Abstract
Despite extensive research, current glioma therapies are still unsatisfactory, and novel approaches are pressingly needed. In recent years, both nonreplicative viral vectors and replicating oncolytic viruses have been developed for brain cancer treatment, and the mechanistic background of their cytotoxicity has been unveiled. A growing number of clinical trials have convincingly established viral therapies to be safe in glioma patients, and maximum tolerated doses have generally not been reached. However, evidence for therapeutic benefit has been limited: new generations of therapeutic vectors need to be developed in order to target not only tumor cells but also the complex surrounding microenvironment. Such therapies could also direct long-lasting immune responses toward the tumor while reducing early antiviral reactions. Furthermore, viral delivery methods are to be improved and viral spread within the tumor will have to be enhanced. Here, we will review the outcome of completed glioma virus therapy trials as well as highlight the ongoing clinical activities. On this basis, we will give an overview of the numerous strategies to enhance therapeutic efficacy of new-generation viruses and novel treatment regimens. Finally, we will conclude with approaches that may be crucial to the development of successful glioma therapies in the future.
Keywords: gene therapy, glioblastoma, oncolysis
In 2013, primary malignant brain tumors and other CNS malignancies were estimated to be diagnosed in 23 130 people and to cause ∼14 080 deaths in the United States.1 Of particular concern is the rising incidence over the past 30 years as well as the wide spectrum of symptoms and complications that considerably influence patients' quality of life.2 Primary brain tumors can be further subdivided into a number of different tumor types, with World Health Organization grade III anaplastic astrocytomas (AAs) and grade IV glioblastoma multiforme (GBM) being the most common types in adults.2 GBM, known for its infiltrative growth and common expansion to both brain hemispheres, is also the most lethal CNS tumor, with 14.6 months median survival and a disillusioning 5-year survival rate as low as 2%.3 This dismal prognosis is in spite of aggressive treatment regimens, including extensive surgical resection, focal irradiation, and optimized chemotherapeutic regimens such as temozolomide.4 Unfortunately, extensive research improving diagnostics by molecular characterization5 and imaging6 in addition to surgical techniques7 and drug delivery methods8 have only slightly improved glioma therapies, and novel treatments effectively complementing the existing arsenal of drugs are pressingly needed.
Novel, increasingly appreciated treatment modalities are based on viruses with natural or engineered tropism and activity against tumors. The idea to utilize viruses for cancer therapy arose from case studies reporting remissions especially of leukemias and lymphomas that coincided with natural virus infections.9,10 However, concerns about serious side effects, technical problems regarding the purity of virus particle preparations, and the advent of chemotherapy halted early pursuits of virus therapy. Its potential was only appreciated toward the end of the twentieth century, supported by extensive gain of knowledge about viral molecular biology and by reverse genetics and recombination systems allowing for rational modification of viral genomes.11 Now, virus therapies are being exploited for many tumor entities, including gliomas, and can be further subdivided into 2 categories: (i) replication-deficient viral vectors, used as delivery vehicles for therapeutic genes with antitumor activities, such as local activation of chemotherapeutic prodrugs or recruitment and activation of immune cells by cytokines12; and (ii) replication-competent oncolytic viruses (OVs) that specifically infect and replicate in cancer cells, destroy their tumor cell hosts in the course of progeny particle release, and spread throughout the tumor. However, OV-induced tumor destruction is not mediated solely by oncolysis, but also by antitumor immune activation and disruption of tumor blood supply, as well as the activity of virally encoded therapeutic transgenes.13 Multiple viruses that employ these modalities of anticancer action have entered clinical trials for gliomas with the goal of establishing their safety and show preliminary signs of therapeutic efficacy. In this review, we will give an overview of completed and ongoing clinical trials, their achievements and failures. On that basis, we will highlight how these bedside experiences may be addressed in order to create new generations of virus therapies at the bench that may eventually have a decisive impact on this dreadful disease.
Clinical Experience With Glioma Gene Therapy—Transfer of Therapeutic Genes Is Safe
Classic gene therapeutic approaches utilize viruses to deliver transgenes of interest into a desired cell population, an approach that has been most widely used to reintroduce deleted or mutated genes in patients with hereditary monogenetic diseases, like blood coagulation factor VIII or IX deficiencies in hemophiliacs.14 The European Commission's recent market authorization of Glybera (alipogene tiparvovec), an adeno-associated viral vector substituting functional lipoprotein lipase in patients with inherited deficiency in this protein, proves that this strategy can be successful and fuels enthusiasm about establishing gene therapies for other diseases.15
In terms of cancer gene therapy, viral vectors deliver therapeutic transgenes that can mediate tumor cell killing directly or indirectly. The most extensively studied approach is based on the transduction of suicide genes, enzymes that can convert innocuous prodrugs into active chemotherapeutics. The most commonly used system employs herpes simplex virus type I thymidine kinase (HSV-TK), which activates ganciclovir (GCV) into its toxic nucleotide metabolites with very high selectivity for incorporation into DNA.16 As an additional advantage, the active GCV triphosphate can be transferred to neighboring nontransduced cells via gap junctions achieving considerable “bystander” cytotoxicity.17 As we discuss in more detail, though, additional data have also shown that HSV-TK can be very immunogenic, and this is likely another important mode of anticancer action. The HSV-TK system has been clinically investigated for its safety and efficacy in glioma treatment in several trials using retroviral or adenoviral vectors to achieve tumor-specific delivery of the transgene (Table 1). In a randomized, open-label phase III clinical trial for newly diagnosed, previously untreated GBM, Rainov18 injected an HSV-TK–expressing retroviral vector delivered by PA317 producer cells into the walls of the surgical cavity after gross resection of the tumor followed by i.v. GCV infusions over 14 days in an adjuvant setting to standard radiotherapy. Incidences of serious adverse events in the postoperative period were equal for the gene therapy and the control arm, in which patients received surgery and radiotherapy only. Recapitulating the results of earlier safety studies, no serious adverse events that could be linked to the gene therapy were noted.19–22 However, neither median survival (365 days vs 354 days) nor progression-free survival (180 days vs 183 days) was significantly improved in the gene therapy group compared with the standard-of-care control cohort.18 The investigator proposed that failure of the protocol was at least in part due to poor delivery of both vector-producing cells and the GCV prodrug.18
Table 1.
Completed glioma gene therapy trials
| Trial Phase | Disease | Virus and Effectors | Delivery and Dosing | Results | Median Survival, mo | Year [Reference] |
|---|---|---|---|---|---|---|
| III | Untreated GBM | Retroviral vector expressing HSV-TK delivered in vector-producing PA317 cells | 1 × 109 cells into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days + radiotherapy for 6 wk | No survival benefit compared with the control group | 12.0 | 2000 [18] |
| IIB | Operable primary or recurrent malignant glioma | Adenoviral vector expressing HSV-TK | 3 × 1010 pfu into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | Treatment well tolerated; clear survival benefit in comparison with historical and standard care control groups | 14.4 | 2004 [25] |
| I/II | Recurrent GBM | Retroviral vector expressing HSV-TK and IL-2 delivered in vector-producing PA317 cells | 3 × 108–109 cells i.t. or into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | No serious adverse events; evidence for tumor transduction as well as local and systemic type 1 T helper cell cytokine elevation | 7.5 | 2005 [36] |
| I/II | Progressive or recurrent GBM | Retroviral vector expressing HSV-TK delivered in vector-producing PA317 cells | 109 cells into resection cavity followed at day 7 by multiple cycles of 109 cells delivered by Ommaya reservoir + 5 mg/kg GCV i.v. b.i.d. for 14 days | 16/30 patients with serious adverse events | 8.4 | 2003 [153] |
| I/II | Progressive or recurrent malignant brain tumors | Retroviral vector expressing HSV-TK delivered in vector-producing PA317 cells | 2.5 × 108–109 cells into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | Limited gene transfer detected; responses only in small tumors | n.d. | 1999 [21] |
| I/II | Recurrent GBM | Retroviral vector expressing HSV-TK delivered in vector-producing PA317 cells | 1.5 × 108–3 × 108 cells into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | No serious adverse events | 8.6 | 1999 [20] |
| I/II | Recurrent GBM | Retroviral vector expressing HSV-TK delivered in vector-producing M11 cells | 8.7 × 106 cells/cm2 into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | No serious adverse events; 1/12 CR | 6.8 | 1998 [19] |
| IB | Primary malignant glioma | Adenoviral vector expressing HSV-TK | 3 × 1010–3 × 1011 vp into resection cavity + 2 g valacyclovir p.o. t.i.d. for 14 days + radiation + temozolomide | No dose-limiting toxicity observed; CD3 + T cell infiltration of the tumor | 12.4 | 2011 [26] |
| I | Recurrent or progressive malignant glioma | Adenoviral vector expressing human IFN-ß | 2 × 1010–2 × 1011 vp i.t. + 2 × 1010–2 × 1011 vp into resection cavity 4–8 days later | Well tolerated ≤6 × 1010 vp; apoptosis induction observed at highest dose; transgene expression in tumor | 4.1 | 2008 [34] |
| I | Poor prognosis brain tumors | Retroviral vector expressing MGMT | 1.2 × 109 ex vivo transduced CD34+ PBMCs i.v. + procarbazine/CCNU/vincristine chemotherapy | No treatment-associated serious adverse events: ex vivo manipulation of hematopoietic progenitor cells without influence on engraftment | 14.8 | 2006 [42] |
| I | Recurrent glioma | Adenoviral vector expressing p53 | 3 × 1010–3 × 1012 vp i.t. 3 days before surgery and intramural after resection | No maximum-tolerated dose reached; p53 expression and activity confirmed within 5 mm of injection site | 9.9 | 2003 [154] |
| I | Recurrent malignant glioma | Adenoviral vector expressing HSV-TK | 2.5 × 1011–9 × 1011 vp into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 7 days | No serious adverse events related to gene therapy; no virus shedding; no tissue toxicity | 12.0 | 2003 [155] |
| I | Recurrent malignant glioma | Adenoviral vector expressing HSV-TK | 4.5 × 108–4.6 × 1011 vp into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | Treatment well tolerated | 4.0 | 2003 [156] |
| I | Primary or recurrent malignant gliomas | Adenoviral vector expressing HSV-TK | 3 × 1010 pfu into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | No serious adverse events; no virus shedding | 15.0 | 2000 [23] |
| Retroviral vector expressing HSV-TK delivered in vector-producing PA317 cells | 109 cells into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | No serious adverse events; survival not different in comparison with control group | 7.4 | |||
| I | Recurrent malignant brain tumors | Adenoviral vector expressing HSV-TK | 2 × 109–2 × 1012 vp i.t. + 5 mg/kg GCV i.v. b.i.d. for 14 days | Well tolerated ≤2 × 1011 vp; no virus shedding | 4.5 | 2000 [157] |
| I | Progressive or recurrent pediatric malignant brain tumors | Retroviral vector expressing HSV-TK delivered in vector-producing PA317 cells | 108–109 cells into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | 4/12 patients with serious adverse effects; 1/11 SD | N/A | 2000 [158] |
| I | Recurrent GBM | Retroviral vector expressing HSV-TK and IL-2 delivered in vector-producing PA317 cells | 1.5 × 108–3 × 108 cells i.t. or into resection cavity + 5 mg/kg GCV i.v. b.i.d. for 14 days | Evidence for tumor transduction and transgene activity | 8.5 | 1999 [22] |
Abbreviations: CCNU, lomustine; CR, complete response; n.d., not determined; PBMCs, peripheral blood mononuclear cells; SD, stable disease.
