Abstract
The phorbol myristate acetate (PMA) stimulated nutrophil respiratory burst has been considered to simply involve the activation of protein kinase C (PKC). However, the PLD activity was also increased by 10-fold in human neutrophils stimulated with 100 nM PMA. Unexpectedly, U73122, an inhibitor of phospholipase C, was found to significantly inhibit PMA-stimulated respiratory burst in human neutrophils. U73122 at the concentrations, which were sufficient to inhibit the respiratory burst completely, caused partial inhibition of the PLD activity but no inhibition on PKC translocation and activation, suggesting that PLD activity is also required in PMA-stimulated respiratory burst. Using 1-butanol, a PLD substrate, to block phosphatidic acid (PA) generation, the PMA-stimulated neutrophil respiratory burst was also partially inhibited, further indicating that PLD activation, possibly its hydrolytic product PA and diacylglycerol (DAG), is involved in PMA-stimulated respiratory burst. Since GF109203X, an inhibitor of PKC that could completely inhibit the respiratory burst in PMA-stimulated neutrophils, also caused certain suppression of PLD activation, it may suggest that PLD activation in PMA-stimulated neutrophils might be, to some extent, PKC dependent. To further study whether PLD contributes to the PMA stimulated respiratory burst through itself or its hydrolytic product, 1,2-dioctanoyl-sn-glycerol, an analogue of DAG , was used to prime cells at low concentration, and it reversed the inhibition of PMA-stimulated respiratory burst by U73122. The results indicate that U73122 may act as an inhibitor of PLD, and PLD activation is required in PMA-stimulated respiratory burst.
Keywords: phospholipase D, neutrophil, respiratory burst, U73122, phorbol myristate acetate
Introduction
The neutrophils constitute the first line of human body defence and kill invading microbes through respiratory burst in which a great amount of superoxide anion, O2−, is released [1]. The respiratory burst of neutrophil can be initiated either by binding of an agonist, such as N-formylmethionylleucylphenylalanine (fMLP), to its receptor on cell membrane or by activating protein kinase C (PKC) with phorbol myristate acetate (PMA). The former signalling involves guanosine triphosphate (GTP)-binding protein mediated activation of a phosphatidylinositol-specific phospholipase C (PLC), which results in the generation of two second messengers: inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 induces the release of Ca2+ from intracellular stores leading to a transient rise in intracellular free Ca2+[2]. DAG remains associated with the membrane and participates in the activation of PKC. The later signalling involves both the translocation of PKC from cytosol to membrane and its direct activation by PMA without the need of DAG and the transient rise of [Ca2+]i[3].
In addition to PLC and PKC, phospholipase D (PLD) is also believed to play a role in neutrophil respiratory burst [4]. The product of PLD, phosphatidic acid (PA) has been proposed to function in stimulation of respiratory burst in neutrophils, regulation of secretion and activation of specific protein kinases. Using 1-butanol as a substrate, the PA generation could be replaced with phosphatidyl-butanol, leading to inhibition of the PA formation [4]. There is increasing evidence that PLD activation is involved in the human neutrophils stimulated by agonists, such as fMLP [5–8], tumour necrosis factor α[9] and PMA as well [10–13]. It has been generally accepted that the activation of PLD by a variety of growth factors and G protein-linked agonists is regulated by PKC [4, 14]. However, the PKC independence was also reported in some G protein-coupled receptor-agonists stimulated PLD activation. For example, the thrombin receptor PAR1 mediated PLD activation in neonatal rat cardiomyocytes is insensitive to PKC inhibition [15].
U73122 is a selective PLC inhibitor and has been widely used to study the involvement of G protein-coupled phospholipase C in receptor-mediated cell activation [16, 17]. A number of studies also demonstrated that receptor-mediated PLD activation can be inhibited by U73122 in E-series prostaglandins-stimulated human erythroleukaemia cells [18], angiotensin II-stimulated vascular smooth muscle cells [19] and in immortalized gonadotropin-releasing hormone neurons [20]. In addition, some investigations reported that U73122 may directly inhibit PMA-stimulated activation of PLD in vascular smooth muscle cells [19] and Chinese hamster cells [21]. However, whether U73122 inhibits the PLD activation in respiratory burst of neutrophils stimulated by PMA has not been reported. Although it is well established that in PMA-stimulated respiratory burst, PKC is predominant and PLC is not involved, we recently found that U73122 strongly inhibits PMA-stimulated neutrophil respiratory burst by interfering with PLD activation without affecting PKC activity, suggesting that PLD activation might also be involved in the respiratory burst of neutrophils.
