Abstract
Traumatic brain injury (TBI) represents a leading cause of death and disability among young persons with ∼1.7 million reported cases in the United States annually. Although acute alcohol intoxication (AAI) is frequently present at the time of TBI, conflicting animal and clinical reports have failed to establish whether AAI significantly impacts short-term outcomes after TBI. The objective of this study was to determine whether AAI at the time of TBI aggravates neurobehavioral outcomes and neuroinflammatory sequelae post-TBI. Adult male Sprague-Dawley rats were surgically instrumented with gastric and vascular catheters before a left lateral craniotomy. After recovery, rats received either a primed constant intragastric alcohol infusion (2.5 g/kg+0.3 g/kg/h for 15 h) or isocaloric/isovolumic dextrose infusion followed by a lateral fluid percussion TBI (∼1.4 J, ∼30 ms). TBI induced apnea and a delay in righting reflex. AAI at the time of injury increased the TBI induced delay in righting reflex without altering apnea duration. Neurological and behavioral dysfunction was observed at 6 h and 24 h post-TBI, and this was not exacerbated by AAI. TBI induced a transient upregulation of cortical interleukin (IL)-6 and monocyte chemotactic protein (MCP)-1 mRNA expression at 6 h, which was resolved at 24 h. AAI did not modulate the inflammatory response at 6 h but prevented resolution of inflammation (IL-1, IL-6, tumor necrosis factor-α, and MCP-1 expression) at 24 h post-TBI. AAI at the time of TBI did not delay the recovery of neurological and neurobehavioral function but prevented the resolution of neuroinflammation post-TBI.
Key words: : alcohol and drug abuse, animal studies, behavior assessments, inflammation
Introduction
Traumatic brain injury (TBI) contributes to a third of all injury-related deaths in the United States. An estimated 1.7 million people sustain a TBI annually: 52,000 die, 275,000 are hospitalized, and nearly 80% are admitted to an emergency department.1 While military personnel are at a high risk,2 TBIs resulting from falls, motor-vehicle accidents, and assaults remain the leading causes of disability and death in young adults.3 Mild TBI and concussions are the most common and frequently occur in contact sports.4 Specifically, professional football players are vulnerable to concussions with an average of 0.41 concussions occurring per National Football League game.4
Alcohol binge drinking leading to acute alcohol intoxication (AAI) has become extremely prevalent.5 AAI increases the risk of injury and is reported to be a contributing factor in 36–51% of all incidents of TBI.6 Although the presence of blood alcohol level (BAL) is associated with increased occurrence of TBI,7 the effect of AAI on clinical outcomes remains poorly defined.8–14 Inconsistent results from animal studies show that a high BAL at the time of TBI is neurotoxic leading to increased mortality and hemodynamic depression,15–17 while low to moderate BAL at the time of injury may be neuroprotective and results in improved neurobehavioral and cognitive outcomes post-TBI.18–20 Factors such as the history of alcohol exposure, severity of injury, and the varied outcomes measured after TBI may contribute to these conflicting clinical and laboratory reports.
The diverse influences of alcohol suggest that a single mechanism is unlikely to explain the effect of AAI on outcomes after TBI. Therefore, a controlled study to examine the overt changes such as neurological and neurobehavioral function in addition to the underlying neuroinflammatory sequelae from TBI is essential in contributing to the understanding of the overall impact of AAI on TBI outcomes.
Acute neuroinflammation is a characteristic feature of TBI pathophysiological sequelae.21 Elevated proinflammatory cytokines and chemokines increase cerebral blood flow to the injured parenchyma and cause local increases in endothelial permeability and infiltration of circulating leukocytes.21,22 Sustained post-traumatic inflammation is increasingly recognized as a relevant mechanism underlying the adverse outcomes from TBI.23–25 As an immunomodulator, alcohol exerts its impact on multiple aspects of the immune response, including the activation of transcription factors involved in inflammatory signaling pathways, expression of cytokines and inflammatory mediators, expression of adhesion molecules, and recruitment of inflammatory cells.26–44
Previous studies from our laboratory have shown that alcohol has the ability to alter the early hemodynamic, proinflammatory, and neuroendocrine responses to traumatic injury and infection.34,39,45–52 Evidence suggests that alcohol intoxication at the time of trauma impairs the host's ability to respond to a secondary challenge, which is accompanied by localized tissue inflammation and decreased effectiveness in resolution of the post-injury inflammatory process.39,49,51 How these alcohol-mediated alterations modulate the neuroinflammatory responses to TBI is poorly understood.
Using a lateral fluid percussion TBI model, we examined the impact of AAI at the time of TBI on the early clinical (neurological and neurobehavioral) and neuroinflammatory responses in rats. Our working hypothesis was that TBI in the AAI host would be associated with worsening of neurological and neurobehavioral manifestations and accentuated neuroinflammation during the early post-TBI period.
Methods
Animals
Adult male Sprague-Dawley rats (Charles River, Raleigh, NC) weighing 275–300 g were housed in the Division of Animal Care at the Louisiana State University Health Sciences Center for 1 week of acclimatization before all experimental procedures. Rats were housed under a 12 h light/12 h dark cycle at 22°C and had free access to water and standard diet (Purina Rat Chow, St. Louis, MO). All animal procedures and experiments were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and were in accordance with the guidelines of the National Institutes of Health.
Surgical procedures
Animals were anesthetized with an intramuscular injection of ketamine/xylazine (90 mg/kg and 9 mg/kg, respectively). Using aseptic surgical technique, a sterile vascular catheter (PE50) was implanted into the left carotid artery for sample blood collection to measure BAL. A separate gastric catheter (PE50) was introduced into the fundus of the stomach for alcohol or dextrose administration, as previously described by our laboratory.51,53,54–56 All catheters were routed subcutaneously to the nape of the neck where they were exteriorized through a small incision and secured with tape. Using a stereotaxic frame, a craniotomy (5.0 mm in diameter) was performed on the left side of the skull (2 mm posterior to bregma and 3 mm lateral from midline). A female Luer Loc connector was affixed to the skull by cyanoacrylate glue and Jet Denture Repair Acrylic (Lang, Wheeling, IL). The dura was kept intact and moist by filling the interior of the Luer Loc with saline before capping. At the completion of all surgical procedures, the animals were returned to their individual cages and allowed to recover for 3 days, during which time they had free access to food and water.
Alcohol administration
Animals were randomly selected to receive an intragastric bolus of 30% ethanol (2.5 g/kg) followed by a 15 h constant infusion (300 mg/kg/h) for a total of approximately 7 g/kg of ethanol (n=27). BAL achieved averaged 266±10 mg/dL at the time of TBI. This protocol models an episode of binge drinking57,58 and has been used previously by our laboratory to examine the impact of AAI on outcomes from traumatic injury and hemorrhagic shock.39,49,51,54–56 Time-matched control animals (n=27) received a 15 h constant infusion with an equal volume of isocaloric 52% dextrose solution (12.2 g/kg).
TBI
TBI was induced 30 min after the discontinuation of the alcohol or dextrose infusion. Animals were anesthetized with isoflurane (4% induction, 3% maintenance). The cranial female Luer Loc was connected to a fluid percussion system. Injury was induced by lateral fluid percussion to the left cortex (ipsilateral cortex). The kinetic energy produced was 1.37 J in 32 ms, resulting in approximately 2 atm of pressure. The lateral fluid percussion model of TBI has been extensively used in animal studies to provide dependable and consistent applications of force that lead to reliable degrees of injury.59–61 After TBI, immediate apnea duration and time of righting reflex were measured. Time-matched sham controls were anesthetized but were not subjected to TBI. After TBI and between neurological/neurobehavioral assessments, animals were returned to their home cages, provided food and water ad libitum, and housed in controlled conditions.
Apnea and righting reflex
TBI animals were examined for signs of immediate post-injury apnea, which represents a delay in the initiation of breathing from the time of TBI until the animal's first breath. Delay in righting reflex reflects a transient unconsciousness, and it was measured in all animals including the sham animals after their removal from isoflurane. It represents the time the animal took to right itself from a supine position to a prone position where all four paws were against a table surface. Animals were returned to their home cages after TBI and provided with food and water before subsequent neurological and neurobehavioral testing.
