Abstract
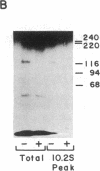
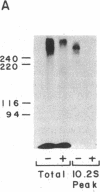
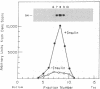
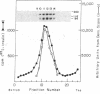
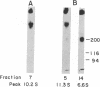
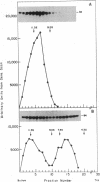
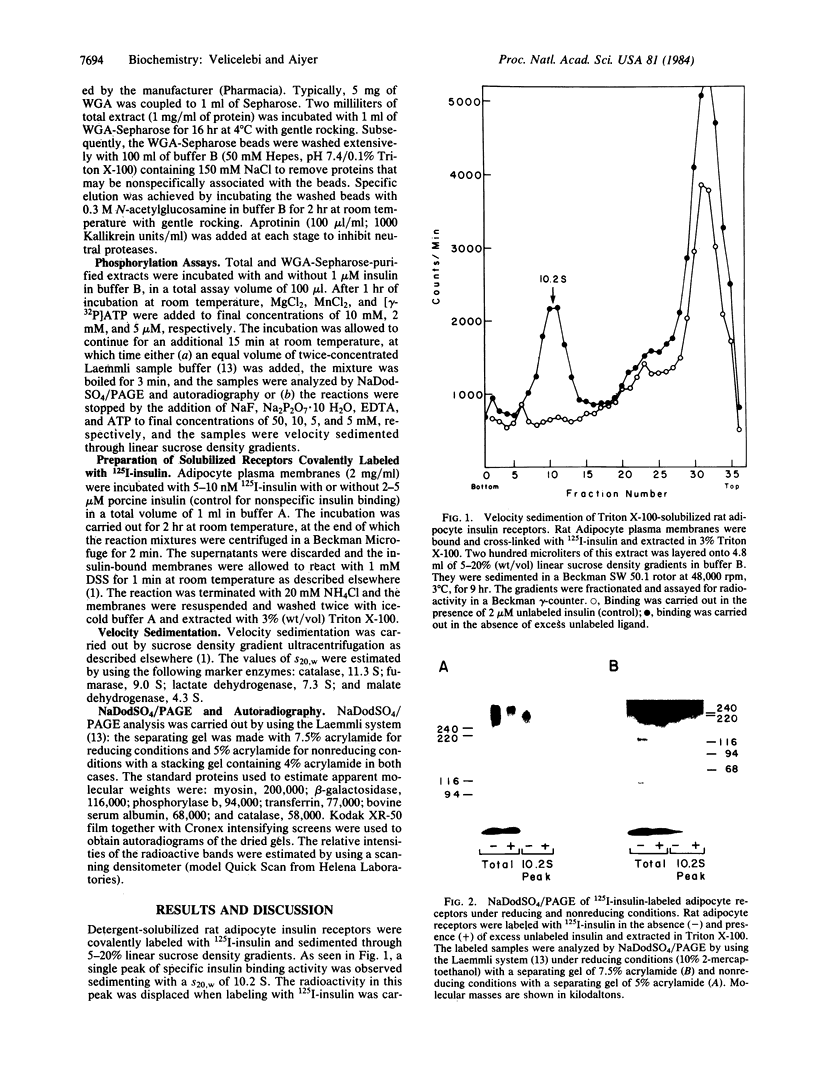
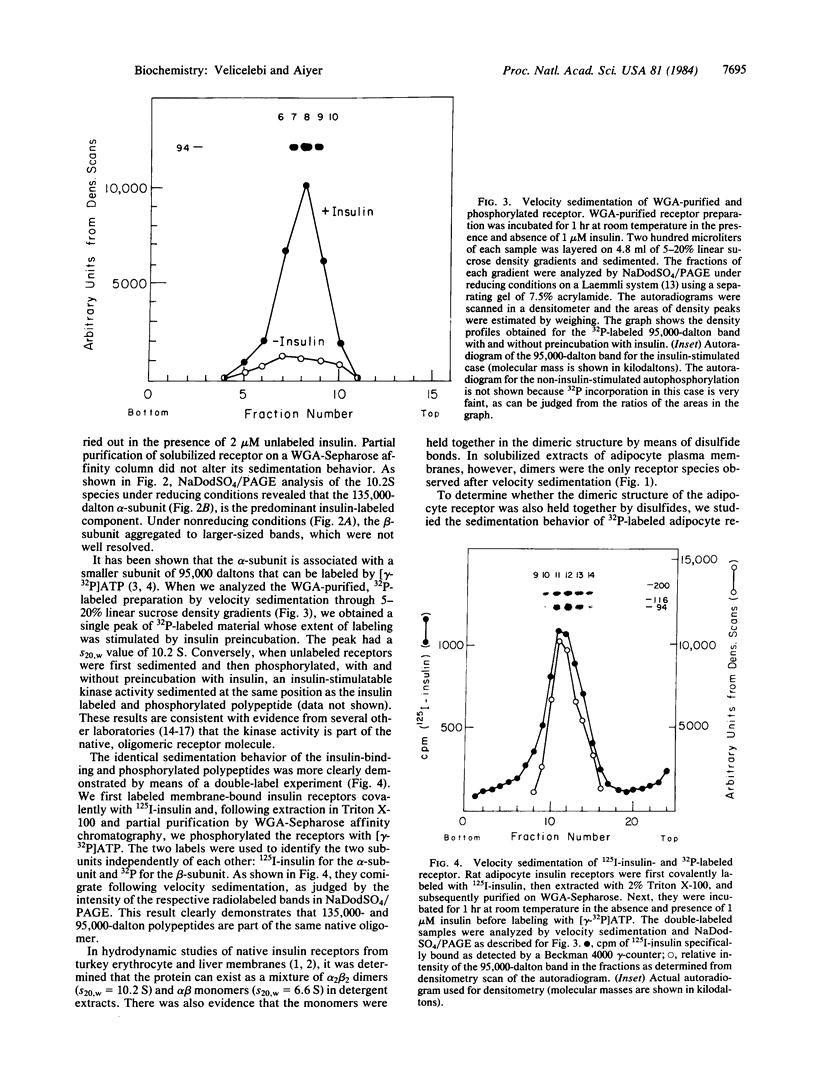
The insulin receptor consists of an insulin-binding subunit (alpha) of 135,000 daltons. More recently, it has been documented that the receptor undergoes insulin-stimulated autophosphorylation that predominantly labels a 95,000-dalton (beta) subunit. We solubilized rat adipocyte insulin receptors in Triton X-100 and partially purified the protein on a wheat germ agglutinin-Sepharose affinity column. Subsequently, we labeled the two subunits of the receptor independently by using 125I-labeled insulin for the 135,000-dalton alpha-subunit and 32P for the 95,000-dalton beta-subunit. Sucrose density gradient sedimentation and NaDodSO4/PAGE were used to characterize the native, oligomeric structure of the receptor. In 0.1% Triton X-100, the receptor sedimented as a single peak of s20,w = 10.2 S as detected by both 125I and 32P. NaDodSO4/PAGE under nonreducing conditions revealed a large species that appeared to be alpha 2 beta 2 and, to a lesser extent, alpha beta. Treatment of the solubilized, partially purified receptor with 10 mM dithiothreitol led to the partial conversion of the 10.2S species to a smaller one sedimenting at 6.6 S. The composition of this species was determined to be alpha beta by nonreducing NaDodSO4/PAGE. Our results suggest that detergent-solubilized insulin receptors can exist as dimers and monomers. The oligomeric structure of receptors functional in the cell membrane cannot be immediately deduced from these results due to the possibility of artifacts arising from membrane disruption and extraction procedures. However, the ability to label the two subunits of the receptor separately should facilitate a detailed study of its oligomeric structure both in solution and in the membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyer R. A. Structural characterization of insulin receptors. I. Hydrodynamic properties of receptors from turkey erythrocytes. J Biol Chem. 1983 Dec 25;258(24):14992–14999. [PubMed] [Google Scholar]
