Abstract
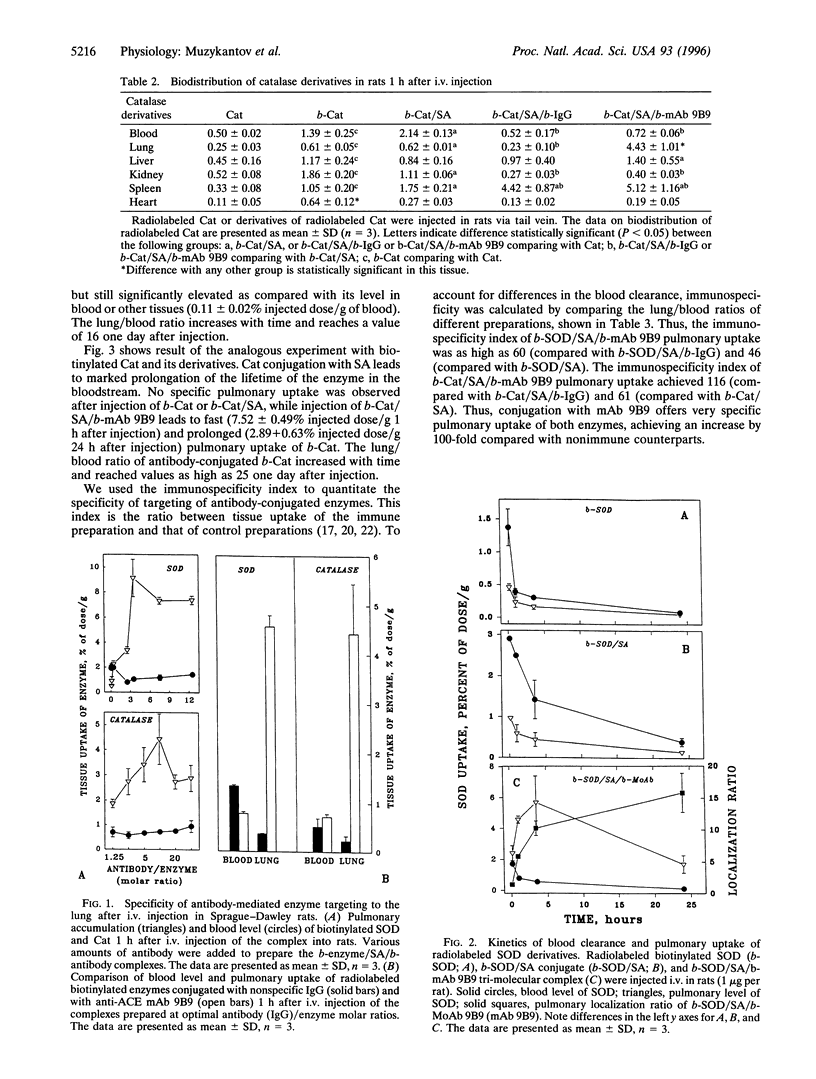
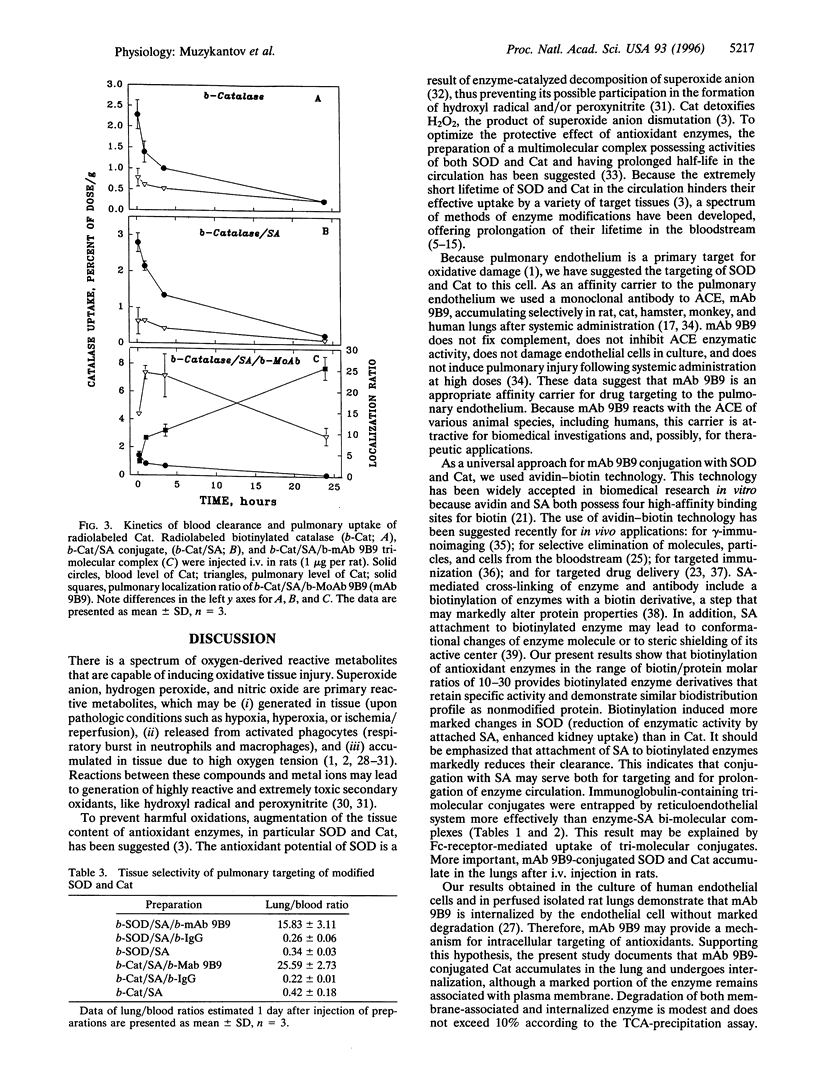
Oxidative injury to the pulmonary endothelium has pathological significance for a spectrum of diseases. Administration of antioxidant enzymes, superoxide dismutase (SOD) and catalase (Cat), has been proposed as a method to protect endothelium. However, neither these enzymes nor their derivatives possess specific affinity to endothelium and do not accumulate in the lung. Previously we have described a monoclonal antibody to angiotensin-converting enzyme (ACE) that accumulates selectively in the lung after systemic injection in rats, hamsters, cats, monkeys, and humans. In the present work we describe a system for selective intrapulmonary delivery of CuZn-SOD and Cat conjugated with biotinylated anti-ACE antibody mAb 9B9 (b-mAb 9B9) by a streptavidin (SA)-biotin bridge. Both enzymes biotinylated with biotin ester at biotin/enzyme ratio 20 retain enzymatic activity and bind SA without loss of activity. We have constructed tri-molecular heteropolymer complexes consisting of b-mAb 9B9, SA, and biotinylated SOD or biotinylated Cat and have studied biodistribution and pulmonary uptake of these complexes in the rat after i.v. injection. Biodistribution of biotinylated enzymes was similar to that of nonmodified enzymes. Binding of SA markedly prolonged lifetime of biotinylated enzymes in the circulation. In contrast to enzymes conjugated with nonspecific IgG, other enzyme derivatives, and nonmodified enzymes, biotinylated enzymes conjugated with b-mAb 9B9 accumulated specifically in the rat lung (9% of injected SOD/g of lung tissue and 7.5% of injected Cat/g of lung tissue). Pulmonary uptake of nonmodified enzymes or derivatives with nonspecific IgG did not exceed 0.5% of injected dose/g. Both SOD and Cat conjugated with b-mAb 9B9 were retained in the rat lung for at least several hours. Trichloracetic acid-precipitable radiolabeled Cat was associated with microsomal and plasma membrane fractions of the lung tissue homogenate. Thus, modification of antioxidant enzymes with biotin and SA-mediated conjugation with b-mAb 9B9 prolongs the circulation of enzymes resulting in selective accumulation in the lung and intracellular delivery of enzymes to the pulmonary endothelium. These results provide the background for an approach to provide protection of pulmonary endothelium against oxidative insults.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., McCoy J. R., Palczuk N. C., van Es T., Davis F. F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977 Jun 10;252(11):3582–3586. [PubMed] [Google Scholar]
