Abstract
Objective:
Dexamethasone (Dex) is a synthetic glucocorticoid that has pro-anabolic and anticatabolic effects in cartilage tissue engineering systems, though the mechanisms by which these effects are mediated are not well understood. We tested the hypothesis that the addition of Dex to chondrogenic medium would affect matrix production and aggrecanase activity of human and bovine bone marrow stromal cells (BMSCs) cultured in self-assembling peptide and agarose hydrogels.
Design:
We cultured young bovine and adult human BMSCs in (RADA)4 self-assembling peptide and agarose hydrogels in medium containing TGF-β1±Dex and analyzed extracellular matrix composition, aggrecan cleavage products, and the effects of the glucocorticoid receptor antagonist RU-486 on proteoglycan content, synthesis, and catabolic processing.
Results:
Dex improved proteoglycan synthesis and retention in agarose hydrogels seeded with young bovine cells but decreased proteoglycan accumulation in peptide scaffolds. These effects were mediated by the glucocorticoid receptor. Adult human BMSCs showed minimal matrix accumulation in agarose, but accumulated ~50% as much proteoglycan and collagen as young bovine BMSCs in peptide hydrogels. Dex reduced aggrecanase activity in (RADA)4 and agarose hydrogels, as measured by anti-NITEGE Western blotting, for both bovine and human BMSC-seeded gels.
Conclusions:
The effects of Dex on matrix production are dependent on cell source and hydrogel identity. This is the first report of Dex reducing aggrecanase activity in a tissue engineering culture system.
Keywords: chondrogenesis, mesenchymal stem cells, degradative enzymes, articular cartilage, extracellular matrix
Introduction
Tissue engineering using bone marrow-derived stromal cells (BMSCs) is an attractive strategy for healing cartilage defects. BMSCs have been shown to differentiate to a cartilage lineage in a variety of scaffolds and create cartilage-like extracellular matrix (ECM).1-4 These cells can be extracted without the need for further damage to the joint and expanded for several passages without losing differentiation potential.5,6 Additionally, BMSCs have been found to synthesize ECM with higher dynamic stiffness, longer sulfated glycosaminoglycan (sGAG) chains, and longer aggrecan core proteins than matrix synthesized by chondrocytes, regardless of donor age.7 Despite progress in cartilage tissue engineering, further optimization is needed to improve integration with native tissue, mechanical function, and maintenance of the chondrocyte phenotype.8,9
One strategy for improving our understanding of these complex systems is to investigate the specific effects of chemical factors added to the cellular microenvironment. Dexamethasone (Dex) is a synthetic glucocorticoid frequently added to culture medium for chondrogenesis studies, motivated by research demonstrating pellet cultures of rabbit BMSCs stimulated with TGF-β1 and Dex (TGF + Dex) grew larger than those cultured with TGF-β1 alone.2 Additional studies found that TGF + Dex increased aggrecan biosynthesis and gene expression in bovine and human BMSCs over TGF-β1 alone.10,11
Dex also has anticatabolic properties. The aggrecanases ADAMTS-4/5 (a disintegrin and metalloproteinase with thrombospondin motifs-4/5) are key destructive enzymes in human osteoarthritis progression and are involved in cytokine-induced aggrecanolysis in cartilage explants.12-14 ADAMTS-4/5–generated aggrecan fragments are also found in BMSC-seeded hydrogels.3 Recently, Dex was found to reduce sGAG loss and rescue proteoglycan synthesis in cartilage explants exposed to inflammatory cytokines.15 The mechanisms through which Dex mediates pro-anabolic and anticatabolic effects is not well understood, though evidence that the glucocorticoid receptor mediates the increase in aggrecan mRNA levels caused by Dex has been reported.16
We have used self-assembling peptide hydrogels for cartilage tissue engineering because they support TGF-β1-induced chondrogenesis of BMSCs in vitro3 and have been successfully used for studies of cartilage repair in animal studies.17,18 Additionally, self-assembling peptides have been used to deliver growth factors,18-21 which is important for designing an optimal chondrogenic microenvironment as well as controlling sustained, local delivery of growth factors. Although scaffold-free, or pellet, cultures offer important culture systems for studying chondrogenesis, our objective was to investigate BMSC matrix production and catabolism in hydrogel scaffolds motivated by the long-term translational challenges of growth factor delivery and integration between neocartilage and adjacent native cartilage. We tested the hypothesis that chondrogenesis of human and bovine BMSCs in self-assembling peptide hydrogels, as well as subsequent matrix production and aggrecanase activity, would be responsive to chondrogenic medium supplemented with Dex. We compared the influence of Dex on chondrogenesis of young bovine BMSCs and adult human BMSCs in (RADA)4 self-assembling peptide hydrogels and agarose hydrogels. By analyzing accumulation of sGAG, DNA, and collagen, the effects of the glucocorticoid receptor antagonist RU-486, and aggrecan cleavage products, we found that cell source and scaffold environment changed the BMSC response to Dex, emphasizing the importance of optimizing these variables for neocartilage generation. Dex reduced ADAMTS-4/5 activity in (RADA)4 self-assembling peptide and agarose hydrogels for both cell types.