Materials and methods
Chemicals
fMLP, PMA, dimethyl sulfoxide (DMSO), Bisindoylmaleimide (GF 109203X), U73122 and Dextran T-500 were purchased from Sigma (St. Louis, MO, USA). [9,10(n)-3H] Palmitic acid was from Amersham (Piscataway, NJ, USA). Phosphatidylethanol (PEt) was from ICN Biochemicals (Irvine, CA, USA). Fura-2/AM was from Molecular Probes (Eugene, OR, USA). PKC Assay system was from Invitrogen/Gibco (Carlsbad, CA, USA). 1,2-dioctanoyl-sn-glycerol (DOG) was from Calbiochem (San Diego, CA, USA). Lymphocytes separating solution (density: 1.007 ± 0.002) was purchased from the Institute of Hematology, Chinese Academy of Medical Sciences (Tianjing, China). Other chemicals were all of analytical grade. Stock solutions of fMLP (10 mM), PMA (1 mM), fura-2/AM (1 mM), U73122 (1 mM), GF 109203X (10 mM) were prepared in DMSO, and further diluted with Hanks’ balanced salt solution (HBSS) in usage. However, the final DMSO concentration in cell suspension never exceeded 0.1%.
Isolation of neutrophils
As previously reported [22], human neutrophils were isolated from the blood of healthy donors firstly by sedimentation of red cells in 0.9% NaCl solution containing 4.5% dextran and then by density-gradient centrifugation of leukocyte-rich plasma in the lymphocytes separating solution. The cells were finally washed twice with saline and suspended in HBSS at the concentration of 1 × 107 cells/ml.
Chemiluminescence measurement of respiratory burst
The respiratory burst of neutrophils was monitored as concomitant chemiluminescence burst [23]. Each 1 ml neutrophil suspension containing 1 μM luminol was added in a quartz cuvette. Two identical cuvettes containing testing or control cell suspension were placed in a rotating sample holder of a laboratory-made photon counter and measured simultaneously at 37°C.
Measurement of intracellular Ca2+
Neutrophils (106 cells/ml) were loaded with fura-2 by incubation with 1 μM fura-2/AM at 37°C for 45 min. Thereafter, the cells were washed twice to remove the extracellular dye and resuspended in HBSS at a density of 106 cells/ml. The fura-2 loaded neutrophils were transferred in a magnetic stirring cuvette and measured at 37°C on a dual excitation fluorescence spectrophotometer (Hitachi F-4500, Tokyo, Japan) that allows simultaneous excitation at 340 nm and 380 nm. During the measurement, fMLP was injected into the cell suspension. The fluorescence of the cytoplasmic Ca2+-binding fura-2 excited at 340 nm and 380 nm, F340 and F380, were simultaneously recorded at the emission of 510 nm. The free intracellular Ca2+ concentration, [Ca2+]i, in cells was calculated according to literature [24].
Radioactive labelling of neutrophil phospholipid pool
Neutrophils were suspended in the buffer (pH 7.3) containing 25 mM Hepes, 125 mM NaCl, 0.7 mM MgCl2, 0.5 mM ethylene glycol tetraacetic acid (EGTA), 10 mM glucose and 1 mg/ml fatty-acid-free bovine serum albumin at the density of 4 × 107 cells/ml at room temperature. Cells were then diluted with an equal volume of the same buffer containing [9,10(n)-3H]Palmitic acid (20 μCi/ml) and incubated for 3 hrs at 37°C in a shaking water bath. Following the incubation, the cells were washed twice and resuspended in the above buffer but with 1.5 mM CaCl2 replacing the EGTA (107 cells/ml).