- Abuchowski A., van Es T., Palczuk N. C., Davis F. F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977 Jun 10;252(11):3578–3581. [PubMed] [Google Scholar]
- Barnard M. L., Baker R. R., Matalon S. Mitigation of oxidant injury to lung microvasculature by intratracheal instillation of antioxidant enzymes. Am J Physiol. 1993 Oct;265(4 Pt 1):L340–L345. doi: 10.1152/ajplung.1993.265.4.L340. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Grootjans J. J., Alon R., Wilchek M. Affinity cleavage and targeted catalysis of proteins using the avidin-biotin system. Biochemistry. 1990 Dec 25;29(51):11274–11279. doi: 10.1021/bi00503a017. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Minor R. L., Jr, White C. W., Repine J. E., Rosen G. M., Freeman B. A. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988 May 15;263(14):6884–6892. [PubMed] [Google Scholar]
- Danilov S. M., Muzykantov V. R., Martynov A. V., Atochina E. N., Sakharov IYu, Trakht I. N., Smirnov V. N. Lung is the target organ for a monoclonal antibody to angiotensin-converting enzyme. Lab Invest. 1991 Jan;64(1):118–124. [PubMed] [Google Scholar]
- Danilov S., Atochina E., Hiemisch H., Churak-ova T., Moldobayeva A., Sakharov I., Deichman G., Ryan U., Muzykantov V. R. Interaction of mAb to angiotensin-converting enzyme (ACE) with antigen in vitro and in vivo: antibody targeting to the lung induces ACE antigenic modulation. Int Immunol. 1994 Aug;6(8):1153–1160. doi: 10.1093/intimm/6.8.1153. [DOI] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. The angiotensin I-converting enzyme. Lab Invest. 1987 Apr;56(4):345–348. [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fisher A. B., Dodia C., Chander A. Alveolar uptake of lipid and protein components of surfactant. Am J Physiol. 1991 Oct;261(4 Pt 1):L334–L340. doi: 10.1152/ajplung.1991.261.4.L334. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Dodia C., Tan Z. T., Ayene I., Eckenhoff R. G. Oxygen-dependent lipid peroxidation during lung ischemia. J Clin Invest. 1991 Aug;88(2):674–679. doi: 10.1172/JCI115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Young S. L., Crapo J. D. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983 Oct 25;258(20):12534–12542. [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Fujita T., Nishikawa M., Tamaki C., Takakura Y., Hashida M., Sezaki H. Targeted delivery of human recombinant superoxide dismutase by chemical modification with mono- and polysaccharide derivatives. J Pharmacol Exp Ther. 1992 Dec;263(3):971–978. [PubMed] [Google Scholar]
- Greenwald R. A. Superoxide dismutase and catalase as therapeutic agents for human diseases. A critical review. Free Radic Biol Med. 1990;8(2):201–209. doi: 10.1016/0891-5849(90)90092-w. [DOI] [PubMed] [Google Scholar]