Neurological and neurobehavioral assessments
Neurological severity (NSS) and neurobehavioral (NBS) scores (summarized in Table 1) were obtained at baseline (1 day before infusion and TBI) and at 6 and/or 24 h after TBI. Sixteen animals were tested at 6 h and immediately sacrificed, 10 animals were tested independently at 24 h, and 25 animals were tested at both time points. Because not all animals were tested at both time points, the NSS and NBS measured at each time point were examined and analyzed independently. Our scoring system was adapted from previously published methods that were routinely used for animal's cognitive and behavioral assessments.62,63 Total scores ranged from 0 to 25 and 0 to 12 for NSS and NBS, respectively. Post-TBI scores obtained at each time point were compared with the animal's individual baseline scores obtained 1 day before injury and are presented as change (Δ) from that pre-injury score. Greater score increases indicate more severe dysfunction resulting from TBI.
Table 1.
Neurological Severity Score and Neurobehavioral Score
| Neurobehavioral Score (NBS) | Points |
|---|---|
| Motor tests | |
| Flexion of limbs when raised by tail | |
| Flexion of limbs and extension of head | 0 |
| No flexion of hind limb | 1 |
| No flexion of forelimb | 1 |
| No neck extension | 1 |
| Body twisting/paretic side weakness | 1 |
| Hemeplegia | |
| Resistance on both sides | 0 |
| Nonresistance on left side | 1 |
| Nonresistance on right side | 2 |
| Walking on flat surface | |
| Normal walk | 0 |
| Abnormal walk/freezing | 1 |
| Inability to walk straight | 2 |
| Circling toward paretic side | 3 |
| Falls down to paretic side | 4 |
| Sensory test–loss of placing reflex | |
| Limbs on table w/palms down | 0 |
| Limbs tucked close to body | 1 |
| Beam walking | |
| Normal ability to walk on all beams | 0 |
| Failure on 2.5 cm wide beam | 1 |
| Failure on 5.0 cm wide beam | 2 |
| Failure on 8.0 cm wide beam | 3 |
| Failure on 10.0 cm wide beam | 4 |
| Beam balancing | |
| >60 sec (balances with steady posture) | 0 |
| <60 sec (balances but unstable and/or freezes) | 1 |
| <40 sec (balances with 1–2 limbs hanging off ) | 2 |
| <20 sec (attempts to balance but fails) | 3 |
| Falls off immediately (does not attempt) | |
| Beam balancing | |
| >60 sec (balances with steady posture) | 0 |
| <60 sec (balances but unstable and/or freezes) | 1 |
| <40 sec (balances with 1–2 limbs hanging off ) | 2 |
| <20 sec (attempts to balance but fails) | 3 |
| Falls off immediately (does not attempt) | 4 |
| Reflexes and abnormal movement | |
| Pinna reflex | 1 |
| Corneal reflex | 1 |
| Startle reflex | 1 |
| Righting reflex | 1 |
| Seizures | 1 |
| NSS Total | 25 |
| Neurobehavioral Score (NBS) | |
| Resistance to lateral pulsion | |
| Normal resistance | 0 |
| Impaired resistance in forelimbs | 1 |
| Impaired resistance in hind limbs | 1 |
| Ability to stand on an inclined plane | |
| Rat successfully stays on at 90 degrees | 0 |
| Impaired ability to grip at 90 degrees | 1 |
| Impaired ability to grip at 45 degrees | 2 |
| Impaired ability to grip at 30 degrees | 3 |
| Impaired–no ability to grip at <30 degrees | 4 |
| Exploratory behavior in home cage | |
| Actively explores top of cage and surroundings | 0 |
| Rears, explores top of cage, not surroundings | 1 |
| No rearing, explores bottom of cage | 2 |
| Inactive | 3 |
| Novel object recognition | |
| Approaches, touches, and sniffs object | 0 |
| Curious, approaches, no touching/smelling | 1 |
| Curious, no approach | 2 |
| Inactive/freezes/does not acknowledge | 3 |
| NBS total | 12 |
NSS tests functions for motor, sensory, reflexes, beam walking, and beam balancing. Animals were pre-trained and allowed to have several attempts (∼5 min) on the beam task before obtaining a baseline score. A score of 0 indicates a normal function in motor, sensory, reflexes, and the ability to walk and balance on all beams. NBS evaluates sensorimotor task, proprioception, exploratory behavior in home cage, and novel object exploration. NBS was assessed immediately after NSS, and therefore animals were stimulated from previous testing procedures before returning to home cages for the measurement of exploratory behavior and novel object exploration. A score of 0 indicates active exploration as well as having a normal ability for proprioception and sensorimotor tasks. A lack of performance in any of the NSS and NBS activities listed in Table 1 received a number of score according to its severity. At each behavior testing session, the total time spent on each animal for assessing both NSS and NBS was approximately 10 min.
Blood alcohol concentration measurement
Blood samples were collected in pre-chilled and heparinized syringes containing 10 μl/mL aprotinin (Sigma, St. Louis, MO) after infusion, immediately preceding TBI. BAL was measured by using an amperometric oxygen electrode and kit (Analox Instruments Limited, London, England).
Tissue collection
Animals were randomly divided into two study groups for 6 h (n=16) or 24 h (n=35) time-point sacrifice. After decapitation and the removal of brain from the skull, the brain's surface was rapidly sprayed by Richard-Allan Scientific Cytocool II (Thermo Scientific, Waltham, MA) and dipped into liquid nitrogen for approximately 6 to 10 sec. The brain was positioned into a pre-frozen standard adult rodent brain slicer matrix (Zivic Instruments, Pittsburgh, PA). Three razor blades were pressed into slice channels at 4, 8, and 13 mm from the tip of the frontal cortex to excise out a width of 4 mm prefrontal cortex and a 5 mm width of the injured area. A fourth razor blade was pressed at midline to separate the ipsilateral injured area from the contralateral region. Brain tissues from ipsilateral, contralateral, and prefrontal regions were collected and stored at −80°C until analyses.
Myeloperoxidase (MPO) activity assay
MPO was measured as an index of inflammatory cell infiltration.64 Tissue samples were homogenized using a PRO 200 homogenizer (PRO scientific, Oxford, CT) in buffer (20 mM potassium phosphate/0.1 mM ethylenediaminetetraaceticacid pH 7.4) and centrifuged at 10,000 revolutions per minute (rpm) for 5 min at 4°C. The pellet was resuspended in suspension buffer (50 mM potassium phosphate/0.5% hexadecyltrimethylammoniom bromide pH 6.0) and vortexed for 30 sec. The suspension was sonicated for 10 sec, followed by two cycles of freeze-thaw, and sonicated again for 10 sec. After 15 min centrifugation at 20,000 rpm, the supernatant was incubated for 3 min with 3,3′,5,5′-tetramethylbenzidine (TMB) Liquid Substrate System (Sigma, Saint Louis, MO) for the measurement of MPO activity. The reaction was stopped by adding 3 M sulfuric acid. The absorbance of the oxidized TMB was detected at 450 nm with Benchmark Plus microplate spectrophotometer (Biorad, Hercules, CA). The color intensity was proportional to the amount of MPO in the sample. MPO activity was expressed as units/min/mg of protein and calculated as: Total activity=([absorbance – blank]/min/g)×dilution factor.