Assay of PLD activity in cells
PLD activity was measured by the formation of [3H] PEt in [3H] Palmitic-acid-labelled neutrophils [25]. Ethanol (1.5%) was added to the cell suspension (4 ml; 1 × 107 cells/ml) in tubes, and the suspension was incubated for 5 min. at 37°C before adding test agent. After additional 10 min. incubation, cells were collected by centrifugation (12,000 ×g, 10 sec.) as pellets. The pellets were immediately resuspended in 2 ml chloroform/methanol (2:1, by vol.). All subsequent procedures were performed at 2°C. An aliquot of 0.04% (mass/vol.) CaCl2 (1 ml) was added and mixed with the cell lysate and allowed to stand for 15 min. After centrifugation (1500 ×g, 10 min.), the upper aqueous phase was removed, the lower organic phase was washed with 1 ml 0.04% CaCl2. The resultant organic phase was evaporated to dryness using a vacuum pump. The dried phospholipid extracts were suspended in 30 μl chloroform/methanol (2:1 by vol.), then immediately spotted onto Silica Gel plates (Merck KgaA, Darmstadt, Germany) using authentic PEt as reference. The thin layer chromatography (TCL) plates were developed in chloroform/methanol/acetic acid (32.5:7.5:1 by vol.) [6]. The phospholipid bands were localized by iodine staining and the PEt band were scraped. Radioactivity in the band was determined by liquid scintillation counting.
Assay of PKC activity in cells
PKC was assayed with Protein Kinase C Assay system using Ac-MBP as substrate and the pseudosubstrate peptide PKC as inhibitor. According to the protocol provided, neutrophils (4 × 107 cells/ml) in HBSS were pre-incubated with or without U73122 for 10 min. and then stimulated with PMA. Reactions were stopped by addition of 3-fold excess of ice-cold HBSS. Cells were collected and resuspended in 1 ml disruption solution (20 mM Tris-HCl, 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β-mercaptoethanol, 25 μg /ml leupeptin and aprotinin). After sonication, the lysate was centrifuged at 100,000 ×g for 1 hr at 4°C. The pellet fractions was resuspended in 0.5 ml extraction buffer containing Triton X-100 (0.5%), 20 mM Tris-HCl, 0.5 mM EDTA and EGTA, 10 mM β-mercaptoethanol and 200 mM NaCl, then incubated at 4°C for 30 min. and centrifuged again. The PKC in cell membrane fraction was obtained as supernatant. The supernatant was mixed with 50 μl of the lipid preparation provided with the assay system (100 μM PMA, 2.8 mg/ml phosphatidyl serine and Triton X-100 mixed micelles). The mixture was then divided into nine aliquots (30 μl) of three groups, the first three aliquots were added with 10 μl water as control, the 10 μl supplied inhibitor solution (100 μM pseudosubstrate peptide PKC and 20 mM Tris pH 7.5) and 10 μl 5 μM U73122 was added to the aliquots of the second and third group respectively. Each aliquot was added with 10 μl of 32P/Substrate (250 μM Ac-MBP, 1000 μM ATP, 5 mM CaCl2, 100 mM MgCl2, 20 mM Tris pH 7.5 and 20 μCi/ml [γ-32P] ATP) and incubated at 30°C for 5 min., then spotted onto phosphocellulose discs. After washing discs with 1% phosphoric acid and water to remove the free 32P-ATP, the radioactivity associated with PKC substrate on the discs was measured by liquid scintillation spectroscopy. The results were obtained as the counts of the control group and the group with U73122 minus the counts of the group with inhibitor.
Results
Inhibitory effect of U73122 on the respiratory burst in fMLP- and PMA-stimulated neutrophils
The inhibitory effects of U73122 on fMLP- and PMA-stimulated neutrophil respiratory burst were investigated. Figure 1 shows that 1 μM U73122 almost completely inhibited the fMLP-stimulated respiratory burst, while 2 μM U73122 was required for achieving the same inhibitory effect on the PMA-stimulated burst.
Fig 1.
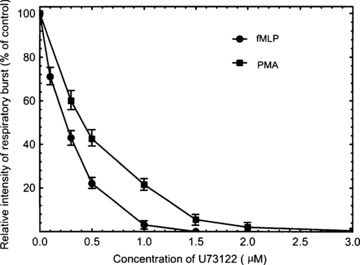
The inhibition of fMLP- and PMA-stimulated respiratory burst by U73122. Neutrophils (1 × 107/ml) were incubated with various concentrations of U73122 for 10 min. and then stimulated with fMLP (100 nM) or PMA (100 nM). The respiratory burst was measured as chemiluminescence burst. Data are means ± S.D. from three independent measurements.