Methods
Bovine BMSC Isolation and Expansion
Bovine BMSCs were extracted from the femora and tibiae of four 1- to 2-week-old calves (Research 87, Marlborough, MA) as described previously.3 Briefly, marrow was isolated aseptically, homogenized in phosphate-buffered saline (PBS), centrifuged, and the cell fraction was plated on tissue culture plastic for 30 minutes to remove rapidly adhering cells. The remaining cell population was plated into flasks at 1 × 106 mononuclear cells/cm2. Remaining red blood cells were removed by a medium change 2 days after plating. Colonies were expanded in low glucose Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Inc, Manassas, VA) with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, or ThermoScientific, Logan, UT), penicillin streptomycin amphotericin (PSA) (Sigma-Aldrich, St. Louis, MO), 4-(2-hydroxyethyl)-l-piperzaineethanesulfonic acid (HEPES) (Invitrogen), and 1 ng/mL basic fibroblast growth factor (bFGF) (R&D Systems, Minneapolis, MN) until reaching 80% confluence as described previously.3 Cells were then removed by 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid (EDTA) (Invitrogen) and frozen for future use (passage 0, P0). After thawing frozen P0 aliquots, cells were seeded at 6,000 cells/cm2 and expanded two passages consisting of 3 days each in expansion medium with 5 ng/mL bFGF. BMSCs were then seeded into hydrogels for chondrogenesis studies.
Human BMSC Isolation and Expansion
Human BMSCs (hBMSCs) were isolated from intramedullary aspirate generated using a Reamer Irrigator Aspirator device during surgical procedures performed at Brigham and Women’s Hospital and Massachusetts General Hospital (Boston, MA).22 The three patients whose cells were used in this study (two males, one female, ages 81, 51, and 37) granted informed consent, and surgical procedures were preapproved by the local institutional review board. The bulk aspirate was centrifuged, the red blood cell fraction lysed briefly in a buffer of 155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA (pH 7.2), and BMSCs isolated from the remaining nucleated cell fraction by differential adhesion to tissue culture plastic as above. These donor cell populations have been confirmed to undergo chondrogenic differentiation in pellet cultures in response to TGF-β1 and Dex.23 Frozen aliquots of P1 or P2 cells were thawed and seeded at 1,000 cells/cm2 in expansion medium with 5 ng/mL bFGF. Cultures received supplements of bFGF on day 4, passaged on day 6, given more bFGF on day 9 or 10, and were seeded into hydrogels on day 12. One patient’s cells required further expansion and were passaged on day 12, given more bFGF on day 14, and cast on day 17.
Hydrogel Encapsulation and Culture
Following BMSC expansion in monolayer, cells were encapsulated in one of two hydrogel materials: (RADA)4 self-assembling peptide hydrogel (RAD, also known as PuraMatrix, a gift from 3DM, Cambridge, MA) and low-melting-point agarose (Invitrogen, catalog number 15517-022). BMSCs were encapsulated in 0.5% (w/v) RAD or 2% (w/v) agarose at 107 cells/mL. These hydrogel concentrations were chosen to match previous studies.3,4,11,24,25 The cell/hydrogel mixture was cast as disks (~6 mm diameter, 1.5 mm thickness) into the center of rings of acellular agarose pre-equilibrated in chondrogenic medium consisting of high glucose DMEM, 1% ITS+1 (Sigma-Aldrich; insulin, transferrin, sodium selenite, bovine serum albumin, and linoleic acid), proline (Sigma-Aldrich), ascorbate-2-phosphate (Wako Chemicals, Richmond, VA), HEPES, PSA, nonessential amino acids (NEAA) (Sigma-Aldrich), and sodium pyruvate (Invitrogen), as described previously.3 The cell-seeded hydrogel disks were cultured in chondrogenic medium with 10 ng/mL recombinant human TGF-β1 (R&D Systems) with or without 100 nM dexamethasone (Sigma-Aldrich), labeled TGF + Dex or TGF, respectively. Hydrogels were cultured for up to 21 days with medium changes every 2 to 3 days. Some hydrogels were immediately cast into buffer containing 50 mM tris(hydroxymethyl)aminomethane (Tris) and 1 mM CaCl2 to measure day 0 DNA levels.