Real-time polymerase chain reaction (PCR) analysis
Neuroinflammation was assessed by detecting pro-inflammatory cytokine and chemokine mRNA expression in brain cortical tissue. Cytokine and chemokine mRNA expression at the site of injury was measured at 6 h (n=16) and 24 h (n=17) after TBI. Total RNA was extracted from brain tissue using an RNeasy Plus Universal Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Total RNA was reverse transcribed using TaqMan Reverse Transcription Reagent kit (Life Technologies Corporation, Carlsbad, CA). The primer sequences (Integrated DNA Technologies, Coralville, IA) used in this study were as follows: interleukin (IL)-6, 5’- AAG CCA GAG TCA TTC AGA GC-3′ (forward) and 5′- GTC CTT AGC CAC TCC TTC TG-3′ (reverse); ribosomal protein (RP) S13, 5′- GAC GTG AAG GAA CAA ATT TAC AAG TTG GCC -3′ (forward) and 5′- GAA TCA CAC CTA TCT GGG AAG GAG TCA -3′ (reverse); tumor necrosis factor (TNF)-α, 5′- CCA ACA AGG AGG AGA AGT TCC CAA -3′ (forward) and 5′- GAG AAG ATG ATC TGA GTG TGA GGG -3′ (reverse); IL-1β, 5′- AGC AGC TTT CGA CAG TGA GGA GAA -3′ (forward) and 5′- TCT CCA CAG CCA CAA TGA GTG ACA -3′ (reverse); Monocyte chemotactic protein (MCP)-1, 5′- TGC TGT CTC AGC CAG ATG CAG TTA -3′ (forward) and 5′- TAC AGC TTC TTT GGG ACA CCT GCT -3′ (reverse). The primer concentrations used were 500 nmol. The RT2 SYBR Green FAST Mastermixes (Qiagen, Valencia, CA) were used for real-time PCR. All reactions were performed on a CFX96 system (Bio-Rad Laboratories, Hercules, CA). RT-qPCR data were analyzed using the ΔΔCT method. Target genes were compared with RPS13 and normalized to control values. RSP13 was chosen as the endogenous control to normalize gene expression because it was stably expressed based on a meta-analysis of 13,629 gene array samples.65
Statistical analysis
All data are presented as mean±standard error of the mean with the number of animals per group indicated. An unpaired, two-tailed t test was used to compare apnea between dextrose/TBI and AAI/TBI groups. For all other measures, statistical analysis was accomplished by two-way analysis of variance at each time point (6 h or 24 h post-TBI). When significant interaction effects were found, post-hoc tests using Bonferroni multiple comparisons were performed in GraphPad Prism software version 5.0. Statistical significance was set at p<0.05.
Results
Apnea and righting reflex post-TBI
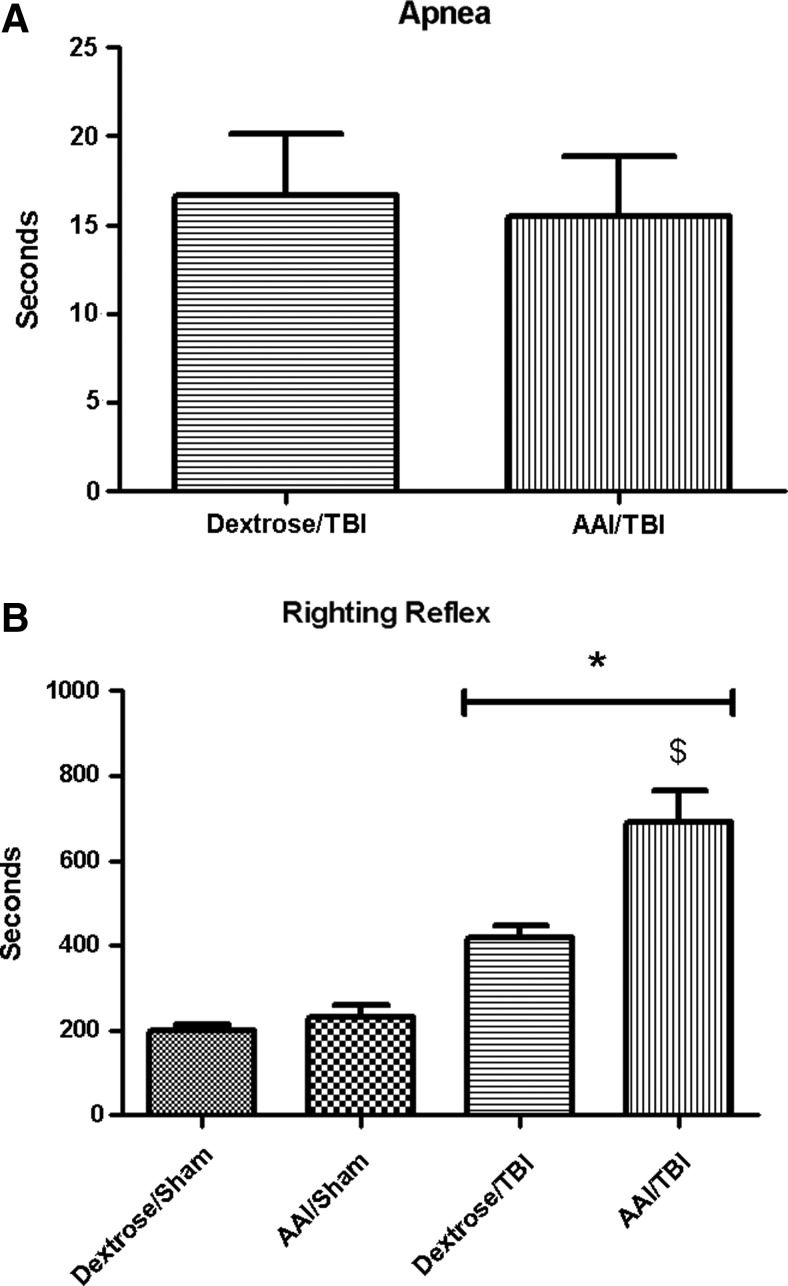
No significant difference in apnea duration was observed between dextrose (17±5 sec) and AAI groups (15±5 sec immediately after TBI (p>0.05; Fig. 1A).
FIG. 1.
Apnea duration (A) and delay in righting reflex (B) measured in seconds immediately after traumatic brain injury (TBI). Values are means±standard error of the mean (dextrose/sham, n=13; acute alcohol intoxication (AAI)/sham, n=10; dextrose/TBI, n=14; AAI/TBI, n=15). *p<0.05 vs. appropriate time-matched sham controls; $p<0.05 vs. dextrose/TBI group. Apnea was analyzed by t test. Delay in righting reflex was analyzed by two-way analysis of variance.
Delay in righting reflex showed 199±16 sec in dextrose/sham, 231±30 sec in AAI/sham, 419±30 sec in dextrose/TBI, and 692±73 sec in AAI/TBI. There was a significant main effect of injury on righting reflex (F(1,48)=51.58; p<0.01) relative to sham controls. There was a significant main effect of AAI on righting reflex (F(1,48)=10.35; p<0.01) relative to dextrose controls. Moreover, there was a significant interaction effect of TBI and AAI on righting reflex (F(1, 48)=6.46; p=0.01). Bonferroni multiple comparisions revealed that AAI/TBI group had significantly longer delay in righting reflex than dextrose/TBI group (p<0.01; Fig. 1B).