PLD activation in PMA- and fMLP-stimulated neutrophils and its inhibition by U73122
To further elucidate the role of PLD and its relevance to the inhibitory effect of U73122 in the PMA- and fMLP-stimulated respiratory burst in human neutrophils, the PLD activities in the stimulated cells either in the absence or in the presence of U73122 of various concentrations were determined. The results are shown in Fig. 2. PLD activity increases in both PMA- and fMLP-stimulated cells. But, the activation of PLD seems more prominent after PMA-stimulation. When cells were pre-incubated with U73122 at various concentrations for 10 min., the PLD activity in both PMA- and fMLP-stimulated cells was significantly reduced in a dose-dependent manner. The activity of PLD in the PMA-stimulated neutrophils was reduced by 20% and 40% in the presence of 0.3 μM and 1 μM of U73122 respectively. Interestingly, it was noticed that 2 μM U73122 almost completely inhibited the PMA-stimulated respiratory burst, but only suppressed PLD activity by about 50%. The fact that half of PMA-stimulated PLD activity still exist in the cells, whose respiratory burst is already completely inhibited by 2 μM U73122, may suggest that either the activation of PLD is only partially responsible for the PMA-stimulated respiratory of human neutrophils or PLD activity needs to be kept over a certain threshold to prime the respiratory burst. Based on such differential effects of U73122, it may be reasonable to conclude that PLD activity may partially contribute to PMA-stimulated respiratory burst.
Fig 2.
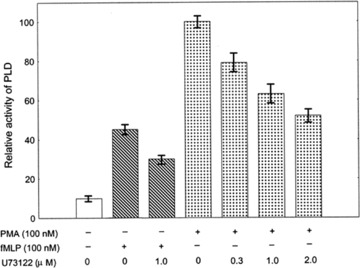
The activities of PLD in PMA- or fMLP-stimulated human neutrophils treated without or with U73122. Neutrophils (1 × 107/ml) were incubated with various indicated concentrations of U73122 at 37 °C for 10 min. and then stimulated with fMLP (100 nM) or PMA (100 nM). 10 min. after stimulation the cells were lysed for the assay of PLD activity. Data are means ± S.D. of three independent assays.
Blockade of PA generation partially blocks PMA-stimulated neutrophil respiratory burst
After showing the activation of PLD in the PMA-stimulated respiratory burst of neutrophils, we tested if blockade of downstream of PLD signalling results in reduced respiratory burst. PLD activation leads to the generation of PA, which can be quickly catalysed into DAG [4]. Since PLD functions through its hydrolytic products PA and DAG, we used 1-butanol, which blocks the PA generation, to pre-treat cells at different concentrations and observe its effects on PMA stimulated respiratory burst. Figure 3 shows that 1-butanol partially blocks the respiratory burst. The above results show that PLD partially contributes to PMA-stimulated respiratory burst probably through its hydrolytic products.
Fig 3.
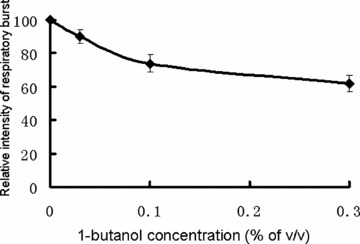
The inhibition of PMA stimulated respiratory burst by 1-butanol. Neutrophils (1 × 107/ml) were incubated with various indicated concentrations of 1-butanol at 37 °C for 10 min. and then stimulated with PMA (100 nM). The respiratory burst was measured as chemiluminescence burst. Data are means ± S.D. from three independent measurements.
Effect of U73122 on protein kinase C translocation and activation in PMA-stimulated neutrophils
To determine if the inhibition of the PMA-stimulated respiratory burst and PLD activation by U73122 is due to inhibition of PKC, the PKC activity in the membrane fraction, that was isolated from the cells pre-incubated either with or without U73122 for 10 min. and then stimulated by PMA, were determined. In order to distinguish between the direct action of U73122 on PKC and a possible action of U73122 on the signalling pathway upstream of PKC, the in vitro inhibitory effect of U73122 on PKC activity was also determined by adding U73122 into the membrane fraction of the lysed PMA-stimulated neutrophils. The results are summarized in Table 1. It can be seen that the membrane PKC activity increased 15-fold by PMA stimulation. However, U73122 did not inhibit the PKC activity either in PMA-stimulated cells or in the PKC-containing membrane fraction isolated from the same stimulated cells. The results indicate that the inhibition of the PMA-stimulated respiratory burst by U73122 is not due to any inhibitory effect on translocation and activation of PKC in cells.
Table 1.
