Abstract
Expression of gnd of Escherichia coli, which encodes the hexose monophosphate shunt enzyme, 6-phosphogluconate dehydrogenase (6PGD; EC 1.1.1.44), is subject to growth rate-dependent regulation: the level of the enzyme is directly proportional to growth rate under a variety of growth conditions. Previous results obtained with strains carrying transcriptional fusions of gnd to the structural genes of the lactose operon suggested that the growth rate-dependent regulation of gnd expression is at the post-transcriptional level. To characterize the regulation further, we prepared with phage MudII a set of eight independent gnd-lac gene (protein) fusions. We showed through genetic analysis and DNA sequencing that each fusion joint was located within the 6PGD-coding sequence between the first and second base pair of a codon, the reading frame required for production of a hybrid 6PGD-beta-galactosidase. Strains harboring the gnd-lac fusion plasmids produced proteins whose mobility in a NaDodSO4/polyacrylamide gel agreed with the molecular weights predicted from the DNA sequence for the respective hybrid proteins. The level of beta-galactosidase was high and relatively growth rate-independent in the fusion whose fusion joint was at codon 48. The level of beta-galactosidase in the other seven fusion strains whose fusion joints were located further downstream in the 6PGD-coding sequence showed the same dependence on growth rate as 6PGD in a normal strain. beta-Galactosidase levels were not affected by the presence of a gnd+ gene in trans to any of the fusions. The results suggest that all sites necessary for growth rate-dependent regulation of 6PGD level lie in gnd upstream from codon 118 and that an essential site of negative control lies within the coding sequence, between codons 48 and 118.
Full text
PDF

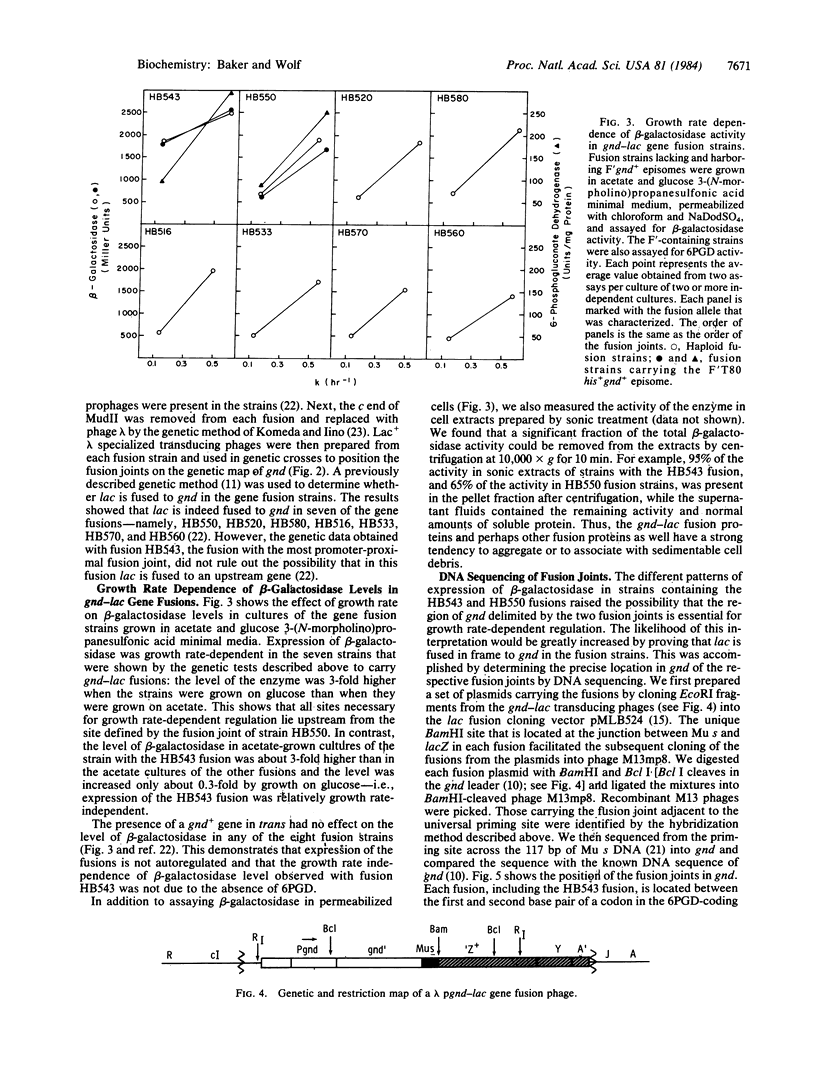
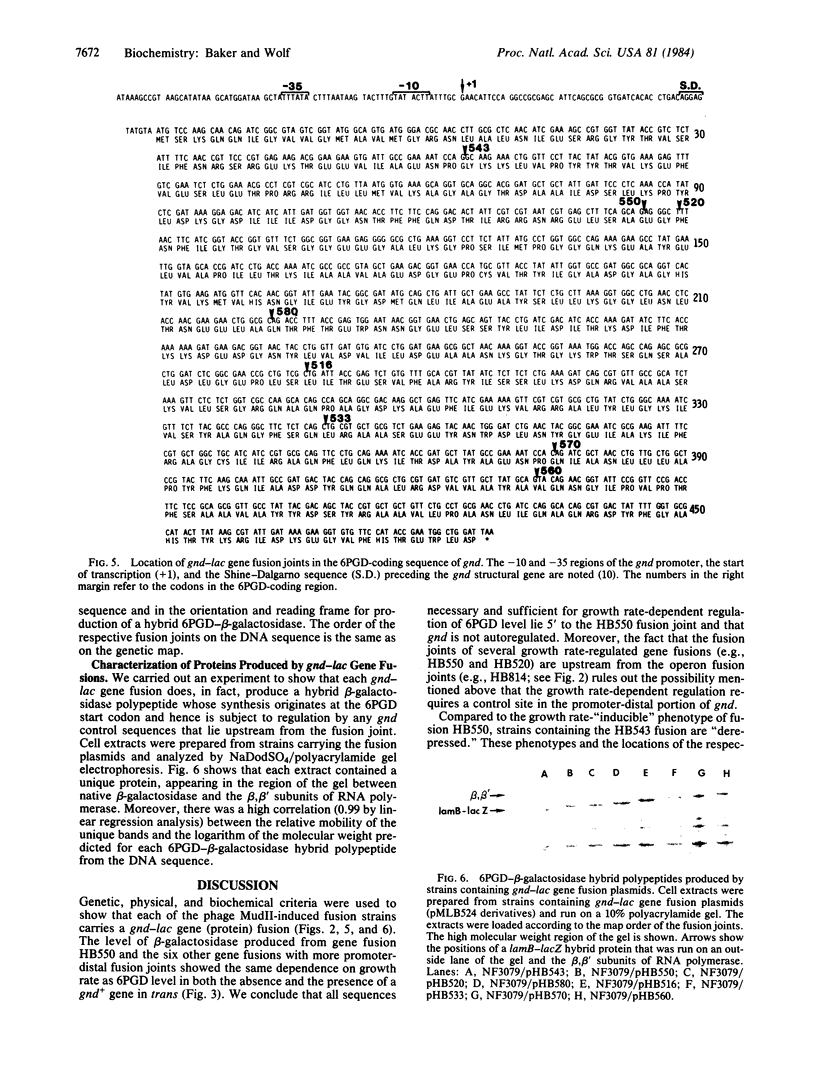

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy S., Squires C. L., Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984 Feb;157(2):363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V., 2nd, Wolf R. E., Jr Growth rate-dependent regulation of 6-phosphogluconate dehydrogenase level in Escherichia coli K-12: beta-galactosidase expression in gnd-lac operon fusion strains. J Bacteriol. 1983 Feb;153(2):771–781. doi: 10.1128/jb.153.2.771-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J. In vivo formation of gene fusions encoding hybrid beta-galactosidase proteins in one step with a transposable Mu-lac transducing phage. Proc Natl Acad Sci U S A. 1984 Jan;81(2):535–539. doi: 10.1073/pnas.81.