In this context, use of adenoviral transfer of the HSV-TK transgene was shown to be a legitimate alternative to the PA317/retrovirus system, as high viral titers can be produced, nonproliferating cells that are especially abundant in the walls of the tumor cavity after surgery can be transduced, and transduction efficacies are considerably higher.23,24 In a seminal phase I trial, both treatments were directly compared in 7 glioma patients each, receiving either 1 × 109 retroviral vector-producing cells or 3 × 1010 plaque-forming units (pfu) of adenoviral vector (AdV-HSV-TK) injected peritumorally into the resection cavity followed by i.v. GCV infusion over 14 days. Within 3 months, all gliomas treated with retrovirally transduced HSV-TK progressed, leading to a mean survival time of 7.4 months (control arm 8.3 mo). In contrast, 3 of 7 patients in the adenovirus group had stable disease for at least 3 months. Furthermore, the mean survival time in this group was significantly prolonged to 15.0 months (P < .012), while the treatment was still well tolerated.23 Following up on these results, the same study group later performed the first randomized, controlled study demonstrating survival prolongation in patients with operable primary or recurrent high-grade glioma using adenovirally transduced HSV-TK/GCV gene therapy compared with the outcome after surgery and radiotherapy.25 Tumor bed injection of 3 × 1010 pfu AdV-HSV-TK after surgical resection and GCV infusion for 14 days resulted in a mean survival of 70.6 weeks, almost double the mean survival of the control group (39.0 wk, P = .0095). In 2 of 17 patients in the AdV-HSV-TK group, viral DNA was transiently detected in serum, and antiviral antibody titers were increased in 6 of the 17 patients, but overall the treatment was well tolerated.25 An alternative protocol is currently being assessed in a phase II trial for recurrent high-grade glioma: intra-arterial cerebral infusion of AdV-HSV-TK followed by i.v. GCV is being compared with surgery, systemic chemotherapy, or palliative care (NCT00870181). Feasibility of the AdV-HSV-TK was recently corroborated by a phase IB clinical trial in malignant glioma patients receiving 3 × 1010 to 3 × 1011 AdV-HSV-TK viral particles (vp) injected into the tumor bed after resection, followed by the orally administered prodrug valacyclovir for 14 days followed by temozolomide, and overlapping radiotherapy.26 While a maximum tolerated dose was not reached in this trial and gene therapy–related adverse events were minimal, median overall survival was 12.4 months. Importantly, CD3+ T-cell and CD68+ macrophage infiltration was detected in re-resected tumors, suggesting an immunostimulatory effect of AdV-HSV-TK/valacyclovir gene therapy.26 Both viral delivery and the immunogenic transgene itself add to this effect based on the mode of cell death that involves the HSV-TK suicide system releasing tumor-associated antigens.27–31 Radiation and chemotherapies will act synergistically in this respect,32,33 which has encouraged the realization of a phase II trial for this therapeutic approach (NCT00589875).26 Additionally, safety of AdV-HSV-TK/valacyclovir gene therapy in combination with radiation is being investigated for pediatric brain tumors, including GBM, AAs, and recurrent ependymomas (NCT00634231).
Interestingly, this immunostimulation can be directly exploited in a second gene therapeutic approach in which immunomodulatory transgenes are virally transduced. The resulting immune activation may present one way of overcoming the problem of insufficient transduction efficacies, as uninfected cells can also be affected. In a recent phase I clinical trial, an adenoviral vector with E1 and partial E3 gene deletions, rendering the virus replication incompetent, was utilized to achieve sustained long-term expression of interferon (IFN)-β in glioma patients34 in order to improve the encouraging outcome of brain tumor treatment with IFN-β recombinant protein.35 Gene therapy was applied intratumorally (i.t.) 4 to 8 days before surgical removal of the tumor as well as into the tumor bed directly after resection.34 Doses ranging from 2 × 1010 to 2 × 1011 vp were generally well tolerated, with the exception of 1 patient in the high-dose group who suffered from severe confusion likely to be related to adenoviral treatment. Detection of the therapeutic transgene both in the tumor and systemically as well as the induction of intratumoral apoptosis, inflammation, and/or necrosis warrant further clinical investigation of this approach, although the exact dosing will have to be carefully chosen.34 Immune-activating transgenes may also enhance the immunostimulatory effects of prodrug-activating enzymes (see above), as exemplified by a phase I/II study using i.t. injection of PA317 cells producing a retroviral vector expressing both HSV-TK and human interleukin (IL)-2 plus i.v. GCV in 12 patients with recurrent GBM.36 Tumor responses were observed in 50% of patients, and the median overall survival was 7.5 months. Importantly, local and systemic levels of IL-2, IL-10, IFN-γ, and tumor necrosis factor–α (TNF-alpha) were persistently elevated, suggesting the induction of a type 1 T helper cell immune response, although functionality of antitumor-specific T-cell response was not analyzed.36 Future applications of this dual-transgene approach might benefit from the use of the intrinsically more immunogenic and more efficiently transducing adenoviral vectors, whose coding capacity after E1 and E3 gene deletion should suffice to insert 2 transgenes. Alternatively, individual delivery systems could be used, as exemplified by the direct administration of IL-2 as an adjuvant to AdV-HSV-TK gene therapy37 or the combination of 2 adenoviral vectors expressing HSV-TK and the monocyte chemoattractant protein chemokine C-C ligand 2 (CCL2).38 The latter concept has recently reached the stage of clinical investigation using AdV-HSV-TK and AdV-Flt3L, an adenoviral vector expressing the fms-like tyrosine kinase–3 ligand (Flt3L), which recruits antigen-presenting cells like dendritic cells and macrophages into the tumor microenvironment and thus triggers cellular and humoral antitumor immune responses.39 In a rat model of GBM recurrence, the combination of these 2 vectors was capable of inducing immunological memory to recognize tumor-associated antigens that were unique to the recurrent tumors but absent in the original neoplasm.40 The phase I dose escalation trial will assess the impact of 1010–1011 vp AdV-HSV-TK and 109–1011 vp AdV-Flt3L administered i.t. after surgical resection in combination with oral valacyclovir, temozolomide, and radiation therapy (NCT01811992). Of note, the same study group has also developed a bicistronic adenoviral vector that encodes both transgenes in an attempt to reduce the administered vector load in a future trial.41
Putting a new twist on the gene therapy approach, viral gene transfer has also been utilized to protect from hematopoietic side effects of aggressive chemotherapy in brain tumor patients.42 The concept was investigated in a pilot study, in which the patients' granulocyte colony-stimulating factor–mobilized peripheral blood progenitor cells were collected, enriched for CD34 expression, and transduced with a retroviral vector expressing methylguanine DNA methyltransferase (MGMT). After reinfusion into the patient, the transduced hematopoietic progenitor cells should be more resistant to alkylator therapy with procarbazine, CCNU, and vincristine, reducing the often dose-limiting myelosuppressive side effects. Although a therapeutic benefit could not be detected due to low patient numbers and variability in the number of treatment cycles, the study confirmed that engraftment of progenitor cells was not influenced by the ex vivo transduction procedure, that transduced cells were present for up to 11 months after the first infusion cycle, and that no serious adverse events occurred that could be linked to the engrafted cells.42 Clearly, transduction efficacy and longevity of engraftment will have to be improved. Currently, a variation of this system is clinically being investigated using a mutated version of MGMT that is incapable of binding the guanine analog O6-benzylguanine while retaining its DNA repair functions.43 Patients will receive O6-benzylguanine in combination with temozolomide and radiation therapy in order to treat newly diagnosed GBM after gross tumor resection. The autologous hematopoietic stem cells reinfused into the patients after ex vivo gene transfer with a retroviral vector expressing the MGMT mutant should be protected from O6-benzylguanine treatment, giving them a selective advantage that should prolong the time these cells remain in the system.44,45 The ongoing phase I clinical trial will compare the safety and feasibility of gene therapy after and prior to concurrent chemoradiotherapy with survival and tumor responses included in the secondary outcome measures (NCT01269424).
Overall, there is a great body of evidence establishing the safety of glioma gene therapy. However, the efficacy of this approach still has to be proven, especially since most studies have been early phase trials and have not included sufficient numbers of subjects to draw conclusions with respect to therapeutic benefits. After 2 decades of clinical investigation, achieving higher transduction efficacies, greater vector stability, and extended transgene expression are still the issues that need to be addressed in order to realize therapeutic success. However, the recent trend toward combination of gene therapy with other treatment modalities is promising in order to more fully attack both the tumor and its microenvironment.
Clinical Experience With Glioma Virotherapy—Employing Replicating Viruses for Glioma Increases Cell Killing Modalities
One way to tackle the problem of low transduction efficiency and rapid vector loss is the use of replication-competent viruses that specifically infect and replicate in tumor cells while sparing normal cells. During progeny particle release, tumor host cells are destroyed and tumor-associated antigens are released, while progeny virions infect neighboring tumor cells. Ultimately, viral infection should spread throughout the tumor, leading to complete tumor destruction by various mechanisms, including direct oncolysis, induction of an antitumor immune response, cancer cell starvation by destruction of tumor vasculature, and the activity of virally encoded therapeutic transgenes.13 Oncolytic viruses have been developed for various types of cancer, with the first-in-human approval of the oncolytic adenovirus H101 for head and neck cancer in China46 and with an advanced efficacy trial of i.t. delivered oncolytic HSV talimogene laherparepvec (formerly known as Onco-VEX) for patients suffering from metastatic melanoma, which is completing a phase III trial with very encouraging results.47
Clinical assessment of OV therapy for glioma is at the phase I/II stage, with various virus species and strains being exploited (Tables 2 and 3). The suicide gene therapy approach discussed in the previous section combined with a replicating virus is exemplified by Toca 511 (also called vocimagene amiretrorepvec), a replicating retroviral vector expressing the prodrug activating enzyme yeast cytosine deaminase, which can convert the innocuous prodrug 5-fluorocytosine (5-FC) into the active chemotherapeutic agent 5-fluorouracil (5-FU).48 Since the activated drug is readily diffusible, this suicide gene system is characterized by an extremely strong bystander effect on uninfected neighboring cells, which, as opposed to the HSV-TK/GCV system, functions independently of cell-cell contacts.49 Of note, 5-FU is not a component of standard chemotherapeutic regimens for gliomas, although derived cells are susceptible to 5-FU in vitro and in preclinical rodent models.50 However, the in situ prodrug activation within cytosine deaminase–transduced tumor cells is thought to be more effective than systemic 5-FU as administered in classic chemotherapy. Furthermore, the replicative nature of the transducing virus is supposed to prolong transgene expression compared with replication-defective gene therapy vectors, thus increasing the therapeutic benefit. Toca 511 is being investigated in a phase I/II trial for recurrent high-grade glioma after i.t. injection in combination with up to 6 orally applied 5-FC cycles (NCT01156584), as well as in a phase I trial in which the virus is injected into the tumor bed after surgical removal of recurrent malignant brain tumors and combined with 3-week-long 5-FC cycles (NCT01470794).