- Hallewell R. A., Laria I., Tabrizi A., Carlin G., Getzoff E. D., Tainer J. A., Cousens L. S., Mullenbach G. T. Genetically engineered polymers of human CuZn superoxide dismutase. Biochemistry and serum half-lives. J Biol Chem. 1989 Mar 25;264(9):5260–5268. [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Hnatowich D. J., Virzi F., Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987 Aug;28(8):1294–1302. [PubMed] [Google Scholar]
- Inoue M., Ebashi I., Watanabe N., Morino Y. Synthesis of a superoxide dismutase derivative that circulates bound to albumin and accumulates in tissues whose pH is decreased. Biochemistry. 1989 Aug 8;28(16):6619–6624. doi: 10.1021/bi00442a013. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Jokiranta T. S., Meri S. Biotinylation of monoclonal antibodies prevents their ability to activate the classical pathway of complement. J Immunol. 1993 Aug 15;151(4):2124–2131. [PubMed] [Google Scholar]
- Mao G. D., Thomas P. D., Lopaschuk G. D., Poznansky M. J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J Biol Chem. 1993 Jan 5;268(1):416–420. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Muzykantov V. R., Atochina E. N., Gavriljuk V., Danilov S. M., Fisher A. B. Immunotargeting of streptavidin to the pulmonary endothelium. J Nucl Med. 1994 Aug;35(8):1358–1365. [PubMed] [Google Scholar]
- Muzykantov V. R., Gavriluk V. D., Reinecke A., Atochina E. N., Kuo A., Barnathan E. S., Fisher A. B. The functional effects of biotinylation of anti-angiotensin-converting enzyme monoclonal antibody in terms of targeting in vivo. Anal Biochem. 1995 Apr 10;226(2):279–287. doi: 10.1006/abio.1995.1226. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R., Martynov A. V., Puchnina E. A., Danilov S. M. In vivo administration of glucose oxidase conjugated with monoclonal antibodies to angiotensin-converting enzyme. The tissue distribution, blood clearance, and targeting into rat lungs. Am Rev Respir Dis. 1989 Jun;139(6):1464–1473. doi: 10.1164/ajrccm/139.6.1464. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R., Sakharov D. V., Smirnov M. D., Samokhin G. P., Smirnov V. N. Immunotargeting of erythrocyte-bound streptokinase provides local lysis of a fibrin clot. Biochim Biophys Acta. 1986 Nov 19;884(2):355–362. doi: 10.1016/0304-4165(86)90184-4. [DOI] [PubMed] [Google Scholar]
- Nakazono K., Watanabe N., Matsuno K., Sasaki J., Sato T., Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury T. D., Piantadosi C. A., Crapo J. D. Cold-induced brain edema in mice. Involvement of extracellular superoxide dismutase and nitric oxide. J Biol Chem. 1993 Jul 25;268(21):15394–15398. [PubMed] [Google Scholar]