NSS and NBS post-TBI
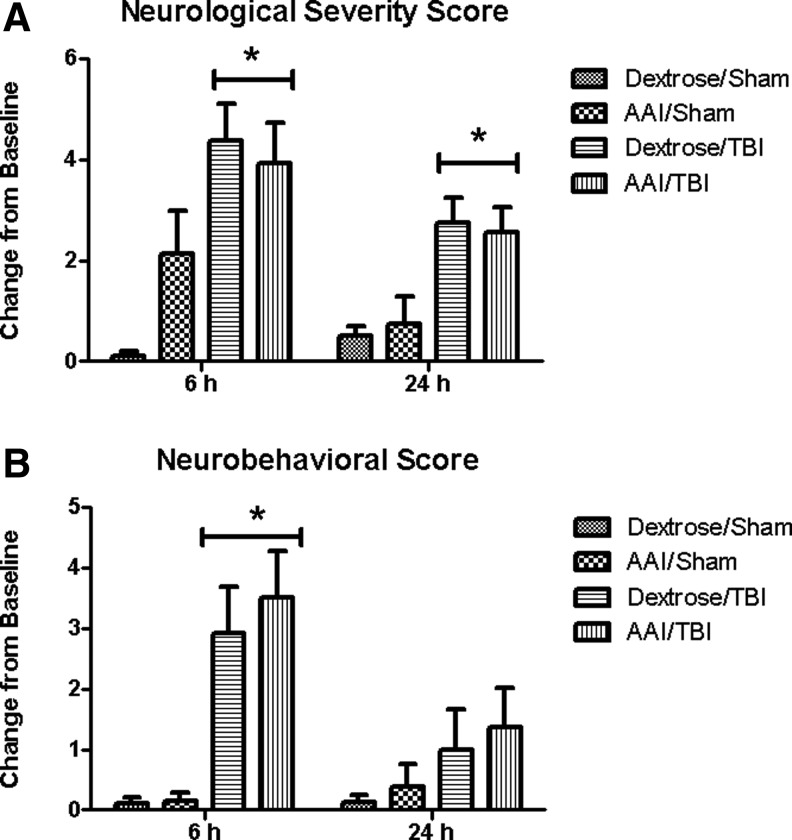
NSS and NBS were obtained at baseline and at 6 h and/or 24 h after TBI. NSS at 6 h revealed Δ0.1±0.1 in dextrose/sham, Δ2.1±0.8 in AAI/sham, Δ4.4±0.7 in dextrose/TBI, and Δ3.9±0.8 in AAI/TBI groups. There was a significant main effect of TBI on NSS at 6 h (F(1,36)=17.96; p<0.01) relative to sham controls. No significant main effect of AAI (F(1,36)=1.25; p=0.27) or interaction effect of TBI and AAI (F(1,36)=3.05; p=0.09) on NSS was found at 6 h after TBI. NBS at 6 h showed Δ0.1±0.1 in dextrose/sham, Δ0.1±0.1 in AAI/sham, Δ2.9±0.8 in dextrose/TBI, and Δ3.5±0.8 in AAI/TBI groups. There was a significant main effect of TBI on NBS at 6 h (F(1,36)=22.53; p<0.01) relative to sham controls. No significant main effect of AAI (F(1,36)=0.24; p=0.63) or interaction effect of TBI and AAI (F(1,36)=0.18; p=0.68) on NBS was found at 6 h post-TBI.
At 24 h after TBI, NSS showed Δ0.5±0.2 in dextrose/sham, Δ0.8±0.5 in AAI/sham, Δ2.8±0.5 in dextrose/TBI, and Δ2.5±0.5 in AAI/TBI groups. There was a significant main effect of TBI on NSS at 24 h (F(1,31)=18.23; p<0.01) relative to sham controls. No significant main effect of AAI (F(1,31)=0.00; p=0.96) or interaction effect of TBI and AAI (F(1,31)=0.23; p=0.63) on NSS was found at 24 h after TBI. NBS at 24 h showed Δ0.1±0.1 in dextrose/sham, Δ0.4±0.4 in AAI/sham, Δ1.0±0.7 in dextrose/TBI, and Δ1.4±0.7 in AAI/TBI groups. There was no significant main effect of TBI on NBS at 24 h (F(1,31)=3.23; p=0.08) relative to sham controls. No significant main effect of AAI (F(1,31)=0.08; p=0.79) or interaction effect of TBI and AAI (F(1,31)=0.02; p=0.89) on NBS was found at 24 h post-TBI. Therefore, no significant differences in NSS and NBS were observed between the dextrose and AAI treated animals at either 6 h or 24 h time point (Fig. 2A, 2B).
FIG. 2.
Neurological severity (NSS) (A) and neurobehavioral (NBS) (B) scores shown as change from baseline at 6 and/or 24 h after traumatic brain injury (TBI) calculated for individual animals. Values are means±standard error of the mean (at 6 h: dextrose/sham, n=10; acute alcohol intoxication (AAI)/sham, n=7; dextrose/TBI, n=11; AAI/TBI, n=12; at 24 h: dextrose/sham, n=8, AAI/sham, n=8; dextrose/TBI, n=8; AAI/TBI, n=11). *p<0.05 vs. time-matched sham controls, by two-way analysis of variance.
MPO activity post-TBI
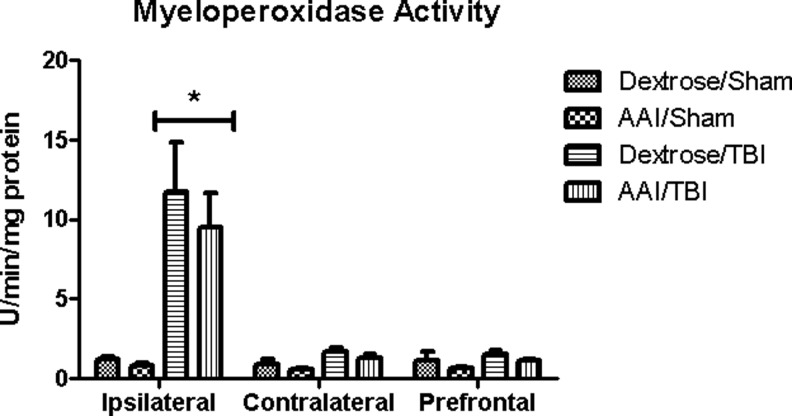
There was a significant main effect of TBI on MPO activity in the ipsilateral cortex when compared with sham controls at 24 h after TBI (F(1,27)=7.16; p=0.01). No significant main effect of AAI (F(1,27)=0.13; p=0.72) or interaction between alcohol and TBI was observed, however (F(1,27)=0.07; p=0.80). Therefore, AAI at the time of injury did not alter the magnitude of the rise in MPO activity induced by TBI. MPO activity was not altered in the contralateral or prefrontal cortex after TBI in any of the experimental groups (Fig. 3).
FIG. 3.
Myeloperoxidase (MPO) activity reflecting neutrophil infiltration measured at ipsilateral, contralateral, and prefrontal brain regions at 24 h after traumatic brain injury (TBI). Values are means±standard error of the mean of activity units per mg of protein (dextrose/sham, n=4; acute alcohol intoxication (AAI)/sham, n=3; dextrose/TBI, n=12; AAI/TBI, n=12). *p<0.05 vs. time-matched sham controls, by two-way analysis of variance.
Neuroinflammation after TBI
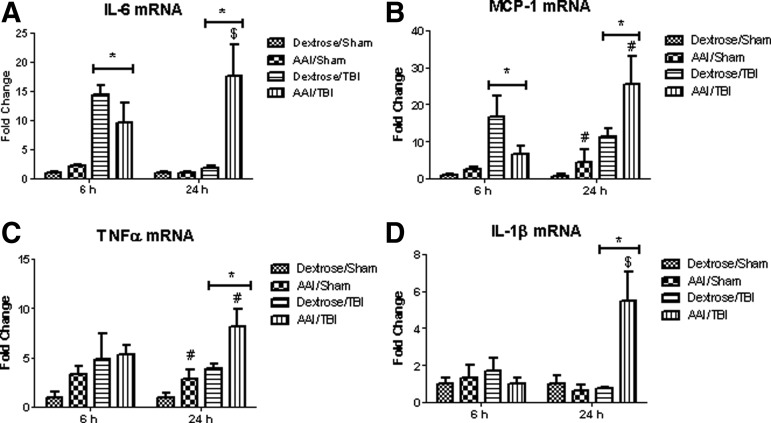
At 6 h after TBI, there was a significant main effect of injury on IL-6 (∼16 fold; F(1,12)=31.12; p<0.01) and MCP-1 (∼17 fold; F(1,12)=10.02; p<0.01) mRNA expression in the ipsilateral cortex when compared with sham controls. There was no significant main effect of alcohol, however, on IL-6 (F(1,12)=0.82; p=0.38) or MCP-1 (F(1,12)=1.77; p=0.21). No significant interaction between alcohol and TBI was observed in IL-6 (F(1,12)=2.46; p=0.14) or MCP-1 (F(1,12)=3.43; p=0.09).