Table 2.
Completed glioma virotherapy trials
| Trial Phase | Disease | Virus and Effectors | Delivery and Dosing | Results | Median Survival, mo | Year [Reference] |
|---|---|---|---|---|---|---|
| I/II | Recurrent GBM | NDV-HUJ | Intrapatient dose escalation with 0.1–11 BIU + 3 cycles of 55 BIU i.v. or 2 × 11 BIU/wk i.v. | Maximum tolerated dose not reached; 1/11 CR, not durable; virus recoverable from body fluids | 7.4 | 2006 [64] |
| IB | Recurrent GBM | G207: ICP6-inactivated and ICP34.5-deleted HSV | 1.5 × 108 pfu i.t. 2–5 days before surgery + 1 × 109 pfu into resection cavity | No dose-limiting toxicities; no CR or PR; immune cell infiltration posttreatment detectable | 6.6 | 2009 [58] |
| I | Recurrent malignant gliomas | Reovirus | 1 × 107–1 × 109 TCID50 i.t. | Maximum tolerated dose not reached; 1/11 SD; virus shedding detectable, but not persistent | 4.8 | 2008 [72] |
| I | Recurrent malignant glioma | ONYX-015: E1B and E3-deleted AdV | 1 × 107–1 × 1010 pfu into resection cavity | Maximum tolerated dose not reached; immune cell infiltration in recurrences of treated tumors | 6.2 | 2004 [60] |
| I | High-grade glioma | HSV1716: ICP34.5-deleted HSV | 1 × 105 pfu into resection cavity + radio- and chemotherapy as needed | No virus-associated toxicity; 3/12 SD | n.d. | 2004 [54] |
| I | Malignant glioma | HSV1716: ICP34.5-deleted HSV | 1 × 105 pfu i.t. + resection 4–9 days later | No adverse effects; 1/12 CR; viral DNA recoverable; indication for intratumoral viral replication | n.d. | 2002 [53] |
| I | Recurrent malignant glioma | HSV1716: ICP34.5-deleted HSV | 1 × 105 pfu i.t + standard of care | Well tolerated; no reactivation of latent HSV | n.d. | 2000 [52] |
| I | Malignant glioma | G207: ICP6-inactivated and ICP34.5-deleted HSV | 1 × 106–3 × 109 pfu i.t. | No virus-associated toxicity; viral DNA recoverable | 6.2 | 2000 [57] |
Abbreviations: BIU, billion infectious units (ie, 1 × 109 50% egg infectious dose); CR, complete response; n.d., not determined; PR, partial response; SD, stable disease; TCID50, 50% tissue cell infectious dose.
Table 3.
Ongoing glioma virotherapy trials
| Trial Phase | Disease | Virus and Effectors | Design | Reference |
|---|---|---|---|---|
| I/II | Recurrent high-grade glioma | Toca 511: replicating retroviral vector expressing CD | Dose escalation of i.t. injected Toca 511 + max. 6× 5-FC p.o. for 6 days every mo starting 3–4 wk later | NCT01156584 |
| I/II | GBM, sarcoma, and neuroblastoma | NDV-HUJ | 1 × 1010 EID50 i.v./day for a minimum of 5 d/wk for at least 1 y until disease progression | NCT01174537 |
| I/II | Progressive primary or recurrent GBM | ParvOryx: rat parvovirus H1-PV | Group I: dose escalation of 5 × 106–5 × 108 pfu i.t., surgery on day 10 + same dose injected into the resection cavity; | NCT01301430 |
| group II: dose escalation of 1 × 105–1 × 108 pfu/day i.v. on 5 consecutive days, surgery on day 10 + same total dose injected into the resection cavity | ||||
| I/II | Recurrent GBM | Delta-24-RGD: partially E1A-deleted AdV retargeted toward integrins via an RGD peptide | Dose escalation of 1 × 107–1 × 1011 vp i.t. by convection-enhanced delivery | NCT01582516 |
| I/II | Recurrent or progressive GBM | G47Δ: ICP6-inactivated, ICP34.5-, and ICP47-deleted HSV | Dose escalation of G47Δ injected i.t. twice 5–14 days apart | JPRN-UMIN000002661 |
| I | Recurrent GBM | MV-CEA: measles virus vaccine strain Edmonston B expressing human CEA | Group I: dose escalation of MV-CEA injected into the resection cavity; | NCT00390299 |
| group II: dose escalation of i.t. injected MV-CEA, surgery on day 5 + another dose injected into the resection cavity | ||||
| I | Recurrent malignant glioma | DNX-2401/Delta-24-RGD: partially E1A-deleted AdV retargeted toward integrins via an RGD peptide | Group I: dose escalation of 1 × 107–3 × 1010 vp i.t.-injected DNX-2401; | NCT00805376 |
| group II: dose escalation of i.t.-injected DNX-2401, surgery on day 14 + another dose injected into the resection cavity | ||||
| I | Recurrent malignant brain tumors | Toca 511: replicating retroviral vector expressing CD | Dose escalation of Toca 511 injected into the resection cavity + 3× 5-FC p.o. for 8 days every 2 mo starting 7 wk later | NCT01470794 |
| I | Recurrent supratentorial GBM | PVS-RIPO: live attenuated poliovirus vaccine (Sabin strain) with a human rhinovirus type 2 internal ribosomal entry site | Dose escalation of 1 × 108–1 × 1010 TCID50 delivered i.t. by convection-enhanced delivery | NCT01491893 |
Abbreviations: CD, cytosine deaminase; TCID50, 50% tissue culture infectious dose.
The most extensively studied viruses in oncolytic glioma therapy, however, are recombinant HSVs with deletions of both viral copies of the ICP34.5 gene, rendering the virus non-neurovirulent.51 The virus designated as HSV1716 was evaluated in a total of 33 patients in 3 consecutive safety trials and was well tolerated after administration into the tumor or the tumor bed after resection.52–54 The initial trial proved that encephalitis did not develop after i.t. injection of 105 pfu of HSV1716,52 and the follow-up trial revealed seroconversion in previously HSV-naïve patients and detectable viral DNA levels at distal tumor sites in 4 of 12 patients.53 Furthermore, in 2 patients, live virus could be recovered from resected tumors 5 to 6 days after HSV1716 injection, and viral DNA copy numbers higher than the administered dose were detected at the injection site in 4 cases, indicating some degree of viral replication in human gliomas.53 HSV1716 injection into the resection cavity in a third trial was similarly safe, and stable disease for up to 22 months was achieved in 3 of 12 GBM patients.54 Similarly encouraging data resulted from 2 trials using another ICP34.5-deleted HSV, G207, whose ICP6 gene encoding the viral ribonucleotide reductase is inactivated in order to achieve an additional layer of safety, since this enzymatic function is cellularly complemented in actively proliferating glioma cells but not in quiescent cells surrounding the tumor.55 This mutation also has been shown to target replication to cells defective in the p16 tumor suppressor gene, regardless of their cell cycle state, thus providing the biologic rationale for tumor-specific replication.56 In an initial dose escalation trial in 21 patients suffering from GBM or AA, up to 3 × 109 pfu of G207 were safely injected i.t. without signs of encephalitis or serious adverse events unquestionably linked to the virus.57 More recently, safety of G207 was confirmed when administered in a 2-step protocol, in which 13% of the total 1.15 × 109 pfu were infused i.t. via a catheter and the remainder of the viral dose was injected 2 or 5 days later into the resection cavity after surgery.58 Although viral replication was convincingly detected in one of the patients, efficacy of the treatment could not be evaluated due to the low numbers of subjects. A third oncolytic HSV has entered a phase I/II GBM trial in Japan that has been escalating the dose of 2 consecutive i.t. injections of G47Δ, which harbors an ICP47 gene and Us11 promoter deletion in addition to those present in G207 (JPRN-UMIN000002661). Due to earlier Us11 expression and hence earlier inhibition of innate antiviral immune responses, this deletion has been shown to increase yields of ICP34.5-deleted viruses in many cancer cells in vitro without increasing neurovirulence.59 Furthermore, lack of ICP47 led to a disinhibition of the transporter associated with antigen processing, resulting in enhanced major histocompatibility complex class I–restricted presentation of endogenous, tumor-associated antigens and thus the stimulation of antitumor immune responses.59 These properties of G47Δ can potentially increase the efficacy of oncolytic HSV treatment, and results of the Japanese trial are highly anticipated.