The effects of U73122 on the membrane PKC activity in PMA-stimulated neutrophils
| Treatment of cells | Membrane PKC activity (% of control) |
|---|---|
| None | 100 |
| PMA (100 nM) | 1510 ± 110 |
| PMA + U73122* | 1530 ± 150 |
| PMA + U73122§ | 1470 ± 130 |
Results are the mean ± S.D. of three separate experiments. *Neutrophils were incubated with 2 μM U73122 for 10 min. and then stimulated with 100 nM PMA. §Neutrophils were incubated with 2 μM U73122 for 10 min. and then stimulated with 100 nM PMA. After cell lysis, 2 μM U73122 more was added in the membrane fraction before PKC assay.
PLD activation and its PKC dependence in the PMA-stimulated respiratory burst
To know if the PMA-stimulated PLD activation is PKC dependent, PLD activity and the respiratory burst in the PMA-stimulated neutrophils were determined in the presence of GF109203X, a selective PKC inhibitor. The results revealed that GF109203X effectively suppressed the PMA-stimulated respiratory burst in a dose-dependent manner (Fig. 4A). It was also found that GF109203X effectively inhibited the PMA-stimulated PLD activation even at a lower concentration of 0.3 μM and almost completely inhibited the stimulated PLD activity at the concentration of 1 μM (Fig. 4B). It was interesting to notice that 0.3 μM GF 109203X could almost completely inhibited the PMA-stimulated PKC activation and respiratory burst in neutrophils, but only partially suppressed the PLD activity (compare Fig. 4A with Fig. 4B). Thus, it may be concluded that only a portion of PLD activation comes from PMA-activated PKC, or in other words, the PMA-stimulated PLD activation is partially PKC dependent. This result is in accordance with Fig. 2, in which 2 μM U73122 completely blocks PMA stimulated respiratory burst but only partially inhibits PLD activity, suggesting that PLD activity needs to be kept above a certain level to sustain the respiratory burst.
Fig 4.
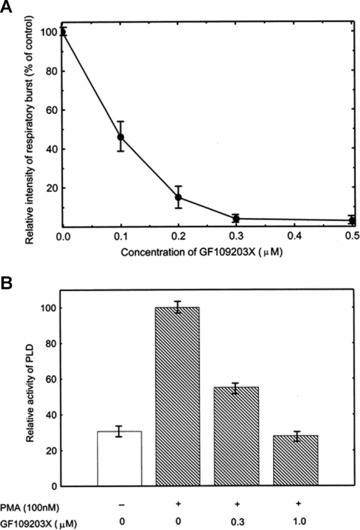
The effect of GF109203X on the respiratory burst and PLD activity in PMA-stimulated neutrophils. Neutrophils (1 × 107/ml) were incubated with various indicated concentrations of GF109203X at 37°C for 10 min. and then stimulated with PMA (100 nM). The respiratory burst was measured as chemiluminescence burst (A). The PLD activity was assayed 10 min. after stimulation (B). Data are means ± S.D. of three independent experiments.
Priming with low concentration of DOG reverses the inhibitory effect of U73122 in PMA-stimulated neutrophil respiratory burst
The above results indicate that PLD is involved in PMA-stimulated respiratory burst. However, the mechanism is unknown. Since PLD activation results in the generation of PA, which can be quickly turned over to DAG, an important activator of PKC and respiratory burst, we used a low concentration of DOG (100 nM), a membrane permeable DAG analogue, to prime cells and then investigated if DOG modulates the PMA-stimulated respiratory burst of neutrophils in either the presence or absence of U73122. The results are shown in Fig. 5. DOG only slightly enhanced the PMA-stimulated respiratory burst in the absence of U73122. However, priming with DOG significantly eliminates the inhibitory effect of U73122 on PMA-stimulated respiratory burst. This result implies that DAG is still required in PMA-stimulated respiratory burst, and PLD might be a major source enzyme to provide it. The obligation of U73122-exerted inhibition of respiratory burst by DOG suggests that U73122 blocks PMA-stimulated respiratory burst possibly through elimination of DAG, the product of PLD activation.
Fig 5.
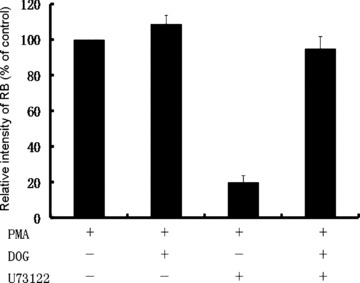
The effect of DOG on the respiratory burst in PMA-stimulated neutrophils treated with or without U73122. Neutrophils (1 × 107/ml) were incubated with or without U73122 (1 μM) at 37°C for 10 min. DOG (100 nM) was added with or without PMA (100 nM) to the cells. The respiratory burst was measured as chemiluminescence burst. Data are means ± S.D. of three independent experiments.