Viability Staining
To assess viability of BMSCs for all culture conditions, hydrogels were viewed using a Nikon Eclipse TE-300 fluorescence microscope at approximately day 4 with 4 µg/mL fluorescein diacetate (live cells) and either 0.875 µg/mL ethidium bromide or 35 µg/mL propidium iodide (dead cells).
Glucocorticoid Receptor Antagonist Culture
RU-486 (Mifepristone; Sigma-Aldrich), a glucocorticoid receptor antagonist with partial agonist activity,26,27 was added to the culture medium to determine whether observed responses to Dex were mediated by the glucocorticoid receptor. A dose–response study confirmed that 1 µM RU-486 would not interfere with BMSC viability or sGAG synthesis. This dose has been used previously.16 Bovine BMSCs were encapsulated in (RADA)4 or agarose hydrogels as described above and cultured in medium with 10 ng/mL TGF-β1 or 10 ng/mL TGF-β1 with 100 nM Dex with and without RU-486 for 21 days.
Hydrogel Biochemistry
For the final 24 hours of culture, hydrogels used for biochemical analysis were radiolabeled with 5 µCi/mL 35S-sulfate and 10 or 20 µCi/mL 3H-thymidine (Perkin Elmer Inc, Waltham, MA) to measure proteoglycan and DNA synthesis, respectively. Unincorporated radiolabel was removed, hydrogels were weighed wet, lyophilized, weighed dry, and digested with Proteinase-K (Roche Applied Science, Indianapolis, IN) as described previously.3 Digested samples were assayed for sGAG content by 1.9-dimethylmethylene blue (DMMB) dye binding,28 DNA content by Hoechst dye binding,29 and radiolabel incorporation by liquid scintillation counting. Hydroxyproline content, as a measure of total collagen, was measured by reaction with p-dimethylaminobenzaldehyde.30 sGAG released to the culture medium was measured by DMMB.
Histology and Immunohistochemistry
Day 21 hydrogels from one experiment were fixed in 4% paraformaldehyde for 2 hours at room temperature and overnight at 4 °C. Osteochondral explants were collected from the distal femurs of 1- to 2-week-old bovine calves for collagen staining controls. Explants were fixed in 4% paraformaldehyde, transferred to 70% ethanol, and demineralized. Gels and explants were then embedded in paraffin, sliced into sections, deparaffinized, and stained as described previously in detail.3 Briefly, for immunohistochemistry, samples were stained for type I or type II collagen; negative controls were incubated without primary antibodies.31 Additional sections were stained for sulfated proteoglycans using toluidine blue dye solution.32 For detection of apoptotic cells, sections were stained with hematoxylin. Cells with and without nuclear blebbing were counted using a Zeiss Axiophot microscope with 40x objective (Zeiss, Wetzlar, Germany). For quantification of nuclear blebbing,33 sections from three different gels per experimental group were evaluated by counting cells in three adjacent, distinct fields of vision in the center of each section. For each individual gel/sample the percentage of apoptotic cells was calculated and the mean value for each experimental group was given (n = 3).
Aggrecan Extraction and Western Blot Analysis
Additional hydrogels were cultured for 21 days, soaked in PBS with Complete Protease Inhibitors (Roche) for ~2 hours, and frozen at −20 °C until extraction. Hydrogels were rotated in 4 M guanidine hydrochloride with 100 mM sodium acetate plus protease inhibitors for 2 days at 4 °C to extract proteoglycans. After centrifugation at 13,000g for 30 minutes, the supernatant was removed and its sGAG content was measured by DMMB. Aggrecan extract was then run through microcentrifuge tubes with a 10,000 MW cut-off (Millipore, Billerica, MA). Retained protein was washed twice with buffer containing 0.05 M Tris, 0.05 M sodium acetate, and 0.01 M EDTA and resuspended in this buffer at a concentration of 1 µg sGAG/μL. The aggrecan was then deglycosylated using protease-free chondroitinase ABC (30 mU/100 µg sGAG), keratanase II (0.5 mU/100 µg sGAG), and endo-β-galactosidase (0.5 mU/100 µg sGAG) (Seikagaku Biobusiness Corporation, Tokyo, Japan). sGAG was loaded into a 4% to 12% Bis-Tris gel (Invitrogen) and run at 200 V for 45 minutes. Proteins were transferred to a polyvinylidene fluoride membrane and probed with the anti-NITEGE monoclonal antibody AGG-C134 (a gift from Dr. Carl Flannery, Pfizer) and the anti-G1 antibody G1-2 (a gift from Dr. John Sandy, Rush University).35 Some membranes were stripped following anti-NITEGE imaging and reprobed with anti-G1 antibody. To ensure the removal of the NITEGE antibody following stripping, membranes were exposed to the secondary antibody again and imaged to ensure no signal.