- Sakharov D. V., Muzykantov V. R., Domogatsky S. P., Danilov S. M. Protection of cultured endothelial cells from hydrogen peroxide-induced injury by antibody-conjugated catalase. Biochim Biophys Acta. 1987 Sep 14;930(2):140–144. doi: 10.1016/0167-4889(87)90025-5. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., van den Berg W. B., van de Putte L. B., Joosten L. A., van den Bersselaar L. Cationization of catalase, peroxidase, and superoxide dismutase. Effect of improved intraarticular retention on experimental arthritis in mice. J Clin Invest. 1985 Jul;76(1):198–205. doi: 10.1172/JCI111946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I. U., Revak S. D., Cochrane C. G. Proteases and oxidants in experimental pulmonary inflammatory injury. J Clin Invest. 1984 Apr;73(4):1175–1184. doi: 10.1172/JCI111303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skea D. L., Barber B. H. Studies of the adjuvant-independent antibody response to immunotargeting. Target structure dependence, isotype distribution, and induction of long term memory. J Immunol. 1993 Oct 1;151(7):3557–3568. [PubMed] [Google Scholar]
- Smirnov V. N., Domogatsky S. P., Dolgov V. V., Hvatov V. B., Klibanov A. L., Koteliansky V. E., Muzykantov V. R., Repin V. S., Samokhin G. P., Shekhonin B. V. Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6603–6607. doi: 10.1073/pnas.83.17.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., White J. E., Gordon R. J., Lumb P. D., Tsan M. F. Polyethylene glycol-conjugated superoxide dismutase protects rats against oxygen toxicity. J Appl Physiol (1985) 1993 Mar;74(3):1425–1431. doi: 10.1152/jappl.1993.74.3.1425. [DOI] [PubMed] [Google Scholar]
- Taylor R. P., Reist C. J., Sutherland W. M., Otto A., Labuguen R. H., Wright E. L. In vivo binding and clearance of circulating antigen by bispecific heteropolymer-mediated binding to primate erythrocyte complement receptor. J Immunol. 1992 Apr 15;148(8):2462–2468. [PubMed] [Google Scholar]
- Tesfamariam B. Free radicals in diabetic endothelial cell dysfunction. Free Radic Biol Med. 1994 Mar;16(3):383–391. doi: 10.1016/0891-5849(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Crapo J. D., Freeman B. A. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest. 1984 Jan;73(1):87–95. doi: 10.1172/JCI111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. R., Brock T. A., Chang L. Y., Crapo J., Briscoe P., Ku D., Bradley W. A., Gianturco S. H., Gore J., Freeman B. A. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988 May 15;171(1):1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]