The influence of antitumor immune activation on the outcome of glioma virotherapy has also been exploited in the context of a phase I dose escalation trial using ONYX-015, an oncolytic adenovirus with selectivity for tumor cells that can substitute for RNA export functions lost by the deletion of the E1B-55K gene in the virus genome.60–62 The virus was injected into the tumor bed after resection at doses ranging from 107 to 1010 pfu: no serious adverse events unequivocally attributed to ONYX-015 were observed, and a maximum tolerated dose was not reached. Interestingly, in 2 patients tumor recurrence was diagnosed on the basis of gadolinium enhancement, and histological analysis of the resected recurrent tumors revealed considerable perivascular lymphocyte infiltration in tumor tissue, but not in the surrounding tumor parenchyma.60 This “pseudoprogression”63,64 is a phenomenon that has also been noticed with immunotherapeutics,65 which raises the question of whether radiologic criteria for activity of biologic agents should be redefined, as recently published with the Response Assessment in Neuro-Oncology criteria.66,67 Using these criteria, initial progressive disease can be monitored serially in order not to miss long-term effects with delayed kinetics induced by immune activation. This should also be considered when evaluating the 2 ongoing clinical trials of recurrent gliomas with the oncolytic adenovirus DNX-2401 (also known as Delta-24-RGD). In addition to the Delta-24 mutation, which allows for replication only in cells that are defective in the retinoblastoma protein tumor suppressor,68 this virus' tumor specificity is initially determined at the level of viral host cell recognition. The cyclic arginine/glycine/aspartic acid (RGD) peptide inserted into the viral fiber knob (ie, the viral capsid region responsible for attachment to host cells) directs the virus toward integrins, which are highly expressed in gliomas, but whose expression of the natural coxsackievirus and adenovirus receptor is relatively low.68 This potentially increases the active dose in the tumor, since the virus is not sequestered in off-target tissues. In both trials using DNX-2401 (NCT00805376, NCT01582516) the virus is injected locally, and this mode of tumor specificity should add to the safety profile of the virus and potentially boost its potency. Preliminary results have been recently announced for one of these trials (NCT00805376): a single dose of i.t. injected DNX-2401 was well tolerated and led to stable disease, partial or complete responses in 52% of GBM patients with survival for up to 4 years.69
The delayed kinetics of therapeutic effects associated with an adaptive antitumor immune response is becoming a recurrent theme in OV trials. The ongoing trial for recurrent GBM using the engineered poliovirus PVS-RIPO provides another example (NCT01491893). The OV is based on the Sabin poliovirus vaccine targeting cancer cells via nectin-5, a receptor overexpressed on several malignancies, including GBM. Its neuropathogenicity is ablated by the exchange of the internal ribosomal entry site of poliovirus with that of human rhinovirus 2, modulating translation initiation.70 While the single dose escalation trial for i.t. infusion of PVS-RIPO by convection-enhanced delivery is ongoing and still recruiting patients, encouraging preliminary results have been recently presented: in 3 of 7 patients, complete responses were achieved, but the reduction in tumor size was delayed by months after virus administration.69 Delayed biopsies of injected tumors revealed extensive glioma necrosis and immune infiltrates comprising macrophages and lymphocytes attributed to an antitumor response, as opposed to direct viral oncolysis, since an antibody response led to viral clearance within 2 weeks.69 Clearly, any conclusion will need to await the published results of this trial, which may also shed further light on the mechanisms underlying the antitumor immune activation induced by transient viral replication and oncolysis.
While the tumor specificity of all the OVs we have mentioned has been engineered, several unmodified viruses with natural tumor tropism have been clinically investigated in the context of brain tumors. One is Reolysin, the Dearing strain reovirus, which relies on Ras signaling pathway activation for productive viral infection and is thus naturally targeted to malignant cells with aberrant Ras activity.71 A phase I clinical trial established the good tolerability of i.t. administration of Reolysin in recurrent malignant gliomas without a maximum tolerated dose being reached.72 There has been one stable disease response, reported for 39 weeks in a patient with anaplastic oligoastrocytoma; evaluation of clinical efficacy was not possible due to low patient numbers, heterogeneous diagnosis, and variable pretreatments. Interestingly, in half the patients whose tumors were reoperated upon, plasma cell aggregates not present in biopsies taken at entrance into the study were found,72 in agreement with similar observations made after treatment with ONYX-015 as discussed above.60 Future trials optimizing administration and assessing efficacy may be worthwhile, especially considering the success with Reolysin treatment in other malignancies that has led to the initiation of an advanced phase III trial investigating the benefit of i.v. infused Reolysin as an adjuvant to chemotherapy in squamous cell carcinoma of the head and neck (NCT01166542).
Unlike the previous trials where i.t. administration was used, systemic OV delivery has been explored for recurrent GBM using the Hebrew University Jerusalem (HUJ) strain of Newcastle disease virus (NDV), another OV with natural tumor selectivity.64 Intravenous infusion of the virus was safe, and a maximum tolerated dose was not reached. Although tumors of all 11 patients eventually progressed, 1 transient remission was observed and perivascular lymphocyte infiltrates were found in biopsies of 3 patients with tumor growth, judged on the basis of gadolinium enhancement.64 NDV-HUJ is currently being more extensively investigated in a phase I/II trial in which GBM, neuroblastoma, and sarcoma patients are being systemically treated with 1010 EID50 (50% egg infectious dose) per day on 5 or more days a week for at least a year (NCT01174537). Although safety has overall been established for OV therapies in general, safety assessment for this avian virus will be important, based on its long-term, repeated administration into the circulation and potential exposure of people in contact with treated subjects. In fact, NDV-HUJ virus was recovered not only from a tumor biopsy, but also from blood, saliva, and urine samples after the development of anti-NDV antibodies, indicating that these were not neutralizing.64
The influence of the administration route is being directly compared in an ongoing trial with ParvOryx (NCT01301430), a non-engineered rat parvovirus H1 with natural tropism for malignant glial cells while being otherwise nonpathogenic.73 Patients will be treated either with a first i.t. dose of the virus applied via a catheter and a second dose into the resection wall after surgery 10 days later or, if no safety concerns arise during dose escalation with this regimen, with 5 viral doses administered i.v. on 5 consecutive days with a 6th dose peritumorally injected into the tumor bed after surgery on day 10.74 Although this phase I trial is not powered enough to determine ParvOryx efficacy, it will provide useful information related to the active viral dose actually distributing within the tumor with the 2 delivery methods and on safety of this virus, which is the first parvovirus to be tested in clinical trials.
In an alternative attempt to identify the fate of injected OVs, an oncolytic measles virus (MV), based on the Edmonston B vaccine strain, has been engineered to express the soluble, extracellular portion of carcinoembryonic antigen (CEA) in order to monitor gene expression as an indicator for viral replication by measuring serum CEA levels.75 This method has been validated in a recent phase I trial using i.p. administered MV-CEA in 21 patients with chemorefractory recurrent ovarian carcinoma, in which serum CEA levels rose in a dose-dependent fashion after each of the 6 viral treatment cycles, albeit at decreasing levels. Since anti-CEA antibodies were nondetectable, the gradual decrease in CEA levels may represent a decline in viral spread after repeated administration, indicating inefficient viral replication in humans.75 At present, the CEA marker is being utilized to monitor viral gene expression as the primary outcome measure in a phase I trial currently at the recruitment stage for patients with recurrent GBM (NCT00390299).
In summary, glioma virotherapy has proven safe and well tolerated, as have the gene therapy approaches. Similarly, clinical investigation of OVs for brain tumors have been or are at the phase I level, for which therapeutic benefit is a secondary outcome measure and patient numbers are generally too low to draw conclusions on antitumor efficacy. However, the ongoing trials utilizing PVS-RIPO and DNX-2401 are encouraging so far and warrant the continuation of their clinical evaluation in advanced efficacy trials.
Taking Clinical Experiences Back to the Bench—Strategies to Enhance Efficacy
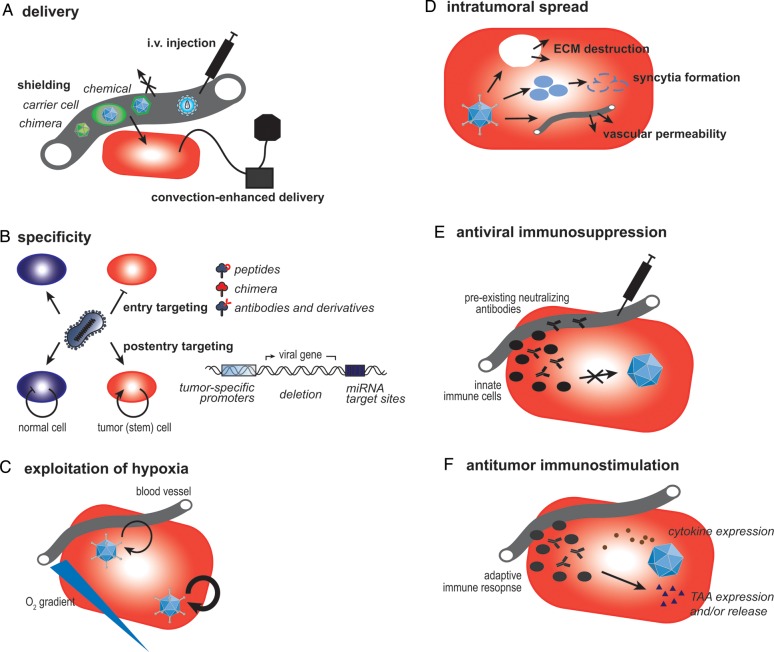
Despite the encouraging results of early clinical trials with virus therapies related to safety, several aspects affecting efficacy still need to be improved before therapeutic viruses can have a significant and meaningful influence on survival from brain malignancies. These include the entire range of steps from virus delivery to intratumoral spread, as well as the role that the hypoxic microenvironment and the immune system play in determining antitumor effects (Fig. 1).
Fig. 1.
Strategies to improve efficacy of virus therapies. Several clinical trials have been performed using therapeutic viruses to treat gliomas. Although well tolerated overall, there is a need for more efficacious viruses, and ongoing bench research is exploring several areas of possible improvement. (A) Intratumoral application of therapeutic viruses may be performed using convection-enhanced delivery. Alternatively, viruses may be administered intravenously, but there is a need for shielding them from host serum factors using chemicals, a chimeric design, or carrier cells, which may also facilitate targeted delivery toward the tumor tissue. (B) Specificity is enhanced during viral entry, allowing the virus to enter tumor cells only by modification of the attachment-mediating surface proteins employing targeting peptides, antibodies and their derivatives, or chimeric capsids/envelopes. Furthermore, specificity can be achieved at a post-entry level with viral replication restricted to target cells utilizing glioma-specific promoters, differentially expressed microRNAs (miRNA), or the deletion of essential viral genes whose function is complemented in tumor cells. Both concepts can be applied to target both tumor bulk and tumor stem cells. (C) When viral genes are engineered to be regulated by hypoxia-responsive promoters, viral replication can be enhanced in areas of low oxygenation, a typical glioma phenotype. (D) If the therapeutic virus does not possess intrinsic properties facilitating intratumoral dissemination like syncytia formation, intratumoral spread can be enhanced by encoding enzymes capable of degrading the tumor's extracellular matrix (ECM) or by combination treatments with drugs that modulate the composition of the tumor stroma or vascular permeability. (E) The immune system plays a complex role during viral therapy of gliomas. Especially in the early phases of treatment, antiviral effects of the innate immune system and preexisting neutralizing factors need to be suppressed by pharmacological intervention. (F) A beneficial adaptive immune response directed against the tumor can be elicited by encoding immunostimulatory transgenes and/or tumor-associated antigens (TAAs).