At 24 h after TBI, there was a significant main effect of injury on mRNA expression (IL-6: F(1,13)=11.35; p<0.01, MCP-1: F(1,13)=15.64; p<0.01, TNF-α: F(1,13)=17.94; p<0.01, and IL-1β: F(1,13)=8.19; p=0.01). In addition, there was a main effect of alcohol on IL-6 (F(1,13)=8.97; p=0.01), MCP-1 (F(1,13)=5.06; p=0.04), TNF-α (F(1,13)=9.31; p<0.01), and IL-1β (F(1,13)=7.42; p=0.02) mRNA expression at 24 h post-TBI. Further, a significant interaction between alcohol and TBI on IL-6 (F(1,13)=8.94; p=0.01) and IL-1β (F(1,13)=10.02; p<0.01) cortical mRNA expression was detected. Post-hoc test performed by Bonferroni multiple comparison revealed that AAI/TBI group had significantly enhanced IL-6 (p<0.01) and IL-1β (p<0.01) mRNA expression at 24 h when compared with dextrose/TBI groups. Therefore, brains of AAI/TBI animals showed sustained inflammation as reflected by IL-6 (∼17 fold), MCP-1 (∼26 fold), TNF-α (∼8 fold), and IL-1β (∼5 fold) mRNA expression at 24 h post-TBI (Fig. 4A–D).
FIG. 4.
Brain interleukin (IL)-6 (A), monocyte chemotactic protein (MCP)-1 (B), tumor necrosis factor (TNF)α (C), and IL-1β (D) cortical mRNA expression at ipsilateral site of injury 6 h and 24 h after traumatic brain injury (TBI). Values are means±standard error of the mean fold change from dextrose/sham group values, (at 6 h: dextrose/sham, n=5; acute alcohol intoxication (AAI)/sham, n=3; dextrose/TBI, n=4; AAI/TBI, n=4; at 24 h: dextrose sham, n=5; AAI/sham, n=3; dextrose/TBI, n=5; AAI/TBI, n=4). *p<0.05 vs. time-matched sham controls; # p<0.05 vs. time-matched dextrose groups; $ p<0.05 vs. dextrose/TBI group, by two-way analysis of variance.
Discussion
The role of AAI in modulating the outcome from TBI has been debated in clinical and animal studies. We examined the impact of AAI on neurological and neurobehavioral dysfunction and the associated neuroinflammatory changes resulting from TBI in a rodent model. Our results showed that TBI produced immediate apnea and a delayed righting reflex with accompanying significant neurological and neurobehavioral dysfunction observed at 6 h and 24 h after TBI. In addition, TBI induced localized and transient neuroinflammation in the ipsilateral cortex. AAI at the time of injury did not aggravate the TBI-induced neurological and neurobehavioral impairments but significantly enhanced IL-6, TNFα, IL-1β, and MCP-1 mRNA expression at 24 h post-injury. These findings suggest that significantly impaired resolution of neuroinflammation is not paralleled by exacerbation of neurological and neurobehavioral dysfunction during the early period post-mild TBI.
Our evaluation of the immediate post-TBI recovery demonstrated that AAI at the time of injury did not alter the apnea duration but significantly delayed the righting reflex, a measure of transient unconsciousness. These findings are in agreement with those of previous studies reporting that AAI prolonged post-traumatic righting reflex.66 The BAL achieved in this study as a result of a 15 h continuous intragastric alcohol infusion was 266±10 mg/dL, which provided moderately intoxicating but not debilitating behavioral effects. Possible physiological responses that occur with highly intoxicating BAL at the time of injury include impaired respiratory control,15 increased concentrations of brain and cerebral venous blood lactate,67 increased hemodynamic and respiratory depression,16 increased volume of cytotoxic brain edema,17 and increased mortality.68 In previous porcine and murine TBI studies, the combination of AAI and TBI has been reported to result in increased apnea, impaired ventilation, and hypercapnic responses.15,16,66 No significant effect of AAI on apnea duration, however, was observed in our study, possibly reflecting a species difference and/or a differential response to the severity of injury.
The injury force generated in our model was considered mild at ∼2 atm.15,16,66 Experimental studies using a controlled cortical impact injury model have suggested that increasing injury severity is associated with an increased incidence of apnea and is directly correlated with neuronal loss in the ipsilateral cortex, hippocampus, thalamus, and cerebellum.69 Therefore, the impact of AAI on apnea may be evident with greater severity of injury, leading to damage in subcortical structures and other brain regions such as the cerebellum. Nevertheless, alcohol exposure can affect central nervous system (CNS) and cause amnesia and/or sedation through the modulation of synaptic efficacy and disruption of the neurotransmitter systems.70–73 AAI decreases the excitatory glutamate transmission and increases the gamma-amino butyric acid-mediated tonic inhibition.71,72,74–78 In the event of TBI, the sedative effect of alcohol can exacerbate neurodepression, which may explain our observed delay in righting reflex post-TBI in our study.
In addition to analyzing normal cognitive performance and neurological functions, animal behavioral tests have been routinely performed to examine the effect of alcohol and TBI. One study observed reduced cognitive deficits, determined by water maze and memory probe tests, 7 days after TBI in alcohol-pretreated rats.18 The cognitive difference observed in this study was produced after an acute oral administration of low-dose alcohol, achieving a BAL less than 100 mg/dL.18 In contrast, higher blood alcohol levels achieved in our study (BAL >200 mg/dL) and earlier time points (6 and 24 h) at which we examined outcomes showed significant motor, cognitive, and neurobehavioral dysfunction associated with both dextrose/TBI and AAI/TBI groups. Whether alcohol could lead to long-term alterations in neurological and neurobehavioral bfunction after TBI remains to be investigated.
Multiple studies have suggested that variations in the degree of alcohol intoxication can lead to inconsistent TBI outcomes. This is supported by a study in which three different doses (low 1g/kg, moderate 2.5 g/kg, and high 3 g/kg) of intraperitoneal alcohol injection resulted in paradoxical outcomes after cortical contusion injury in rats.19 The injured rats receiving low or moderate doses of alcohol had significantly less impairment in beam-walking ability compared with the control or high-dose alcohol group. In addition, the low or moderate alcohol dose group had morphologically smaller brain lesion volumes compared with other groups.19 The improvement in functional recovery seen in the low-dose alcohol model is suggested to result from the blockade of N-methyl-D-aspartate receptor-mediated excitotoxicity by alcohol.79,80 This neuroprotective effect of alcohol is lost, however, at higher BAL.19
Consistent with these findings, the intoxicating level of BAL achieved in our study did not reverse neurological or neurobehavioral impairment after TBI. This indicates that the deleterious effect of alcohol predominates at higher concentrations, and therefore higher degrees of alcohol intoxication do not improve cognition, motor, and behavioral dysfunction after TBI.
The important roles of proinflammatory cytokines and chemokines produced in the CNS in contributing to the pathophysiological sequelae and adverse outcomes of TBI have been extensively documented.21,23,25,81 TBI induces an immediate upregulation of proinflammatory cytokines and chemokines, and the most studied mediators include IL-6, TNF-α, MCP-1, and IL-1β.82–84 A previous study demonstrated a marked increase in IL-1β and TNF-α in the injured cortex of animals receiving a cortical impact injury. In addition, oral alcohol pre-treatment (1.5 or 3.0 g/kg) 1 h before injury attenuated the cytokine levels in a dose-dependent manner when examined 4 h post-TBI.84 Similarly, a recent study demonstrated an upregulation of IL-6, keratinocyte chemoattractant (KC) MCP-1, and MIP-1α levels within 18 h post-closed head injury in mice, and a single 5 g/kg dose of 32% (vol/vol) intragastric alcohol administration 1 h before TBI reduced these inflammatory mediators.66
Our early observation at 6 h post-TBI provides support to these findings by indicating a marked increase of IL-6 and MCP-1 mRNA expression in the ipsilateral cortex of dextrose-treated animals. The inflammation was localized, because no significant increases of these mediators were observed in the contralateral or prefrontal region (data not shown). Consistent with others, our findings confirmed that AAI at the time of TBI did not exacerbate proinflammatory cytokine and chemokine expression at 6 h after TBI. This early time point was based on previous reports that indicated 4–8 h post-TBI to be the time window for the peak elevation of cerebral cortical levels of early proinflammatory cytokines and chemokines.85,86 In addition, our data showed that the early upregulation of cytokine and chemokine expression led to massive neutrophil infiltration to the injury site as detected by ∼10 fold increase of MPO activity at 24 h after TBI in both dextrose and AAI animals.