Discussion
PMA-stimulated neutrophil respiratory burst has been considered simply as a PKC-dependent signal pathway. However, the present investigation demonstrated, probably for the first time, that the stimulated PLD take considerable responsibility for the respiratory burst in PMA-stimulated neutrophils. The observation that U73122 at a reasonable concentration (2 μM) completely inhibited the PMA-stimulated respiratory burst and the PMA-stimulated PLD activation evidently indicates a role played by PLD in PMA-stimulated respiratory burst. It suggests that PMA stimulates respiratory burst may not only through direct activation of PKC, a well-established signalling pathway [3], but also by activating PLD.
It was interesting to find that U73122 inhibited PMA-stimulated PLD activation but had no inhibitory effect on the PMA-stimulated PKC activity in the stimulated cells, suggesting that U73122 inhibits PMA-stimulated PLD activation downstream of PKC. One recent study on the mechanisms of regulation of PLD1 by PKCα showed that PKC activated PLD through protein-protein interaction but not phosphorylation of PLD [26]. U73122 might also inhibit PLD activation by interfering with PKC and PLD interaction. The mechanism on how U73122 inhibit PMA stimulated PLD activation needs to be further studied.
The present investigation showed that the specific inhibitor of PKC, GF109203X, could completely inhibit the respiratory burst and PLD activation in PMA-stimulated neutrophils. However, the complete inhibition of the respiratory burst appeared much earlier than the complete inhibition of the PLD activation (compare Fig. 4A with Fig. 4B). It has been well established that the respiratory burst (or the generation of O2−) is due to the phosphorylation of the subunit p67phox of the NADPH oxidase by activated PKC in PMA-stimulated neutrophils [27]. Many studies showed that GF109203X is a more specific inhibitor of PKC, which strongly inhibits the PMA-induced phosphorylation of p67phox[27], and significantly reduces a number of inflammatory process resulting from PKC activation by the topical application of PMA to mouse ears [28]. Unlike Staurosporine, a non-specific PKC inhibitor which primes the fMLP-stimulated respiratory burst of human neutrophils by potentiating PLD activation, GF109203X neither stimulated PLD nor primed fMLP-induced PLD or respiratory burst [29]. It is reasonable to believe that the inhibition of PKC by GF109203X should be synchronized with the inhibition of the respiratory burst by GF109203X. The earlier appearance of the complete inhibition of the PMA-stimulated respiratory burst by GF109203X suggests that the PKC activation might be a dominant factor for the PMA-stimulated respiratory burst.
U73122 has been considered as a selective inhibitor of phospholipase C and used to demonstrate the involvement of PLC in receptor-mediated signal transduction. The finding of its inhibitory effect on PLD activation in this study and a previous report on its inhibition of cholecytokinin-(26–33)-peptide amide (CCK-8)-induced PLD activation [21] suggest that U73122 may not be only specific for phospholipase C. Caution must be taken in identifying PLC involvement just based on inhibitory effect of U73122. As discussed above, U73122 might inhibit PMA-stimulated PLD activation.
The inhibitory effect of U73122 on PMA-stimulated respiratory burst discloses the requirement of PLD activities in PMA-stimulated respiratory burst. However, how PLD is involved in PMA-stimulated respiratory burst still remains unclear. PLD play important roles in lipids signalling and cellular metabolism. PLD hydrolyses PC and generate PA, which can be further catalysed into DAG. 1-butanol, a high-affinity PLD substrate, blocks PA generation by generating PtdButanol instead. Our results show that 1-butanol partially blocked PMA-stimulated respiratory burst (Fig. 3), suggesting that PLD helps activate PMA-stimulated respiratory burst through PA or its catalytic product DAG. To further test this hypothesis, we used low concentration (100 nM) of membrane permeable DAG analogue DOG to prime the cells. Figure 5 shows that this DAG analogue, at the concentration which is not sufficient to activate respiratory burst [22], completely eliminates the inhibitory effect of U73122 on PMA stimulated respiratory burst. The result indicates that DAG is required for PMA stimulated respiratory burst. PA and DAG have been suggested to activate the respiratory burst of neutrophils at a step downstream of PKC [30], possibly via the activation of NADPH oxidase. Our results indicate that besides PKC activation, PA and DAG also play roles in PMA stimulated respiratory burst.
