collected before or after anti-OX40 (Figure 6A and B).
Figure 6. Anti-OX40 infusion increases tumor-specific immune response.
(A-B) PBMC from two patients with melanoma, before and after anti-OX40, were co-cultured with either autologous, HLA-mis-matched melanoma cell lines or flu. IFN-γ in the supernatant was measured by ELISA. Anti-tumor-specific antibodies were measured by Western blot (C) serum from a patient with melanoma was used to probe lysates (15μg of protein/lane) from a melanoma cell line (FEMX) or a human embryonic kidney cell line (HEK 293) at different times after anti-OX40.
Serum from one melanoma patient was used to probe protein lysates from a melanoma cell line (FMEX) and an embryonic kidney cell line (HEK293) by Western blot before and days 0, 8, 15, 29, 36 after anti-OX40. (Figure 6C). There is a 27kd band present in the melanoma lysate lanes that increases in intensity over time after anti-OX40 (peaking at day 15), but is absent in kidney lysate lanes. The 27kd band was also detected in two other melanoma cell lines with the day 15 sera from this patient (data not shown). It appears that anti-OX40 increased a tumor-associated Ab response in this patient.
Discussion
Antibodies that enhance T-cell proliferation, survival, and effector function are currently being assessed in clinical trials for cancer patients. Ipilimumab, an anti-CTLA-4 mAb, was recently approved by the FDA for the treatment of advanced melanoma (7). Preclinical studies indicate that anti-OX40 and anti-CTLA-4 have similar abilities to stimulate T cells in both basic and tumor immunology models (4, 13, 29, 30); anti-OX40 by co-stimulation and anti-CTLA-4 by blocking inhibition. Here we report the first clinical and immunologic assessment of anti-OX40 in cancer patients. Anti-OX40 was well tolerated; mild to moderate side effects included a brief period of lymphopenia, fatigue, fever/chills, and mild rashes. The MTD was not reached within the dose levels tested. There was tumor shrinkage in 12/30 patients following just one cycle, although there were no responses using RECIST. The major limitation of this mouse mAb was the induction of HAMA, which precluded the administration of multiple cycles. Despite this limitation, much was learned about the immunomodulatory effects of OX40. The results of this proof-of-principle study will help in the design and monitoring of future clinical trials using humanized OX40 agonists that we anticipate will lead to increased immunologic activation and greater clinical activity.
The immune stimulatory effects of anti-OX40 observed in humans are similar to those described in mice and monkeys (31, 32). A significant increase in proliferation of both CD4+ and CD8+ T cells was observed starting seven days after the initial infusion and lasting for at least 15 days, and sometimes up to a month. There was a dose-dependent increase in CD4+ and CD8+ T-cell proliferation when the dose was increased from 0.1 to 0.4 mg/kg. However, at 2 mg/kg, a different kinetic pattern of CD4+ T-cell proliferation and a reduced increase in CD8+ T-cell proliferation was observed. We do not know the biological reason for the difference observed at the highest anti-OX40 dose. Anti-OX40 did not appear to increase CD4+/FoxP3+ Treg proliferation possibly due to the differences in OX40 expression between PBL and TIL. Ki-67 expression by T cells has also recently been assessed in prostate cancer patients treated with anti-CTLA-4 (33). Anti-CTLA-4 increased proliferation of both FoxP3+ and FoxP3- CD4+ T cells in a dose-dependent manner (33). The pattern of immunologic response seen with anti-OX40 could be of benefit to cancer patients, because Treg are known to dampen immunity to tumor (34). Our observation that TIL Treg express more OX40 than PBL Treg, suggests that tumor biopsy studies will be important in understanding OX40 biology and anti-tumor effects.
Anti-OX40 induced proliferation of CD8+ T cells including CD8+ T cells expressing the activation markers CD38 and HLA-DR, increasing the immunologic potency of these cells. We speculate that a percentage of the Ki-67+ T cells in these cancer patients are tumor-specific. Although the antigens recognized by the proliferating T cells have not been identified, we have evidence that anti-OX40 increased CD8+ T cell IFNγ production in 2 out of 3 melanoma patients where autologous tumor lines were used to assess tumor reactivity. Similar enhanced anti-tumor effects have been recently described in prostate cancer patients receiving anti-CTLA-4 (35).