The overall impact of AAI on secondary brain injury cannot be concluded from a single observation at an early time point post-TBI. Insufficient animal models have examined the effect of AAI on the expression of proinflammatory mediators at a time point beyond their normal peak induction. Therefore, we examined the expression of IL-6, TNF-α, MCP-1, and IL-1β at 24 h after TBI. This allowed us to better understand the impact of AAI on TBI at a time-point beyond that reported by other investigators but still within the clinically relevant time frame for patient management.87 Our results demonstrated a sustained elevation of pro-inflammatory cytokine and chemokine expression in AAI animals compared with dextrose-treated animals at 24 h post-TBI. This suggests that alcohol intoxication may be associated with a delayed rise of proinflammatory mediators and/or an impaired resolution of neuroinflammation.
The implications of prolonged neuroinflammation are significant, because enhanced oxidative damage, disruption of blood-brain barrier, and additional biochemical cascades leading to neuronal death have been described.21,23,83,88–91 The effect of alcohol exposure and enhanced neuroinflammation on long-term recovery from TBI remains to be investigated and is the focus of current studies in our laboratory.
Taken together, these results indicate that AAI at the time of injury leads to dissociation between neuroinflammation and behavioral outcomes after TBI. Intoxicated TBI victims may not have additional neurological and neurobehavioral impairment as a result of AAI in the short-term after injury. The presence of BAL, however, at the time of injury may result in sustained neuroinflammatory milieu. The clinical implications of enhanced neuroinflammation for long-term recovery in the intoxicated TBI victims warrant further investigation. Moreover, post-TBI management in AAI persons may necessitate particular attention to events or behaviors that can exacerbate inflammation and oxidative injury to prevent the possibility of neurodegenerative changes post-TBI.
Acknowledgments
The authors would like to thank Renata Impastato, Curtis Vande Stouwe, and Emily Rogers for their technical assistance, as well as Drs. Peter Winsauer, Nicholas Gilpin, Kathleen McDonough, Chu Chen, Janet Rossi, Paige Katz, Jesse Sulzer, Nicole LeCapitaine, and Robert Siggins for their scientific guidance and discussions.
This research was supported by grants NIAAA-AA7577 and LEQSF-EPS(2012)-PFUND-283.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Available at: http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf Accessed November18, 2013 [Google Scholar]
- 2.French L.M. (2009). TBI in the military. Preface. J. Head Trauma Rehabil. 24, 1–3 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC), N.C.f.I.P.a.C (2003). Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Available at: http://www.cdc.gov/ncipc/pub-res/mtbi/mtbireport.pdf Accessed November18, 2013 [Google Scholar]
- 4.Pellman E.J., Powell J.W., Viano D.C., Casson I.R., Tucker A.M., Feuer H., Lovell M., Waeckerle J.F. and Robertson D.W. (2004). Concussion in professional football: epidemiological features of game injuries and review of the literatur—part 3. Neurosurgery 54, 81–96 [DOI] [PubMed] [Google Scholar]
- 5.CDC (2012) Vital signs: binge drinking prevalence, frequency, and intensity among adults—United States, 2010. MMWR Morb Mortal Wkly Rep 61, 14–19 [PubMed] [Google Scholar]
- 6.Corrigan J.D. (1995). Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch. Phys. Med. Rehabil. 76, 302–309 [DOI] [PubMed] [Google Scholar]
- 7.Hurst P.M., Harte D., and Frith W.J. (1994). The Grand Rapids dip revisited. Accid. Anal. Prev. 26, 647–654 [DOI] [PubMed] [Google Scholar]
- 8.Brooks N., Symington C., Beattie A., Campsie L., Bryden J., and McKinlay W. (1989). Alcohol and other predictors of cognitive recovery after severe head injury. Brain Inj. 3, 235–246 [DOI] [PubMed] [Google Scholar]
- 9.Kraus J.F., Morgenstern H., Fife D., Conroy C., and Nourjah P. (1989). Blood alcohol tests, prevalence of involvement, and outcomes following brain injury. Am. J. Public Health 79, 294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luna G.K, Maier R.V., Sowder L., Copass M.K., and Oreskovich M.R. (1984). The influence of ethanol intoxication on outcome of injured motorcyclists. J. Trauma 24, 695–700 [DOI] [PubMed] [Google Scholar]
- 11.Waller P.F., Stewart J.R., Hansen A.R., Stutts J.C., Popkin C.L., and Rodgman E.A. (1986). The potentiating effects of alcohol on driver injury. JAMA 256, 1461–1466 [PubMed] [Google Scholar]
- 12.Ward R.E., Flynn T.C., Miller P.W., and Blaisdell W.F. (1982). Effects of ethanol ingestion on the severity and outcome of trauma. Am. J. Surg. 144, 153–157 [DOI] [PubMed] [Google Scholar]
- 13.Ponsford J., Tweedly L., and Taffe J. (2013). The relationship between alcohol and cognitive functioning following traumatic brain injury. J. Clin. Exp. Neuropsychol. 35, 1–3–112 [DOI] [PubMed] [Google Scholar]
- 14.Chen C.M., Yi H.Y., Yoon Y.H., and Dong C. (2012). Alcohol use at time of injury and survival following traumatic brain injury: results from the National Trauma Data Bank. J. Stud. Alcohol Drugs 73, 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zink B.J., and Feustel P.J. (1995). Effects of ethanol on respiratory function in traumatic brain injury. J. Neurosurg. 82, 822–828 [DOI] [PubMed] [Google Scholar]
- 16.Zink B.J., Walsh R.F., and Feustel P.J. (1993). Effects of ethanol in traumatic brain injury. J. Neurotrauma 10, 275–286 [DOI] [PubMed] [Google Scholar]
- 17.Katada R., Nishitani Y., Honmou O., Okazaki S., Houkin K., and Matsumoto H. (2009). Prior ethanol injection promotes brain edema after traumatic brain injury. J. Neurotrauma 26, 2015–2025 [DOI] [PubMed] [Google Scholar]
- 18.Janis L.S., Hoane M.R., Conde D., Fulop Z., and Stein D.G. (1998). Acute ethanol administration reduces the cognitive deficits associated with traumatic brain injury in rats. J. Neurotrauma 15, 105–115 [DOI] [PubMed] [Google Scholar]
- 19.Kelly D.F., Lee S.M., Pinanong P.A., and Hovda D.A. (1997). Paradoxical effects of acute ethanolism in experimental brain injury. J. Neurosurg. 86, 876–882 [DOI] [PubMed] [Google Scholar]
- 20.Wang T., Chou D.Y., Ding J.Y., Fredrickson V., Peng C., Schafer S., Guthikonda M., Kreipke C., Rafols J.A., and Ding Y. (2013). Reduction of brain edema and expression of aquaporins with acute ethanol treatment after traumatic brain injury. J. Neurosurg. 118, 390–396 [DOI] [PubMed] [Google Scholar]
- 21.Arvin B., Neville L.F., Barone F.C., and Feuerstein G.Z. (1996). The role of inflammation and cytokines in brain injury. Neurosci. Biobehav. Rev. 20, 445–452 [DOI] [PubMed] [Google Scholar]
- 22.Faden A.I. (1993). Experimental neurobiology of central nervous system trauma. Crit. Rev. Neurobiol. 7, 175–186 [PubMed] [Google Scholar]
- 23.Feuerstein G.Z., Wang X., and Barone F.C. (1998). The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation 5, 143–159 [DOI] [PubMed] [Google Scholar]