In summary, our study suggests that PLD activation is involved in PMA-stimulated respiratory burst of human neutrophils, though PKC activation is dominant. A possible function of PLD is to provide DAG for maintaining the respiratory burst. PKC and DAG might be both required to activate NADPH oxidase. This study might provide new insight into the mechanism involved in neutrophil respiratory burst and suggest new pharmaceutical target for treatment of related diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China No. 39970197 (to X.S.), 30971524 (to T.H.). The 985 project grant of Xiamen University, the Ministry of Science and Technology grant 2009CB522200, 2006AA02A303 (to T.H.).
References
- 1.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–68. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 2.Thelen M, Dewald B, Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol Rev. 1993;73:797–821. doi: 10.1152/physrev.1993.73.4.797. [DOI] [PubMed] [Google Scholar]
- 3.Nauseef WM, Volpp BD, McCormick S, et al. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991;266:5911–7. [PubMed] [Google Scholar]
- 4.Exton JH. Phospholipase D: enzymology, mechanisms of regulation, and function. Physiol Rev. 1997;77:303–20. doi: 10.1152/physrev.1997.77.2.303. [DOI] [PubMed] [Google Scholar]
- 5.Billah MM, Eckel S, Mullmann TJ, et al. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989;264:17069–77. [PubMed] [Google Scholar]
- 6.Kessels GC, Gervaix A, Lew PD, et al. The chymotrypsin inhibitor carbobenzyloxy-leucine-tyrosine-chloromethylketone interferes with phospholipase D activation induced by formyl-methionyl-leucyl-phenylalanine in human neutrophils. J Biol Chem. 1991;266:15870–5. [PubMed] [Google Scholar]
- 7.Fensome A, Whatmore J, Morgan C, et al. ADP-ribosylation factor and Rho proteins mediate fMLP-dependent activation of phospholipase D in human neutrophils. J Biol Chem. 1998;273:13157–64. doi: 10.1074/jbc.273.21.13157. [DOI] [PubMed] [Google Scholar]
- 8.Kessels GC, Krause KH, Verhoeven AJ. Protein kinase C activity is not involved in N-formylmethionyl-leucyl-phenylalanine-induced phospholipase D activation in human neutrophils, but is essential for concomitant NADPH oxidase activation: studies with a staurosporine analogue with improved selectivity for protein kinase C. Biochem J. 1993;292:781–5. doi: 10.1042/bj2920781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauldry SA, Bass DA, Cousart SL, et al. Tumor necrosis factor alpha priming of phospholipase D in human neutrophils. Correlation between phosphatidic acid production and superoxide generation. J Biol Chem. 1991;266:4173–9. [PubMed] [Google Scholar]
- 10.Gelas P, Ribbes G, Record M, et al. Differential activation by fMet-Leu-Phe and phorbol ester of a plasma membrane phosphatidylcholine-specific phospholipase D in human neutrophil. FEBS Lett. 1989;251:213–8. doi: 10.1016/0014-5793(89)81457-7. [DOI] [PubMed] [Google Scholar]
- 11.Bourgoin S, Poubelle PE, Liao NW, et al. Granulocyte-macrophage colony-stimulating factor primes phospholipase D activityin human neutrophils in vitro: role of calcium, G-proteins and tyrosine kinases. Cell Signal. 1992;4:487–500. doi: 10.1016/0898-6568(92)90018-4. [DOI] [PubMed] [Google Scholar]
- 12.Gay JC, Raddassi K, Truett AP, 3rd, et al. Phosphatase activity regulates superoxide anion generation and intracellular signaling in human neutrophils. Biochim Biophys Acta. 1997;1336:243–53. doi: 10.1016/s0304-4165(97)00034-2. [DOI] [PubMed] [Google Scholar]
- 13.Tou JS. Differential regulation of neutrophil phospholipase d activity and degranulation. Biochem Biophys Res Commun. 2002;292:951–6. doi: 10.1006/bbrc.2002.6765. [DOI] [PubMed] [Google Scholar]
- 14.Lopez I, Arnold RS, Lambeth JD. Cloning and initial characterization of a human phospholipase D2 (hPLD2). ADP-ribosylation factor regulates hPLD2. J Biol Chem. 1998;273:12846–52. doi: 10.1074/jbc.273.21.12846. [DOI] [PubMed] [Google Scholar]