With the exception of oncolytic NDV-HUJ64 and peripheral hematopoietic cells retrovirally transduced with MGMT,42 all virus therapies have been administered locally into the tumor or the resection cavity after surgery (cf Tables 1 and 2). This mode of administration directly brings the virus into the target region, circumventing the blood–brain barrier and exposure to blood-neutralizing antibodies if the subject has been pre-immunized. Free-hand injections, routinely performed in many clinical trials up to now, are being substituted in more recent trial designs with convection-enhanced delivery systems (NCT01582516, NCT01491893). In these, the virus is infused slowly over several hours, and the positive interstitial pressure that prevents viral distribution throughout the tumor is overcome.76 However, local virus treatment reduces the possibility of getting to distant (micro)metastases, and repeated dosing schedules when administered i.t. are less convenient, ultimately favoring systemic delivery. Although several trials using Reolysin77 and the oncolytic vaccinia virus JX-594 (Pexa-Vec)78 in non-CNS malignancies have proven that i.v. injection of OVs is feasible and well tolerated, several factors have been identified that can impede efficient distribution of the OV into the target tumor. These include virus neutralization by preexisting antibodies, complement and coagulation factors,79–82 and virus sequestration in the spleen and in Kupffer cells in the liver.83 Attempts to overcome these problems (Fig. 1A) are based on pharmacological interventions, such as using polyinosinic acid, and on chemical shielding of viral surfaces using clodronate or polyethylene glycol.84,85 Furthermore, a variety of chimeric viruses with neutralizing epitopes exchanged with nonreactive serotype epitopes have been engineered in order to evade neutralization.86 Another increasingly utilized alternative relies on “carrier” cells, such as mesenchymal stem cells, immune cells, or other cells with tumor-homing capacities, transporting the virus to the tumor while concealing it from serum-neutralizing factors.13 Preclinically, the applicability of this strategy in the context of brain tumors has been shown with the delivery of a glioma-targeted oncolytic adenovirus by neural stem cells to U87 glioma xenografts in rodent models.87 The study confirmed not only tumor-directed delivery of the virus by loaded carrier cells, but also transduction of tumor foci in the contralateral hemisphere and reduced off-site infectivity compared with injection of naked virus.87 The evaluation of carrier cell–delivered virotherapy in humans is in preparation88 and follows the example of an ongoing trial evaluating carrier cell–mediated nonviral gene therapy for recurrent high-grade glioma, in which treatment with neural stem cells engineered to express cytosine deaminase in combination with oral 5-FC is evaluated (NCT01172964). Importantly, preclinical studies showed a two-thirds reduction in tumor mass as a result of this treatment89 and no influence of radiation, corticosteroid, or alkylator pre- or cotreatments on carrier cell tropism,89,90 suggesting that carrier cell administration is compatible with established glioma therapies. Additional mechanistic insight into the tumor-homing capacities of carrier cells will further support the application of this technology.
Once the virus has reached the tumor, target cell specificity has to be ensured in order to minimize side effects. Although safety has not been an issue in the completed glioma trials, it will still need to be closely monitored in the future, as the administered doses will likely rise due to improved manufacturing techniques that will allow for virus preparations with higher titers. Furthermore, new and more potent OVs are being engineered and tested. On the one hand, tumor cell targeting has been achieved at the level of viral entry (Fig. 1B), as discussed above in the context of DNX-2401, specifically recognizing integrins on the tumor cell surface.68 Typically, entry targeting involves both the destruction of natural receptor binding and the introduction of novel tropism to a surface molecule exclusively present or overexpressed on tumor cells. In addition to peptide-mediated targeting, as in DNX-2401, retargeting can be achieved by use of chimeric surface proteins exploiting distinct tissue tropisms of alternative serotypes or viruses86 or by introduction of antibody-derived moieties in case of enveloped viruses like MV and HSV, allowing for targeting of virtually any surface protein of interest.91 The flexibility of antibody-derived molecules can be specifically exploited for gene therapeutic approaches when combined into bispecific adaptors that can bridge the viral vector to the target cell.92
On the other hand, tumor specificity can be achieved at a post-entry level limiting (trans)gene expression and/or viral replication and cell lysis to malignant cells (Fig. 1B). Several examples are provided by the clinically investigated viruses HSV1716,52–54 G207,55 G47Δ,59 ONYX-015,60,93 and DNX-2401,68 in which deletion of essential viral genes is exclusively complemented in tumor cells but not in surrounding healthy tissue. Alternatively, viral replication can be limited to tumor cells by the introduction of microRNA target sites into viral genomes, silencing transgene expression or virus replication in off-target cells that express the corresponding microRNA, while viral gene expression is unharmed in tumor cells in which the microRNA is downregulated.94 Feasibility of this approach for gliomas has been established using a miR7-regulated MV95 and an oncolytic Semliki Forest virus with miR124-mediated attenuation of neurovirulence.96 Additional microRNAs that could be exploited in this context include miR128 and miR137.97,98 Importantly, microRNA-mediated targeting is a highly universal tool that should be applicable to all virus families and to both nonreplicating and oncolytic viruses. Furthermore, microRNAs overexpressed in GBM, like miR21 or miR221,97 can be utilized to silence genes capable of negatively influencing viral replication, making microRNA targeting an extremely flexible tool for future safe and more potent virus therapies.
A third possibility for post-entry targeting relies on tissue- or cell type–specific promoters that drive viral replication and/or transgene expression. In fact, transcriptional targeting is the most widely investigated approach for regulation of viral gene expression and has been recently reviewed.99 The most notable examples with respect to glioma virus therapy include a conditionally replicating adenovirus whose E1 gene expression is regulated by the promoter of the glial fibrillary acidic protein in addition to the E4 gene under the control of a Ki67 promoter100 and oncolytic HSVs whose ICP34.5 virulence gene expression is controlled by the promoters of the RNA binding protein Musashi1101 or of the intermediate filament nestin.102 Of note, the nestin promoter has been found to contain several methylation sites, limiting promoter activity, ICP34.5 expression, and consequently replication and cell lysis.103 However, combination of virotherapy with demethylating agents like 5-azacytidine reversed this effect in vitro and in vivo, resulting in significant enhanced survival prolongation compared with virotherapy alone.103 Transcriptional targeting will most likely be included in the design of many next-generation viruses with potentially improved therapeutic efficacy.
GBM is characterized by hypoxia-mediated necrosis and vascular proliferation with hypercellular “pseudopalisades” encircling necrotic nests.104 Indeed, hypoxia is associated with worse clinical outcomes and is believed to influence the biology of GBM stem cells.105 At the molecular level, hypoxia is associated with the intracellular accumulation of hypoxia-inducible factor (HIF)1α, enabling the formation of a HIF-1α/HIF-1β heterodimer and hence transcription initiation of a plethora of genes involved in survival, angiogenesis, metastasis, chemo and radiation resistance, and others.105 Virus therapies, however, can take advantage of this tumor-specific hypoxic environment in line with the concept of transcriptional targeting described above (Fig. 1C). Both adenovirus106,107 and HSV108 have been controlled by HIF-1α response elements to limit viral replication to hypoxic tumor cells, while a replication-deficient adeno-associated virus has been developed to express its transgene in a hypoxia/HIF-1α–dependent fashion.109 Additionally, viral yields of the oncolytic HSV G207 were higher when grown under hypoxic, as opposed to normoxic, conditions both in vitro and in vivo, likely due to an evolutionary adaption of HSV to hypoxic environments in brain or oral mucosa during natural virus infection.110 In summary, virus therapies not only may be less affected by hypoxia in GBM than chemo- or radiotherapeutic approaches, but can instead capitalize on this condition.
A key problem underlying the lack of efficacy in early clinical trials is limited viral biodistribution within the tumor (Fig. 1D). This issue starts with the need for increased vascular permeability in the tumor, as dense tumor stroma and inadequate lymphatic drainage counteract vascular leakiness in tumors and thus hinder virus extravasation and diffusion.13 In this context, pre-administration of systemic IL-2, bradykinin analogs, or tumor necrosis factor–α has been proposed to support viral spread.13 Furthermore, viral mobility through the tumor is inhibited by substantial tumor stroma,111 and pharmacological or enzymatic destruction of the extracellular matrix has been beneficial in preclinical models: the angiotensin II receptor antagonist losartan could indirectly support intratumoral spread of oncolytic HSV by reducing the collagen I content in several tumor xenografts.112 Similarly, oncolytic adenoviruses and HSVs expressing matrix-degrading enzymes like hyaluronidase113 or a chondroitinase ABC (ChaseABC),114 respectively, were characterized by enhanced spread and therapeutic activity. Of note, some viruses not as extensively studied in glioma trials intrinsically possess properties that facilitate intratumoral spread. These include MV dispersing via cell-to-cell fusion and vaccinia virus subspecies penetrating neighboring cells via an actin tail propeller, both of which have been proven safe for oncolytic applications in clinical trials for non-CNS tumor entities.13
Although the brain is classically considered an immunoprivileged organ, the immune system clearly influences viral therapies of glioma. It is important to note that the immune system plays an ambivalent role in this context, and the details of when which aspect of the immune system acts in synergy with or against therapeutic viruses are still under intense investigation. On the one hand, preexisting immunity toward the virus negatively impacts delivery and spread as discussed before, although it can be considered a safety feature often leading to seropositivity as a “safety” inclusion criterion for early clinical trials. In addition to viral engineering approaches,86 carrier cells have been used to shield therapeutic viruses from preexisting immune factors besides facilitating targeted delivery.13 Importantly, premature lysis of the carrier cells before reaching the target tissue needs to be avoided. The recently developed adenoviral vector proAdΔ24.GFP, which is initially replication defective but can be genetically activated into a replication-competent form, provides a potential tool to ensure minimal toxicity to carrier cells.115 Another strategy to overcome early antiviral effects by the host could be temporary immunosuppression/immunomodulation (Fig. 1E). Metronomic cyclophosphamide (CPA) regimens were shown to enhance therapeutic effects of several OVs in rodent disease models116; for example, CPA pretreatment enhanced oncolytic HSV replication and therapeutic efficacy in a rat glioma model.117 As a result, CPA administration is included in ongoing clinical trials using oncolytic adeno- and reoviruses and MVs in several malignancies (NCT01598129, NCT01240538, NCT00450814). However, CPA can also have immunostimulatory activities and in rare cases even induce a cytokine storm within tumors.118 In addition to preexisting antibodies, the innate immune response—designed to fight off natural virus infections—can interfere with virotherapy. Recently, early deleterious effects on replication of oncolytic HSV in a GBM mouse model were found to originate from activated natural killer cells infiltrating and lysing infected tumor cells.119 This effect relied on defined natural cytotoxicity receptors upregulated in infected GBM cells, which ultimately reduced the efficacy of HSV treatment in vivo.119 Furthermore, HSV replication was negatively impacted by both peripheral CD163+ and brain-resident CD68+ monocytic cells, both found to be upregulated in GBM treated with OV.120 Therefore, pharmacological inhibition of innate immune reactions seems to be beneficial at least at early stages of virus therapies, and these sorts of regimens should be explored in clinical settings.