- Aiyer R. A. Structural characterization of insulin receptors. II. Subunit composition of receptors from turkey erythrocytes. J Biol Chem. 1983 Dec 25;258(24):15000–15003. [PubMed] [Google Scholar]
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Häring H. U., Kasuga M., Kahn C. R. Insulin receptor phosphorylation in intact adipocytes and in a cell-free system. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1538–1545. doi: 10.1016/s0006-291x(82)80082-x. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Machicao F., Urumow T., Wieland O. H. Phosphorylation--dephosphorylation of purified insulin receptor from human placenta. Effect of insulin. FEBS Lett. 1982 Nov 22;149(1):96–100. doi: 10.1016/0014-5793(82)81079-x. [DOI] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. Role of disulfides in the subunit structure of the insulin receptor. Reduction of class I disulfides does not impair transmembrane signalling. J Biol Chem. 1982 Jun 25;257(12):6729–6738. [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Stadtmauer L. A., Rosen O. M. Phosphorylation of exogenous substrates by the insulin receptor-associated protein kinase. J Biol Chem. 1983 Jun 10;258(11):6682–6685. [PubMed] [Google Scholar]
- Van Obberghen E., Kowalski A. Phosphorylation of the hepatic insulin receptor: stimulating effect of insulin on intact cells and in a cell-free system. FEBS Lett. 1982 Jul 5;143(2):179–182. doi: 10.1016/0014-5793(82)80094-x. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Rossi B., Kowalski A., Gazzano H., Ponzio G. Receptor-mediated phosphorylation of the hepatic insulin receptor: evidence that the Mr 95,000 receptor subunit is its own kinase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):945–949. doi: 10.1073/pnas.80.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]
- Zick Y., Whittaker J., Roth J. Insulin stimulated phosphorylation of its own receptor. Activation of a tyrosine-specific protein kinase that is tightly associated with the receptor. J Biol Chem. 1983 Mar 25;258(6):3431–3434. [PubMed] [Google Scholar]