- 15.Fahimi-Vahid M, Gosau N, Michalek C, et al. Distinct signaling pathways mediate cardiomyocyte phospholipase D stimulation by endothelin-1 and thrombin. J Mol Cell Cardiol. 2002;34:441–53. doi: 10.1006/jmcc.2002.1525. [DOI] [PubMed] [Google Scholar]
- 16.Babich M, Alford GE, Nissenson RA. A novel phospholipase C inhibitor and phorbol esters reveal selective regulation of thrombin- and parathyroid hormone-stimulated signaling pathways in rat osteosarcoma cells. J Pharmacol Exp Ther. 1994;269:172–7. [PubMed] [Google Scholar]
- 17.Bleasdale JE, Thakur NR, Gremban RS, et al. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–68. [PubMed] [Google Scholar]
- 18.Wu H, James-Kracke MR, Halenda SP. Direct relationship between intracellular calcium mobilization and phospholipase D activation in prostaglandin E-stimulated human erythroleukemia cells. Biochemistry. 1992;31:3370–7. doi: 10.1021/bi00128a010. [DOI] [PubMed] [Google Scholar]
- 19.Freeman EJ, Chisolm GM, Tallant EA. Role of calcium and protein kinase C in the activation of phospholipase D by angiotensin II in vascular smooth muscle cells. Arch Biochem Biophys. 1995;319:84–92. doi: 10.1006/abbi.1995.1269. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L, Krsmanovic LZ, Vergara LA, et al. Dependence of intracellular signaling and neurosecretion on phospholipase D activation in immortalized gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA. 1997;94:1573–8. doi: 10.1073/pnas.94.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch RR, Patel AM, Van Emst-de Vries SE, et al. U73122 and U73343 inhibit receptor-mediated phospholipase D activation downstream of phospholipase C in CHO cells. Eur J Pharmacol. 1998;346:345–51. doi: 10.1016/s0014-2999(98)00070-3. [DOI] [PubMed] [Google Scholar]
- 22.Hu TH, Bei L, Qian ZM, et al. Intracellular free calcium regulates the onset of the respiratory burst of human neutrophils activated by phorbol myristate acetate. Cell Signal. 1999;11:355–60. doi: 10.1016/s0898-6568(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 23.Allen RC, Stjernholm RL, Steele RH. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972;47:679–84. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- 24.Tsien RY, Rink TJ, Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985;6:145–57. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- 25.Hardy SJ, Robinson BS, Poulos A, et al. The neutrophil respiratory burst. Responses to fatty acids, N-formylmethionylleucylphenylalanine and phorbol ester suggest divergent signalling mechanisms. Eur J Biochem. 1991;198:801–6. doi: 10.1111/j.1432-1033.1991.tb16084.x. [DOI] [PubMed] [Google Scholar]
- 26.Hu T, Exton JH. Mechanisms of regulation of phospholipase D1 by protein kinase Calpha. J Biol Chem. 2003;278:2348–55. doi: 10.1074/jbc.M210093200. [DOI] [PubMed] [Google Scholar]
- 27.Benna JE, Dang PM, Gaudry M, et al. Phosphorylation of the respiratory burst oxidase subunit p67(phox) during human neutrophil activation. Regulation by protein kinase C-dependent and independent pathways. J Biol Chem. 1997;272:17204–8. doi: 10.1074/jbc.272.27.17204. [DOI] [PubMed] [Google Scholar]
- 28.Kuchera S, Barth H, Jacobson P, et al. Anti-inflammatory properties of the protein kinase C inhibitor, 3-[1-[3-(dimethylamino)propyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H- pyrrole-2,5-dione monohydrochloride (GF109203X) in the PMA-mouse ear edema model. Agents Actions. 1993;39:C169–73. doi: 10.1007/BF01972756. [DOI] [PubMed] [Google Scholar]
- 29.Rais S, Pedruzzi E, Dang MC, et al. Priming of phosphatidic acid production by staurosporine in f-Met-Leu-Phe-stimulated human neutrophils–correlation with respiratory burst. Cell Signal. 1998;10:121–9. doi: 10.1016/s0898-6568(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 30.Mitsuyama T, Takeshige K, Minakami S. Phosphatidic acid induces the respiratory burst of electropermeabilized human neutrophils by acting on a downstream step of protein kinase C. FEBS Lett. 1993;328:67–70. doi: 10.1016/0014-5793(93)80967-y. [DOI] [PubMed] [Google Scholar]