- 24.Ziebell J.M., Bye N., Semple B.D., Kossmann T., and Morganti-Kossmann M.C. (2011). Attenuated neurological deficit, cell death and lesion volume in Fas-mutant mice is associated with altered neuroinflammation following traumatic brain injury. Brain Res. 1414, 94–105 [DOI] [PubMed] [Google Scholar]
- 25.Helmy A., Carpenter K.L., Menon D.K., Pickard J.D., and Hutchinson P.J. (2011). The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J. Cereb. Blood Flow Metab. 31, 658–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crews F.T., Bechara R., Brown L.A., Guidot D.M., Mandrekar P., Oak S., Qin L., Szabo G., Wheeler M., and Zou J. (2006). Cytokines and alcohol. Alcohol Clin. Exp. Res. 30, 720–730 [DOI] [PubMed] [Google Scholar]
- 27.Crews F., Nixon K., Kim D., Joseph J., Shukitt-Hale B., Qin L., and Zou J. (2006). BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin. Exp. Res. 30, 1938–1949 [DOI] [PubMed] [Google Scholar]
- 28.Pandey S.C., Chartoff E.H., Carlezon W.A., Jr., Zou J., Zhang H., Kreibich A.S., Blendy J.A., and Crews F.T. (2005). CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcohol Clin. Exp. Res. 29, 176–184 [DOI] [PubMed] [Google Scholar]
- 29.Crews F.T., and Braun C.J. (2003). Binge ethanol treatment causes greater brain damage in alcohol-preferring P rats than in alcohol-nonpreferring NP rats. Alcohol Clin. Exp. Res. 27, 1075–1082 [DOI] [PubMed] [Google Scholar]
- 30.Obernier J.A., White A.M., Swartzwelder H.S., and Crews F.T. (2002). Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol. Biochem. Behav. 72, 521–532 [DOI] [PubMed] [Google Scholar]
- 31.Nixon K., and Crews F.T. (2002). Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J. Neurochem. 83, 1087–1093 [DOI] [PubMed] [Google Scholar]
- 32.Obernier J.A., Bouldin T.W., and Crews F.T. (2002). Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin. Exp. Res. 26, 547–557 [PubMed] [Google Scholar]
- 33.Crews F.T., Braun C.J., Hoplight B., Switzer R.C., , 3rd, and Knapp D.J. (2000). Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin. Exp. Res. 24, 1712–1723 [PubMed] [Google Scholar]
- 34.Molina P.E., Happel K.I., Zhang P., Kolls J.K., and Nelson S. (2010). Focus on: alcohol and the immune system. Alcohol Res. Health 33, 97–108 [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P., Bagby G.J., Happel K.I., Summer W.R., and Nelson S. (2002). Pulmonary host defenses and alcohol. Front. Biosci 7, d1314–1330 [DOI] [PubMed] [Google Scholar]
- 36.Zhang P., Bagby G.J., Boe D.M., Zhong Q., Schwarzenberger P., Kolls J.K., Summer W.R., and Nelson S. (2002). Acute alcohol intoxication suppresses the CXC chemokine response during endotoxemia. Alcohol Clin. Exp. Res. 26, 65–73 [PubMed] [Google Scholar]
- 37.Szabo G. (1998). Monocytes, alcohol use, and altered immunity. Alcohol Clin. Exp. Res. 22, Suppl 5, 216S–219S [DOI] [PubMed] [Google Scholar]
- 38.Standiford T.J., and Danforth J.M. (1997). Ethanol feeding inhibits proinflammatory cytokine expression from murine alveolar macrophages ex vivo. Alcohol Clin. Exp. Res. 21, 1212–1217 [PubMed] [Google Scholar]
- 39.Zambell K.L., Phelan H., Vande Stouwe C., Zhang P., Shellito J.E., and Molina P.E. (2004). Acute alcohol intoxication during hemorrhagic shock: impact on host defense from infection. Alcohol Clin. Exp. Res. 28, 635–642 [DOI] [PubMed] [Google Scholar]
- 40.Shellito J.E. (1998). Alcohol and host defense against pulmonary infection with Pneumocystis carinii. Alcohol Clin. Exp. Res. 22, Suppl 5, 208S-211S [DOI] [PubMed] [Google Scholar]
- 41.Shellito J.E., and Olariu R. (1998). Alcohol decreases T-lymphocyte migration into lung tissue in response to Pneumocystis carinii and depletes T-lymphocyte numbers in the spleens of mice. Alcohol Clin. Exp. Res. 22, 658–663 [DOI] [PubMed] [Google Scholar]
- 42.Patel M., Keshavarzian A., Kottapalli V., Badie B., Winship D., and Fields J.Z. (1996). Human neutrophil functions are inhibited in vitro by clinically relevant ethanol concentrations. Alcohol Clin. Exp. Res. 20, 275–283 [DOI] [PubMed] [Google Scholar]
- 43.D'Souza N.B., Bagby G.J., Nelson S., Lang C.H., and Spitzer J.J. (1989). Acute alcohol infusion suppresses endotoxin-induced serum tumor necrosis factor. 13, 295–298 [DOI] [PubMed] [Google Scholar]
- 44.Arbabi S., Garcia I., Bauer G.J., and Maier R.V. (1999). Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J. Immunol. 162, 7441–7445 [PubMed] [Google Scholar]
- 45.Molina P.E., Sulzer J.K., and Whitaker A.M. (2013). Alcohol abuse and the injured host: dysregulation of counterregulatory mechanisms review. Shock 39, 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greiffenstein P., and Molina P.E. (2008). Alcohol-induced alterations on host defense after traumatic injury. J. Trauma 64, 230–240 [DOI] [PubMed] [Google Scholar]
- 47.Greiffenstein P., Mathis K.W., Stouwe C.V., and Molina P.E. (2007). Alcohol binge before trauma/hemorrhage impairs integrity of host defense mechanisms during recovery. Alcohol Clin. Exp. Res. 31, 704–715 [DOI] [PubMed] [Google Scholar]
- 48.Mathis K.W., Zambell K., Olubadewo J.O., and Molina P.E. (2006). Altered hemodynamic counter-regulation to hemorrhage by acute moderate alcohol intoxication. Shock 26, 55–61 [DOI] [PubMed] [Google Scholar]
- 49.Molina P.E., Zambell K.L., Norenberg K., Eason J., Phelan H., Zhang P., Stouwe C.V., Carnal J.W., and Porreta C. (2004). Consequences of alcohol-induced early dysregulation of responses to trauma/hemorrhage. Alcohol 33, 217–227 [DOI] [PubMed] [Google Scholar]
- 50.Molina P.E., Hoek J.B., Nelson S., Guidot D.M., Lang C.H., Wands J.R., and Crawford J.M. (2003). Mechanisms of alcohol-induced tissue injury. Alcohol Clin. Exp. Res. 27, 563–575 [DOI] [PubMed] [Google Scholar]
- 51.Phelan H., Stahls P., Hunt J., Bagby G.J. and Molina P.E. (2002). Impact of alcohol intoxication on hemodynamic, metabolic, and cytokine responses to hemorrhagic shock. J. Trauma 52, 675–682 [DOI] [PubMed] [Google Scholar]
- 52.Molina P.E., McClain C., Valla D., Guidot D., Diehl A.M., Lang C.H., and Neuman M. (2002). Molecular pathology and clinical aspects of alcohol-induced tissue injury. Alcohol Clin. Exp. Res. 26, 120–128 [PubMed] [Google Scholar]
- 53.Molina P.E., and Abumrad N.N. (2000). Differential effects of hemorrhage and LPS on tissue TNF-alpha, IL-1 and associate neuro-hormonal and opioid alterations. Life Sci. 66, 399–409 [DOI] [PubMed] [Google Scholar]
- 54.Sulzer J.K., Whitaker A.M., and Molina P.E. (2013). Hypertonic saline resuscitation enhances blood pressure recovery and decreases organ injury following hemorrhage in acute alcohol intoxicated rodents. J. tTrauma Acute Care Surg 74, 196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitaker A.M., Sulzer J.K., and Molina P.E. (2011). Augmented central nitric oxide production inhibits vasopressin release during hemorrhage in acute alcohol-intoxicated rodents. Am J. Physiol. Regul. Integr. Comp. Physiol. 301, R1529–R1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sulzer J.K., and Molina P.E. (2011). Delayed resuscitation with physostigmine increases end organ damage in alcohol intoxicated rats. Shock 35, 74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naimi T.S., Brewer R.D., Mokdad A., Denny C., Serdula M.K., and Marks J.S. (2003). Binge drinking among US adults. JAMA 289, 70–75 [DOI] [PubMed] [Google Scholar]
