Abstract
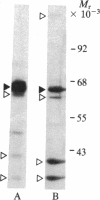
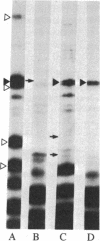
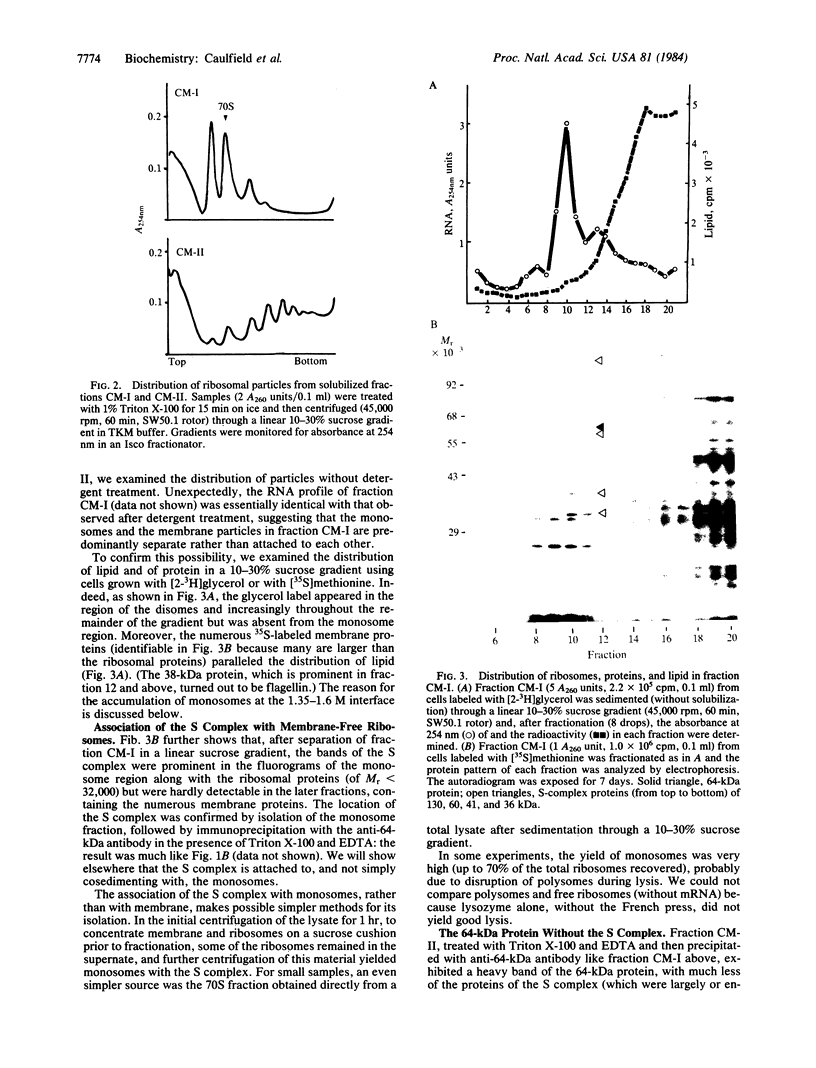
The 64-kDa membrane protein of Bacillus subtilis is evidently involved in the attachment of secreting ribosomes to membrane. On immunoprecipitation with antibody to this protein, the solubilized particulate fraction, with or without prior chemical cross-linking, yields a complex of four proteins (64, 60, 41, and 36 kDa). This "S complex" was found to be associated with membrane-free ribosomes rather than with membrane, but the 64-kDa protein is also present, without the other proteins of the S complex, in the membrane-ribosome fraction and in the cytosol. Only the form present in the membrane-ribosome fraction is protected from protease. These findings suggest a cycle in which the complex participates in initiation of secretion but not in the later stages. It is not yet clear whether the 64-kDa protein found in the membrane-ribosome complexes is retained from the S complex after initiation and later recycled via the cytosol or whether it is a separate pool.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caulfield M. P., Tai P. C., Davis B. D. Association of penicillin-binding proteins and other enzymes with the ribosome-free membrane fraction of Bacillus subtilis. J Bacteriol. 1983 Oct;156(1):1–5. doi: 10.1128/jb.156.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Marty-Mazars D., Tai P. C., Davis B. D. Localization and quantitation of proteins characteristic of the complexed membrane of Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1215–1221. doi: 10.1128/jb.154.3.1215-1221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Tai P. C., Davis B. D. A 64-kilodalton membrane protein of Bacillus subtilis covered by secreting ribosomes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3287–3291. doi: 10.1073/pnas.80.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Marty-Mazars D., Horiuchi S., Tai P. C., Davis B. D. Proteins of ribosome-bearing and free-membrane domains in Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1381–1388. doi: 10.1128/jb.154.3.1381-1388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Tai P. C., Davis B. D. Energy-requiring translocation of the OmpA protein and alkaline phosphatase of Escherichia coli into inner membrane vesicles. J Bacteriol. 1984 Jul;159(1):63–70. doi: 10.1128/jb.159.1.63-70.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Davis B. D. Isolation of polysomes free of initiation factors. Methods Enzymol. 1979;59:362–371. doi: 10.1016/0076-6879(79)59097-1. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]