Anti-OX40 significantly increased Ab titers and T-cell recall responses to tetanus immunization. While similar effects have been observed in mouse models with anti-OX40 and other immune stimulating Abs (36), to our knowledge this is the first verification of these activities in humans. This effect might be mediated by follicular helper T cells that were transiently decreased in the PBL in OX40 treated patients compared to normal controls. This decrease might suggest that these cells trafficked to lymph nodes where they influenced B-cell mediated antibody production. This observation provides a rationale for testing anti-OX40 in conjunction with vaccination to increase T and B cell responses to immunizing Ags in future clinical studies. Anti-OX40 could also serve as adjuvant after disruption of a tumor by radiation or chemotherapy to release tumor Ags.
In summary, administration of anti-OX40 mAb was well tolerated and enhanced both humoral and cellular immunity in patients with cancer. Anti-OX40 increased proliferation of peripheral blood CD4+ and CD8+ T cells, increased responses to recall and naive reporter Ags, and increased endogenous tumor-specific immune responses. We anticipate that multiple doses of a humanized anti-OX40 agonist will lead to stronger immunologic activation and greater tumor regression.
Supplementary Material
Acknowledgments
We thank Julie Cramer, Katrina Herz and Scot Lary who assisted in many clinical research activities, Iliana Gonzales and Gwen Kramer for the cryopreservation of PBMC and Jonna Vercellini for technical assistance. We would like to thank Drs. Nora Disis, John Smith II, and John Thompson for their help in safety monitoring during the clinical trial.
Grant support: R-01 CA109563-06 and the Providence Portland Medical Foundation.
Footnotes
Conflict of Interests: Drs. A. Weinberg an N. Morris have patents related to the use of OX40 agonists in cancer patients.
References
- 1.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 2.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–83. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 3.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–9. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 11.Sznol M, Hodi FS, Margolin K, McDermott DF, Ernstoff MS, Kirkwood JM, et al. Phase I study of BMS-663513, a fully human anti-CD137 gonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J Clin Oncol. 2008 May 20;26(suppl) abstr 3007. [Google Scholar]
- 12.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–21. [PubMed] [Google Scholar]
- 13.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–9. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 14.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–15. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 15.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–39. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–90. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 17.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. Embo J. 1990;9:1063–8. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calderhead DM, Buhlmann JE, van den Eertwegh AJ, Claassen E, Noelle RJ, Fell HP. Cloning of mouse Ox40: a T cell activation marker that may mediate T-B cell interactions. J Immunol. 1993;151:5261–71. [PubMed] [Google Scholar]
- 19.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–7. [PubMed] [Google Scholar]
- 20.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–12. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–72. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 23.Ruby CE, Weinberg AD. The effect of aging on OX40 agonist-mediated cancer immunotherapy. Cancer Immunol Immunother. 2009;58:1941–7. doi: 10.1007/s00262-009-0687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrausch U, Haley D, Miller W, Floyd K, Urba WJ, Walker E. Polychromatic flow cytometry: a rapid method for the reduction and analysis of complex multiparameter data. Cytometry A. 2006;69:1162–73. doi: 10.1002/cyto.a.20342. [DOI] [PubMed] [Google Scholar]
- 25.Siebert JC, Wang L, Haley DP, Romer A, Zheng B, Munsil W, et al. Exhaustive expansion: A novel technique for analyzing complex data generated by higher-order polychromatic flow cytometry experiments. J Transl Med. 2010;8:106. doi: 10.1186/1479-5876-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VL, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–68. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 28.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, Wong S, et al. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–85. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 32.Redmond WL, Weinberg AD. Targeting OX40 and OX40L for the treatment of autoimmunity and cancer. Crit Rev Immunol. 2007;27:415–36. doi: 10.1615/critrevimmunol.v27.i5.20. [DOI] [PubMed] [Google Scholar]
- 33.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–83. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–7. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 35.Kwek SS, Dao V, Roy R, Hou Y, Alajajian D, Simko JP, et al. Diversity of antigen-specific responses induced in vivo with ctla-4 blockade in prostate cancer patients. J Immunol. 2012;189:3759–66. doi: 10.4049/jimmunol.1201529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167:6804–11. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 37.Morris NP, Peters C, Montler R, Hu HM, Curti BD, Urba WJ, et al. Development and characterization of recombinant human Fc:OX40L fusion protein linked via a coiled-coil trimerization domain. Mol Immunol. 2007;44:3112–21. doi: 10.1016/j.molimm.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.