On the other hand, virus-induced cell death exposes not only viral antigens to the patient's immune system, but also tumor-associated antigens (TAAs) capable of triggering an antitumor immune response and thus facilitating tumor eradication (Fig. 1F). This epitope spread has been extensively studied in the context of malignant melanomas—for example, studies using murine disease models treated with Reolysin revealed that virus-mediated tumor cell killing could activate both innate and adaptive immune effector cells121 and that in some systems oncolysis itself was not even necessary for this effect.122 Additionally, for oncolytic therapy with vesicular stomatitis virus (VSV), a strong correlation was established among viral gene expression, induction of an intratumoral pro-inflammatory reaction, and therapeutic efficacy in vivo.123 Similar concepts seem to hold true in brain tumors, as indicated by studies using oncolytic minute virus of mice, related to H1 parvovirus.124 Therapeutic activity achieved in glioma models did not rely only on direct oncolysis, but also on breakage of immune tolerance and the development of a tumor-specific, T cell–mediated long-term memory response.124 Viruses that have been used in attempts to induce antitumor immune responses (Fig. 1F) have been equipped with immunostimulatory transgenes like the chemokine C-C ligand 5,125 promoting immune effector cell recruitment, or granulocyte-monocyte colony-stimulating factor (GM-CSF),126–128 supporting dendritic cell differentiation and activation of cytotoxic T cells. The GM-CSF–armed vaccinia virus JX-594, one of the trailblazers in clinical translation of oncolytic cancer therapies, was recently preclinically evaluated in 2 immunocompetent animal models establishing therapeutic benefit as well as safety with respect to intracranial treatments triggering a predictable degree of GM-CSF–dependent inflammation and necrosis.129 Combination treatment with the mammalian target of rapamycin (mTOR) inhibitor rapamycin additionally improved therapeutic outcome, encouraging further evaluation in nonhuman primates and eventual translation to the clinic.129 As an alternative to immune activators, the TAAs themselves can be encoded in the virus, efficiently priming T cell responses, extensively studied in the context of oncolytic VSV.130 This type of vaccination with highly immunogenic VSV vectors carrying a cDNA library of normal tissue (ie, a plethora of unknown, untargeted antigens) from a particular tissue cured established tumors derived from the same histologic type,131 and tumor eradication was found to be dependent on an IL-17 response.132 Of note, combinatorial use of 3 individual VSV clones resembled the therapeutic activity of the full library, establishing this technology as a powerful tool to identify TAA for a given tumor type or stage.132 Interestingly, topoisomerase-IIα was identified as a recurrence-specific TAA independently of both tumor type and frontline treatment, although it was not relevant in the primary tumors.133 In light of the high recurrence rates in GBM, this technology may be highly useful to develop distinct vaccines and pharmacological treatments for the various grades of gliomas with almost exclusive effectiveness specific to a particular glioma stage (ie, newly diagnosed vs recurrent). Other TAAs relevant for immunotherapies of gliomas include the ephrin receptor tyrosine kinase EphA2134 and fibronectin I.135 Of note, both immunomodulatory transgenes and TAAs could be delivered by an initially nonreplicative adenovirus designed to be analogous to the above mentioned proAdΔ24.GFP.115 The immunostimulation achieved via this gene transfer could then be boosted at a later stage by a switch to the replication-competent oncolytic phenotype.
Certainly, the interplay among a therapeutic virus, the immune system, the tumor, and its microenvironment will determine the benefit patients can derive from viral therapies. As discussed above and more extensively reviewed elsewhere,136 therapeutic outcome and immunological responses to therapy vary with the virus used and the tumor type treated. A balance between the immunogenicity of the virus and TAAs is necessary in order to achieve robust antitumoral responses, as opposed to antiviral immune reactions. However, it is still not known how much viral replication is needed to achieve an effective immunostimulatory response. The timing and the exact nature of immune activation are variables that require additional investigation in this context.
Future Perspectives—Avenues Back to the Patient Bedside
Several clinical trials have unambiguously demonstrated the safety of oncolytic virus therapies for many tumor entities. While several advanced efficacy trials were able to show therapeutic benefit of virotherapy in non-CNS tumors like malignant melanoma, the potency of virus-based therapies for gliomas remains to be substantiated. In their attempt to raise virus efficacy, researchers have undoubtedly exploited a wide variety of approaches to improve the clinical activity of therapeutic viruses in gliomas, including arming with prodrug-activating enzymes, using novel delivery methods, using one of the GBM hallmark phenotypes (hypoxia), destroying the extracellular matrix for improved spread, and directing the immune system toward a generalized and prolonged antitumor response. Certainly, further characterization of the interaction of therapeutic viruses with the tumor, its microenvironment, and the host immune system will help to derive further improvements to the current arsenal of viruses.
However, from today's standpoint, 2 important aspects may prove key to a meaningful assault on gliomas. Firstly, virus therapies will have to attack those cells within the complex tumor microenvironment that function as tumor-initiating or glioma stem cells (GSCs; most commonly characterized by CD133 surface expression), which are believed to confer resistance to common chemo- and radiation therapies, and thus are thought to potentially be the source of recurrence.137 Although there is still controversy on the exact origin of GSCs and the hierarchy of tumor cells within gliomas, the existence of a cell population that initiates and maintains gliomas is generally accepted137 and unequivocally leads to their great potential as therapeutic targets, eliminating the necessity to reach all cells in the tumor mass. Due to their distinct cell killing mechanisms, which differ from those of classic pharmacological or radiation approaches, therapeutic viruses may represent a treatment modality that can actually reach and destroy these cells. Proof of concept for the usefulness of GSC targeting has been provided using a CD133 entry-targeted MV in an orthotopic glioma mouse model,138 oncolytic HSVs replicating under the control of the nestin102 or CD133 promoters69 or expressing soluble tumor necrosis factor–related apoptosis-inducing ligand,139 and an RGD-targeted oncolytic adenovirus whose replication is dependent on nuclear expression of Y-box binding protein 1 due to a specific deletion in the E1A13S protein.140 Further investigation of how GSCs can be specifically targeted by therapeutic viruses without inducing resistance will likely result in the development of novel, more potent viral therapies.
Secondly, observations of many monotherapies failing due to development of resistance encourage the development of combination therapies with OVs and other treatment modalities, despite the numerous modes of cell killing employed by therapeutic viruses themselves. In fact, a phase IB trial using nonreplicating AdV-TK in combination with intensive timing radiation and chemotherapy in addition to surgery has already been concluded (see above and Table 1) and a follow-up phase II trial is ongoing.26 Therapeutic viruses have been demonstrated to act on gliomas in synergy with various anticancer drugs, including the alkylator temozolomide,141–143 the histone deacetylase inhibitors valproic acid and Trichostatin A enhancing HSV144 and vaccinia virus145 replication, as well as adenovirus infectivity,146 the anti-angiogenic antibodies B20–4.1.1147 and bevacizumab,148 both targeting vascular endothelial growth factor A, and the phosphinositide-3-kinase inhibitor L294002.149 Additional compounds successfully tested in the context of other malignancies, like doxorubicin, irinotecan, or erlotinib,69 are also available to be evaluated in brain tumors in combination with OVs. Furthermore, brain irradiation capable of disrupting the blood–brain barrier can support viral delivery: this was employed with systemically administered Reolysin.69 Viral transduction of the theranostic sodium-iodide symporter (NIS) in combination with iodine-131 is another alternative to enhance antitumor activities of HSV,150 vaccinia virus,151 and MV,152 which allows for noninvasive imaging of transgene expression and thus indirectly allows measurement of viral spread at the same time. Indeed, NIS-based radiovirotherapy with MV showed clear antitumor activity against GBM in both hetero- and orthotopic xenograft models.152 Combinatorial activities will have to be carefully assessed with respect to dosing and timing. Translation of optimized regimens in animal models, which still remain to be honed with respect to proper recapitulation of human disease properties and viral permissiveness, may also not be straightforward. However, the multimodal attack of chemoviro- and/or radiovirotherapy may hold more promise than single agent treatments, matching up glioma heterogeneity and the variety of cell types influencing tumor growth with a medley of therapeutic agents and anticancer mechanisms. Ultimately, such a holistic approach may lead to a multimodal treatment regimen from which patients can derive significant and meaningful benefits, the long strived for but yet unmet goal in glioma therapy.
Funding
This work was supported by the National Institutes of Health (grant 1P01CA16320501A1, as well as grants 7U01NS061811, CA163205, and CA069246 to E.A.C.).