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T., Johnsen M., Fiil N. P., Friesen J. D. RNA secondary structure and translation inhibition: analysis of mutants in the rplJ leader. EMBO J. 1984 Jul;3(7):1609–1612. doi: 10.1002/j.1460-2075.1984.tb02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrish E. E., Baker H. V., 2nd, Wolf R. E., Jr Different control circuits for growth rate-dependent regulation of 6-phosphogluconate dehydrogenase and protein components of the translational machinery in Escherichia coli. J Bacteriol. 1982 Nov;152(2):584–594. doi: 10.1128/jb.152.2.584-594.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen D., Iserentant D., Derom C., Fiers W. Systematic alteration of the nucleotide sequence preceding the translation initiation codon and the effects on bacterial expression of the cloned SV40 small-t antigen gene. Gene. 1982 Jan;17(1):55–63. doi: 10.1016/0378-1119(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Castilho B. A., Casadaban M. J. In vivo DNA cloning and adjacent gene fusing with a mini-Mu-lac bacteriophage containing a plasmid replicon. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1480–1483. doi: 10.1073/pnas.81.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980 Apr;9(1-2):1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Jinks-Robertson S., Gourse R. L., Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983 Jul;33(3):865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979 Sep;139(3):721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Expression of ribosomal genes in bacteria. Adv Genet. 1982;21:53–121. doi: 10.1016/s0065-2660(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasoff M. S., Baker H. V., 2nd, Wolf R. E., Jr DNA sequence of the Escherichia coli gene, gnd, for 6-phosphogluconate dehydrogenase. Gene. 1984 Mar;27(3):253–264. doi: 10.1016/0378-1119(84)90070-2. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Vaughn V., Phillips T. A., Bloch P. L. Gene-protein index of Escherichia coli K-12. Microbiol Rev. 1983 Jun;47(2):231–284. doi: 10.1128/mr.47.2.231-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. T., Blum P. H., Artz S. W. Effects of the hisT mutation of Salmonella typhimurium on translation elongation rate. J Bacteriol. 1983 Jan;153(1):357–363. doi: 10.1128/jb.153.1.357-363.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. Differential translation efficiency explains discoordinate expression of the galactose operon. Cell. 1981 Jul;25(1):241–249. doi: 10.1016/0092-8674(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Prather D. M., Shea F. M. Growth-rate-dependent alteration of 6-phosphogluconate dehydrogenase and glucose 6-phosphate dehydrogenase levels in Escherichia coli K-12. J Bacteriol. 1979 Sep;139(3):1093–1096. doi: 10.1128/jb.139.3.1093-1096.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