- 58.Alcoholism N.I.o.A.A.a. (2004). NIAAA council approves definition of binge drinking. NIAAA Newsletter 2004; No. 3, p. 3 [Google Scholar]
- 59.Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou A., Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 60.Thompson H.J., Lifshitz J., Marklund N., Grady M.S., Graham D.I., Hovda D.A., and McIntosh T.K. (2005). Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75 [DOI] [PubMed] [Google Scholar]
- 61.McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 62.Boyko M., Ohayon S., Goldsmith T., Novack L., Novack V., Perry Z.H., Gruenbaum B.F., Gruenbaum S.E., Steiner O., Shapira Y., Teichberg V.I., and Zlotnik A. (2011). Morphological and neuro-behavioral parallels in the rat model of stroke. Behav. Brain Res. 223, 17–23 [DOI] [PubMed] [Google Scholar]
- 63.Ling G.S., Lee E.Y., and Kalehua A.N. (2004). Traumatic brain injury in the rat using the fluid-percussion model. Curr. Protoc. Neurosci. Chapter 9, Unit9.2 [DOI] [PubMed] [Google Scholar]
- 64.Schneider B.S., and Tiidus P.M. (2007). Neutrophil infiltration in exercise-injured skeletal muscle: how do we resolve the controversy? Sports Med. 37, 837–856 [DOI] [PubMed] [Google Scholar]
- 65.de Jonge H.J., Fehrmann R.S., de Bont E.S., Hofstra R.M., Gerbens F., Kamps W.A., de Vries E.G., van der Zee A.G., te Meerman G.J., and ter Elst A. (2007). Evidence based selection of housekeeping genes. PloS One 2, e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodman M.D., Makley A.T., Campion E.M., Friend L.A., Lentsch A.B., and Pritts T.A. (2013). Preinjury alcohol exposure attenuates the neuroinflammatory response to traumatic brain injury. J. Surg. Res. 184, 1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zink B.J., Schultz C.H., Wang X., Mertz M., Stern S.A. and Betz A.L. (1999). Effects of ethanol on brain lactate in experimental traumatic brain injury with hemorrhagic shock. Brain Res. 837, 1–7 [DOI] [PubMed] [Google Scholar]
- 68.Yamakami I., Vink R., Faden A.I., Gennarelli T.A., Lenkinski R., and McIntosh T.K. (1995). Effects of acute ethanol intoxication on experimental brain injury in the rat: neurobehavioral and phosphorus-31 nuclear magnetic resonance spectroscopy studies. J. Neurosurg. 82, 813–821 [DOI] [PubMed] [Google Scholar]
- 69.Igarashi T., Potts M.B., and Noble-Haeusslein L.J. (2007). Injury severity determines Purkinje cell loss and microglial activation in the cerebellum after cortical contusion injury. Exp. Neurol. 203, 258–268 [DOI] [PubMed] [Google Scholar]
- 70.Wakita M., Shin M.C., Iwata S., Nonaka K., and Akaike N. (2012). Effects of ethanol on GABA(A) receptors in GABAergic and glutamatergic presynaptic nerve terminals. J. Pharmacol. Exp. Ther. 341, 809–819 [DOI] [PubMed] [Google Scholar]
- 71.Koob G.F., and Volkow N.D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzales R.A., and Jaworski J.N. (1997). Alcohol and glutamate. Alcohol Health Res. World 21, 120–127 [PMC free article] [PubMed] [Google Scholar]
- 73.Gonzales R.A. (1990). NMDA receptors excite alcohol research. Trends Pharmacol. Sci. 11, 137–139 [DOI] [PubMed] [Google Scholar]
- 74.Hirono M., Yamada M., and Obata K. (2009). Ethanol enhances both action potential-dependent and action potential-independent GABAergic transmission onto cerebellar Purkinje cells. Neuropharmacology 57, 109–120 [DOI] [PubMed] [Google Scholar]
- 75.Santhakumar V., Wallner M., and Otis T.S. (2007). Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol 41, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryabinin A.E. (1998). Role of hippocampus in alcohol-induced memory impairment: implications from behavioral and immediate early gene studies. Psychopharmacology (Berl)139, 34–43 [DOI] [PubMed] [Google Scholar]
- 77.Mihic S.J., Ye Q., Wick M.J., Koltchine V.V., Krasowski M.D., Finn S.E., Mascia M.P., Valenzuela C.F., Hanson K.K., Greenblatt E.P., Harris R.A., and Harrison N.L. (1997). Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389, 385–389 [DOI] [PubMed] [Google Scholar]
- 78.Mihic S.J., and Harris R.A. (1997). GABA and the GABAA receptor. Alcohol Health Res. world 21, 127–131 [PMC free article] [PubMed] [Google Scholar]
- 79.Chandler L.J., Sumners C., and Crews F.T. (1993). Ethanol inhibits NMDA receptor-mediated excitotoxicity in rat primary neuronal cultures. Alcohol Clin. Exp. Res. 17, 54–60 [DOI] [PubMed] [Google Scholar]
- 80.Takadera T., Suzuki R., and Mohri T. (1990). Protection by ethanol of cortical neurons from N-methyl-D-aspartate-induced neurotoxicity is associated with blocking calcium influx. Brain Res. 537, 109–114 [DOI] [PubMed] [Google Scholar]
- 81.Tehranian R., Andell-Jonsson S., Beni S.M., Yatsiv I., Shohami E., Bartfai T., Lundkvist J., and Iverfeldt K. (2002). Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J. Neurotrauma 19, 939–951 [DOI] [PubMed] [Google Scholar]
- 82.Lenzlinger P.M., Morganti-Kossmann M.C., Laurer H.L., and McIntosh T.K. (2001). The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 24, 169–181 [DOI] [PubMed] [Google Scholar]
- 83.Lucas S.M., Rothwell N.J., and Gibson R.M. (2006). The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 147, Suppl 1, S232–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gottesfeld Z., Moore A.N., and Dash P.K. (2002). Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J. Neurotrauma 19, 317–326 [DOI] [PubMed] [Google Scholar]
- 85.Shohami E., Novikov M., Bass R., Yamin A., and Gallily R. (1994). Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J. Cereb. Blood Flow Metab. 14, 615–619 [DOI] [PubMed] [Google Scholar]
- 86.Knoblach S.M., Fan L., and Faden A.I. (1999). Early neuronal expression of tumor necrosis factor-alpha after experimental brain injury contributes to neurological impairment. J. Neuroimmunol. 95, 115–125 [DOI] [PubMed] [Google Scholar]
- 87.Green J.A., Pellegrini D.C., Vanderkolk W.E., Figueroa B.E., and Eriksson E.A. (2013). Goal directed brain tissue oxygen monitoring versus conventional management in traumatic brain injury: an analysis of in hospital recovery. Neurocrit. Care 18, 20–25 [DOI] [PubMed] [Google Scholar]
- 88.Venters H.D., Dantzer R., and Kelley K.W. (2000). A new concept in neurodegeneration: TNFalpha is a silencer of survival signals. Trends Neurosci. 23, 175–180 [DOI] [PubMed] [Google Scholar]
- 89.Abdul-Muneer P.M., Schuetz H., Wang F., Skotak M., Jones J., Gorantla S., Zimmerman M.C., Chandra N., and Haorah J. (2013). Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 60, 282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmad A., Crupi R., Campolo M., Genovese T., Esposito E., and Cuzzocrea S. (2013). Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PloS One 8, e57208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Campolo M., Ahmad A., Crupi R., Impellizzeri D., Morabito R., Esposito E., and Cuzzocrea S. (2013). Combination therapy with melatonin and dexamethasone in a mouse model of traumatic brain injury. J Endocrinol 217, 291–301 [DOI] [PubMed] [Google Scholar]