Conflict of interest statement. Dr Chiocca serves as a consultant to DNAtrix, Inc., which is developing Delta-24-RGD. For this he receives stock options. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Nabors LB, Ammirati M, Bierman PJ, et al. Central nervous system cancers. J Natl Compr Cancer Netw. 2013;11:1114–1151. doi: 10.6004/jnccn.2013.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams H, Chaichana KL, Avendaño J, et al. Adult cerebellar glioblastoma: understanding survival and prognostic factors using a population-based database from 1973 to 2009. World Neurosurg. 2013;80:e237–e243. doi: 10.1016/j.wneu.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JW, Chi AS, Cahill DP. Tailored therapy in diffuse gliomas: using molecular classifiers to optimize clinical management. Oncol Williston Park N. 2013;27:504–514. [PubMed] [Google Scholar]
- 6.Dhermain FG, Hau P, Lanfermann H, et al. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Rey-Dios R, Roberts DW, et al. Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.06.014. doi:10.1016/j.wneu.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Nagpal S. The role of BCNU polymer wafers (Gliadel) in the treatment of malignant glioma. Neurosurg Clin N Am. 2012;23:289–295, ix. doi: 10.1016/j.nec.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Bluming AZ, Ziegler JL. Regression of Burkitt's lymphoma in association with measles infection. Lancet. 1971;2:105–106. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 10.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther J Am Soc Gene Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 11.Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- 12.Tobias A, Ahmed A, Moon K-S, et al. The art of gene therapy for glioma: a review of the challenging road to the bedside. J Neurol Neurosurg Psychiatry. 2013;84:213–222. doi: 10.1136/jnnp-2012-302946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.High KA. The gene therapy journey for hemophilia: are we there yet? Blood. 2012;120:4482–4487. doi: 10.1182/blood-2012-05-423210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran N. First gene therapy approved. Nat Biotechnol. 2012;30:1153. doi: 10.1038/nbt0912-807. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Hwang R, Chiang Y, et al. Mechanisms for ganciclovir resistance in gastrointestinal tumor cells transduced with a retroviral vector containing the herpes simplex virus thymidine kinase gene. Clin Cancer Res. 1998;4:731–741. [PubMed] [Google Scholar]
- 17.Fick J, Barker FG, 2nd, Dazin P, et al. The extent of heterocellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro. Proc Natl Acad Sci U S A. 1995;92:11071–11075. doi: 10.1073/pnas.92.24.11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 19.Klatzmann D, Valéry CA, Bensimon G, et al. A phase I/II study of herpes simplex virus type 1 thymidine kinase “suicide” gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther. 1998;9:2595–2604. doi: 10.1089/hum.1998.9.17-2595. [DOI] [PubMed] [Google Scholar]
- 20.Shand N, Weber F, Mariani L, et al. A phase 1-2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum Gene Ther. 1999;10:2325–2335. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- 21.Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 22.Palù G, Cavaggioni A, Calvi P, et al. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: a pilot study in humans. Gene Ther. 1999;6(3):330–337. doi: 10.1038/sj.gt.3300805. [DOI] [PubMed] [Google Scholar]
- 23.Sandmair AM, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 24.Puumalainen AM, Vapalahti M, Agrawal RS, et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 25.Immonen A, Vapalahti M, Tyynelä K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Chiocca EA, Aguilar LK, Bell SD, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agard C, Ligeza C, Dupas B, et al. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001;8:128–136. doi: 10.1038/sj.cgt.7700281. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112:1969–1977. doi: 10.1002/jcb.23126. [DOI] [PubMed] [Google Scholar]
- 29.Kuriyama S, Kikukawa M, Masui K, et al. Cancer gene therapy with HSV-tk/GCV system depends on T-cell-mediated immune responses and causes apoptotic death of tumor cells in vivo. Int J Cancer. 1999;83:374–380. doi: 10.1002/(sici)1097-0215(19991029)83:3<374::aid-ijc13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Miles BJ, Shalev M, Aguilar-Cordova E, et al. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum Gene Ther. 2001;12:1955–1967. doi: 10.1089/104303401753204535. [DOI] [PubMed] [Google Scholar]
- 31.Vile RG, Nelson JA, Castleden S, et al. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- 32.Chhikara M, Huang H, Vlachaki MT, et al. Enhanced therapeutic effect of HSV-tk+GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther. 2001;3:536–542. doi: 10.1006/mthe.2001.0298. [DOI] [PubMed] [Google Scholar]
- 33.Fujita T, Teh BS, Timme TL, et al. Sustained long-term immune responses after in situ gene therapy combined with radiotherapy and hormonal therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2006;65:84–90. doi: 10.1016/j.ijrobp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Chiocca EA, Smith KM, McKinney B, et al. A phase I trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther. 2008;16:618–626. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 35.Yung WK, Prados M, Levin VA, et al. Intravenous recombinant interferon beta in patients with recurrent malignant gliomas: a phase I/II study. J Clin Oncol. 1991;9:1945–1949. doi: 10.1200/JCO.1991.9.11.1945. [DOI] [PubMed] [Google Scholar]
- 36.Colombo F, Barzon L, Franchin E, et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther. 2005;12:835–848. doi: 10.1038/sj.cgt.7700851. [DOI] [PubMed] [Google Scholar]
- 37.Terao S, Shirakawa T, Goda K, et al. Recombinant interleukin-2 enhanced the antitumor effect of ADV/RSV-HSV-tk/ACV therapy in a murine bladder cancer model. Anticancer Res. 2005;25:2757–2760. [PubMed] [Google Scholar]
- 38.Kakinoki K, Nakamoto Y, Kagaya T, et al. Prevention of intrahepatic metastasis of liver cancer by suicide gene therapy and chemokine ligand 2/monocyte chemoattractant protein-1 delivery in mice. J Gene Med. 2010;12:1002–1013. doi: 10.1002/jgm.1528. [DOI] [PubMed] [Google Scholar]
- 39.Ghulam Muhammad AKM, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King GD, Muhammad AKMG, Larocque D, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol Ther. 2011;19:1793–1801. doi: 10.1038/mt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puntel M, AKM GM, Farrokhi C, et al. Safety profile, efficacy, and biodistribution of a bicistronic high-capacity adenovirus vector encoding a combined immunostimulation and cytotoxic gene therapy as a prelude to a phase I clinical trial for glioblastoma. Toxicol Appl Pharmacol. 2013;268:318–330. doi: 10.1016/j.taap.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornetta K, Croop J, Dropcho E, et al. A pilot study of dose-intensified procarbazine, CCNU, vincristine for poor prognosis brain tumors utilizing fibronectin-assisted, retroviral-mediated modification of CD34+ peripheral blood cells with O6-methylguanine DNA methyltransferase. Cancer Gene Ther. 2006;13:886–895. doi: 10.1038/sj.cgt.7700963. [DOI] [PubMed] [Google Scholar]
- 43.Maze R, Kurpad C, Pegg AE, et al. Retroviral-mediated expression of the P140A, but not P140A/G156A, mutant form of O6-methylguanine DNA methyltransferase protects hematopoietic cells against O6-benzylguanine sensitization to chloroethylnitrosourea treatment. J Pharmacol Exp Ther. 1999;290:1467–1474. [PubMed] [Google Scholar]
- 44.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 45.Pollok KE, Hartwell JR, Braber A, et al. In vivo selection of human hematopoietic cells in a xenograft model using combined pharmacologic and genetic manipulations. Hum Gene Ther. 2003;14:1703–1714. doi: 10.1089/104303403322611728. [DOI] [PubMed] [Google Scholar]
- 46.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 47.FirstWord Pharma. Amgen presents new data from phase 3 study of talimogene laherparepvec in patients with metastatic melanoma. http://www.firstwordpharma.com/node/1142597 . Accessed October 25, 2013. [Google Scholar]
- 48.Huang TT, Hlavaty J, Ostertag D, et al. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013;20:544–551. doi: 10.1038/cgt.2013.51. [DOI] [PubMed] [Google Scholar]
- 49.Huber BE, Austin EA, Richards CA, et al. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994;91:8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez OD, Logg CR, Hiraoka K, et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20:1689–1698. doi: 10.1038/mt.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKie EA, MacLean AR, Lewis AD, et al. Selective in vitro replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants in primary human CNS tumours—evaluation of a potentially effective clinical therapy. Br J Cancer. 1996;74:745–752. doi: 10.1038/bjc.1996.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 53.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 54.Harrow S, Papanastassiou V, Harland J, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 55.Mineta T, Rabkin SD, Yazaki T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 56.Aghi M, Visted T, Depinho RA, et al. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene. 2008;27:4249–4254. doi: 10.1038/onc.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 58.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Todo T, Martuza RL, Rabkin SD, et al. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 61.O'Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 62.O'Shea CC, Soria C, Bagus B, et al. Heat shock phenocopies E1B-55 K late functions and selectively sensitizes refractory tumor cells to ONYX-015 oncolytic viral therapy. Cancer Cell. 2005;8:61–74. doi: 10.1016/j.ccr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Sze DY, Iagaru AH, Gambhir SS, et al. Response to intra-arterial oncolytic virotherapy with the herpes virus NV1020 evaluated by [18F]fluorodeoxyglucose positron emission tomography and computed tomography. Hum Gene Ther. 2012;23:91–97. doi: 10.1089/hum.2011.141. [DOI] [PubMed] [Google Scholar]
- 64.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 65.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 66.Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) working group. Neurosurgery. 2012;70:234–243. doi: 10.1227/NEU.0b013e318223f5a7. [DOI] [PubMed] [Google Scholar]
- 67.Linhares P, Carvalho B, Figueiredo R, et al. Early pseudoprogression following chemoradiotherapy in glioblastoma patients: the value of RANO evaluation. J Oncol. 2013;2013:690585. doi: 10.1155/2013/690585. Article ID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 69.Pol JG, Marguerie M, Arulanandam R, et al. Panorama from the Oncolytic Virotherapy Summit. Mol Ther. 2013;21:1814–1818. doi: 10.1038/mt.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goetz C, Gromeier M. Preparing an oncolytic poliovirus recombinant for clinical application against glioblastoma multiforme. Cytokine Growth Factor Rev. 2010;21:197–203. doi: 10.1016/j.cytogfr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coffey MC, Strong JE, Forsyth PA, et al. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 72.Forsyth P, Roldán G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 73.Herrero Y, Calle M, Cornelis JJ, et al. Parvovirus H-1 infection of human glioma cells leads to complete viral replication and efficient cell killing. Int J Cancer. 2004;109:76–84. doi: 10.1002/ijc.11626. [DOI] [PubMed] [Google Scholar]
- 74.Geletneky K, Huesing J, Rommelaere J, et al. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99. doi: 10.1186/1471-2407-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galanis E, Hartmann LC, Cliby WA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saito R, Tominaga T. Convection-enhanced delivery: from mechanisms to clinical drug delivery for diseases of the central nervous system. Neurol Med Chir (Tokyo) 2012;52:531–538. doi: 10.2176/nmc.52.531. [DOI] [PubMed] [Google Scholar]
- 77.Lal R, Harris D, Postel-Vinay S, et al. Reovirus: rationale and clinical trial update. Curr Opin Mol Ther. 2009;11:532–539. [PubMed] [Google Scholar]
- 78.Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 79.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 80.Ikeda K, Wakimoto H, Ichikawa T, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wakimoto H, Ikeda K, Abe T, et al. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol Ther. 2002;5:275–282. doi: 10.1006/mthe.2002.0547. [DOI] [PubMed] [Google Scholar]
- 82.Short JJ, Rivera AA, Wu H, et al. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Mol Cancer Ther. 2010;9:2536–2544. doi: 10.1158/1535-7163.MCT-10-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Paolo NC, Shayakhmetov DM. Adenovirus de-targeting from the liver. Curr Opin Mol Ther. 2009;11:523–531. [PubMed] [Google Scholar]
- 84.Alvarez-Breckenridge C, Kaur B, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chem Rev. 2009;109:3125–3140. doi: 10.1021/cr900048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haisma HJ, Bellu AR. Pharmacological interventions for improving adenovirus usage in gene therapy. Mol Pharm. 2011;8:50–55. doi: 10.1021/mp100310h. [DOI] [PubMed] [Google Scholar]
- 86.Kaufmann JK, Nettelbeck DM. Virus chimeras for gene therapy, vaccination, and oncolysis: adenoviruses and beyond. Trends Mol Med. 2012;18:365–376. doi: 10.1016/j.molmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Thaci B, Ahmed AU, Ulasov IV, et al. Pharmacokinetic study of neural stem cell–based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther. 2012;19:431–442. doi: 10.1038/cgt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med. 2013;11:20. doi: 10.1186/1479-5876-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aboody KS, Najbauer J, Metz MZ, et al. Neural stem cell–mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5:184ra59. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tobias AL, Thaci B, Auffinger B, et al. The timing of neural stem cell–based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl Med. 2013;2:655–666. doi: 10.5966/sctm.2013-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Navaratnarajah CK, Miest TS, Carfi A, et al. Targeted entry of enveloped viruses: measles and herpes simplex virus I. Curr Opin Virol. 2012;2:43–49. doi: 10.1016/j.coviro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nettelbeck DM, Rivera AA, Kupsch J, et al. Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int J Cancer. 2004;108:136–145. doi: 10.1002/ijc.11563. [DOI] [PubMed] [Google Scholar]
- 93.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 94.Kelly EJ, Russell SJ. MicroRNAs and the regulation of vector tropism. Mol Ther. 2009;17:409–416. doi: 10.1038/mt.2008.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leber MF, Bossow S, Leonard VHJ, et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol Ther. 2011;19:1097–1106. doi: 10.1038/mt.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ylösmäki E, Martikainen M, Hinkkanen A, et al. Attenuation of Semliki Forest virus neurovirulence by microRNA-mediated detargeting. J Virol. 2013;87:335–344. doi: 10.1128/JVI.01940-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novakova J, Slaby O, Vyzula R, et al. MicroRNA involvement in glioblastoma pathogenesis. Biochem Biophys Res Commun. 2009;386:1–5. doi: 10.1016/j.bbrc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 98.Peruzzi P, Bronisz A, Nowicki MO, et al. MicroRNA-128 coordinately targets Polycomb Repressor Complexes in glioma stem cells. Neuro-Oncol. 2013;15:1212–1224. doi: 10.1093/neuonc/not055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev. 2009;61:554–571. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 100.Hoffmann D, Meyer B, Wildner O. Improved glioblastoma treatment with Ad5/35 fiber chimeric conditionally replicating adenoviruses. J Gene Med. 2007;9:764–778. doi: 10.1002/jgm.1076. [DOI] [PubMed] [Google Scholar]
- 101.Kanai R, Tomita H, Shinoda A, et al. Enhanced therapeutic efficacy of G207 for the treatment of glioma through Musashi1 promoter retargeting of gamma34.5-mediated virulence. Gene Ther. 2006;13:106–116. doi: 10.1038/sj.gt.3302636. [DOI] [PubMed] [Google Scholar]
- 102.Kambara H, Okano H, Chiocca EA, et al. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65:2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 103.Okemoto K, Kasai K, Wagner B, et al. DNA demethylating agents synergize with oncolytic HSV1 against malignant gliomas. Clin Cancer Res. 2013;19:5952–5959. doi: 10.1158/1078-0432.CCR-12-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 105.Bar EE. Glioblastoma, cancer stem cells and hypoxia. Brain Pathol. 2011;21:119–129. doi: 10.1111/j.1750-3639.2010.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hernandez-Alcoceba R, Pihalja M, Qian D, et al. New oncolytic adenoviruses with hypoxia- and estrogen receptor–regulated replication. Hum Gene Ther. 2002;13:1737–1750. doi: 10.1089/104303402760293574. [DOI] [PubMed] [Google Scholar]
- 107.Post DE, Devi NS, Li Z, et al. Cancer therapy with a replicating oncolytic adenovirus targeting the hypoxic microenvironment of tumors. Clin Cancer Res. 2004;10:8603–8612. doi: 10.1158/1078-0432.CCR-04-1432. [DOI] [PubMed] [Google Scholar]
- 108.Longo SL, Griffith C, Glass A, et al. Development of an oncolytic herpes simplex virus using a tumor-specific HIF-responsive promoter. Cancer Gene Ther. 2011;18:123–134. doi: 10.1038/cgt.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruan H, Su H, Hu L, et al. A hypoxia-regulated adeno-associated virus vector for cancer-specific gene therapy. Neoplasia. 2001;3:255–263. doi: 10.1038/sj.neo.7900157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aghi MK, Liu T-C, Rabkin S, et al. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol Ther. 2009;17:51–56. doi: 10.1038/mt.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yun C-O. Overcoming the extracellular matrix barrier to improve intratumoral spread and therapeutic potential of oncolytic virotherapy. Curr Opin Mol Ther. 2008;10:356–361. [PubMed] [Google Scholar]
- 112.Diop-Frimpong B, Chauhan VP, Krane S, et al. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guedan S, Rojas JJ, Gros A, et al. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol Ther. 2010;18:1275–1283. doi: 10.1038/mt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dmitrieva N, Yu L, Viapiano M, et al. Chondroitinase ABC I-mediated enhancement of oncolytic virus spread and antitumor efficacy. Clin Cancer Res. 2011;17:1362–1372. doi: 10.1158/1078-0432.CCR-10-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakashima H, Chiocca EA. Switching a replication-defective adenoviral vector into a replication-competent, oncolytic adenovirus. J Virol. 2014;88:345–353. doi: 10.1128/JVI.02668-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peng K-W, Myers R, Greenslade A, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013;20:255–261. doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alvarez-Breckenridge CA, Yu J, Price R, et al. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat Med. 2012;18:1827–1834. doi: 10.1038/nm.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fulci G, Dmitrieva N, Gianni D, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67:9398–9406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steele L, Errington F, Prestwich R, et al. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-κB mediated and supports innate and adaptive anti-tumour immune priming. Mol Cancer. 2011;10:20. doi: 10.1186/1476-4598-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prestwich RJ, Ilett EJ, Errington F, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galivo F, Diaz RM, Wongthida P, et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grekova SP, Raykov Z, Zawatzky R, et al. Activation of a glioma-specific immune response by oncolytic parvovirus Minute Virus of Mice infection. Cancer Gene Ther. 2012;19:468–475. doi: 10.1038/cgt.2012.20. [DOI] [PubMed] [Google Scholar]
- 125.Lapteva N, Aldrich M, Weksberg D, et al. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J Immunother. 2009;32:145–156. doi: 10.1097/CJI.0b013e318193d31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu BL, Robinson M, Han Z-Q, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 127.Kim JH, Oh JY, Park BH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 128.Lei N, Shen FB, Chang JH, et al. An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer Gene Ther. 2009;16:33–43. doi: 10.1038/cgt.2008.46. [DOI] [PubMed] [Google Scholar]
- 129.Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol Ther. 2010;18:1927–1936. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Diaz RM, Galivo F, Kottke T, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 131.Kottke T, Errington F, Pulido J, et al. Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nat Med. 2011;17:854–859. doi: 10.1038/nm.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pulido J, Kottke T, Thompson J, et al. Using virally expressed melanoma cDNA libraries to identify tumor-associated antigens that cure melanoma. Nat Biotechnol. 2012;30:337–343. doi: 10.1038/nbt.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boisgerault N, Kottke T, Pulido J, et al. Functional cloning of recurrence-specific antigens identifies molecular targets to treat tumor relapse. Mol Ther. 2013;21:1507–1516. doi: 10.1038/mt.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chow KKH, Naik S, Kakarla S, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ohnishi T, Hiraga S, Izumoto S, et al. Role of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissues. Clin Exp Metastasis. 1998;16:729–741. doi: 10.1023/a:1006532812408. [DOI] [PubMed] [Google Scholar]
- 136.Prestwich RJ, Errington F, Diaz RM, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heywood RM, Marcus HJ, Ryan DJ, et al. A review of the role of stem cells in the development and treatment of glioma. Acta Neurochir (Wien) 2012;154:951–969. doi: 10.1007/s00701-012-1338-9. [DOI] [PubMed] [Google Scholar]
- 138.Bach P, Abel T, Hoffmann C, et al. Specific elimination of CD133+ tumor cells with targeted oncolytic measles virus. Cancer Res. 2013;73:865–874. doi: 10.1158/0008-5472.CAN-12-2221. [DOI] [PubMed] [Google Scholar]
- 139.Tamura K, Wakimoto H, Agarwal AS, et al. Multimechanistic tumor targeted oncolytic virus overcomes resistance in brain tumors. Mol Ther. 2013;21:68–77. doi: 10.1038/mt.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mantwill K, Naumann U, Seznec J, et al. YB-1 dependent oncolytic adenovirus efficiently inhibits tumor growth of glioma cancer stem like cells. J Transl Med. 2013;11:216. doi: 10.1186/1479-5876-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ulasov IV, Sonabend AM, Nandi S, et al. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Br J Cancer. 2009;100:1154–1164. doi: 10.1038/sj.bjc.6604969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanai R, Rabkin SD, Yip S, et al. Oncolytic virus–mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst. 2012;104:42–55. doi: 10.1093/jnci/djr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stedt H, Samaranayake H, Pikkarainen J, et al. Improved therapeutic effect on malignant glioma with adenoviral suicide gene therapy combined with temozolomide. Gene Ther. 2013;20:1165–1171. doi: 10.1038/gt.2013.46. doi:10.1038/gt.2013-46. [DOI] [PubMed] [Google Scholar]
- 144.Otsuki A, Patel A, Kasai K, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 145.MacTavish H, Diallo J-S, Huang B, et al. Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PloS One. 2010;5:e14462. doi: 10.1371/journal.pone.0014462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Watanabe Y, Hashimoto Y, Kagawa S, et al. Enhanced antitumor efficacy of telomerase-specific oncolytic adenovirus with valproic acid against human cancer cells. Cancer Gene Ther. 2012;19:767–772. doi: 10.1038/cgt.2012.57. [DOI] [PubMed] [Google Scholar]
- 147.Thaci B, Ulasov IV, Ahmed AU, et al. Anti-angiogenic therapy increases intratumoral adenovirus distribution by inducing collagen degradation. Gene Ther. 2013;20:318–327. doi: 10.1038/gt.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang W, Fulci G, Buhrman JS, et al. Bevacizumab with angiostatin-armed oHSV increases antiangiogenesis and decreases bevacizumab-induced invasion in U87 glioma. Mol Ther. 2012;20:37–45. doi: 10.1038/mt.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kanai R, Wakimoto H, Martuza RL, et al. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin Cancer Res. 2011;17:3686–3696. doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li H, Nakashima H, Decklever TD, et al. HSV-NIS, an oncolytic herpes simplex virus type 1 encoding human sodium iodide symporter for preclinical prostate cancer radiovirotherapy. Cancer Gene Ther. 2013;20:478–485. doi: 10.1038/cgt.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Belin LJ, Ady JW, Lewis C, et al. An oncolytic vaccinia virus expressing the human sodium iodine symporter prolongs survival and facilitates SPECT/CT imaging in an orthotopic model of malignant pleural mesothelioma. Surgery. 2013;154:486–495. doi: 10.1016/j.surg.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Opyrchal M, Allen C, Iankov I, et al. Effective radiovirotherapy for malignant gliomas by using oncolytic measles virus strains encoding the sodium iodide symporter (MV-NIS) Hum Gene Ther. 2012;23:419–427. doi: 10.1089/hum.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Prados MD, McDermott M, Chang SM, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol. 2003;65:269–278. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 154.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 155.Germano IM, Fable J, Gultekin SH, et al. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65:279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 156.Smitt PS, Driesse M, Wolbers J, et al. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther. 2003;7:851–858. doi: 10.1016/s1525-0016(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 157.Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 158.Packer RJ, Raffel C, Villablanca JG, et al. Treatment of progressive or recurrent pediatric malignant supratentorial brain tumors with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration. J Neurosurg. 2000;92:249–254. doi: 10.3171/jns.2000.92.2.0249. [DOI] [PubMed] [Google Scholar]