Statistical Analysis
Results are reported as mean ± standard error of the mean. A linear mixed model of variance with animal/patient as a random factor and medium condition and time point as fixed effects was used to analyze data for experiments testing the effects of Dex on sGAG content, DNA content, proteoglycan synthesis, DNA synthesis, sGAG retention, and hydroxyproline content for bovine and human BMSCs. Data from the agarose and RAD scaffolds were analyzed separately and not compared statistically. Data from experiments with RU-486 were analyzed using the same model with only the medium condition as a fixed effect. Residual plots for all of the above comparisons were investigated and data were transformed as necessary to ensure normality. Apoptotic cell data were analyzed using a general linear model with medium condition as an independent variable. A Kolmogorov-Smirnov test was used to ensure normality and data were transformed as necessary. Tukey post hoc tests with P < 0.05 were used to evaluate statistical significance for all pairwise comparisons. Statistical tests were performed using Systat 12 software.
Results
Matrix and Cellular Content of Bovine BMSC-Seeded Hydrogels
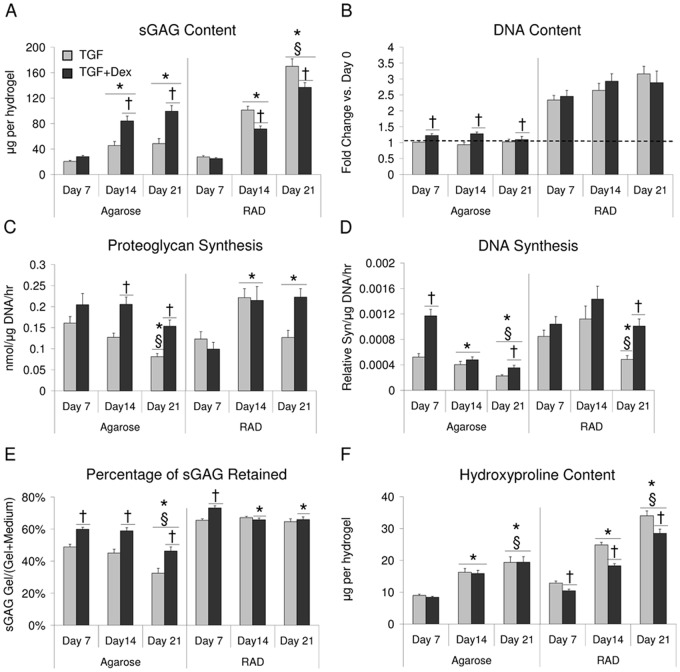
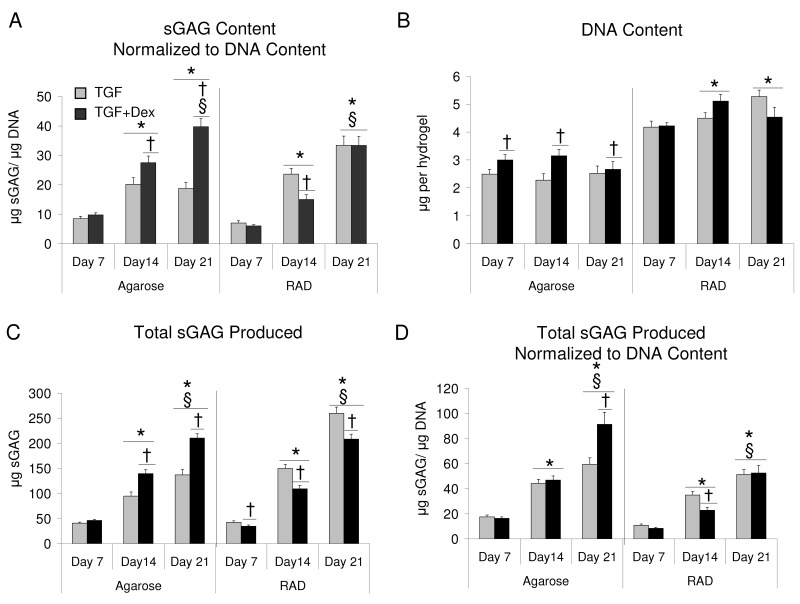
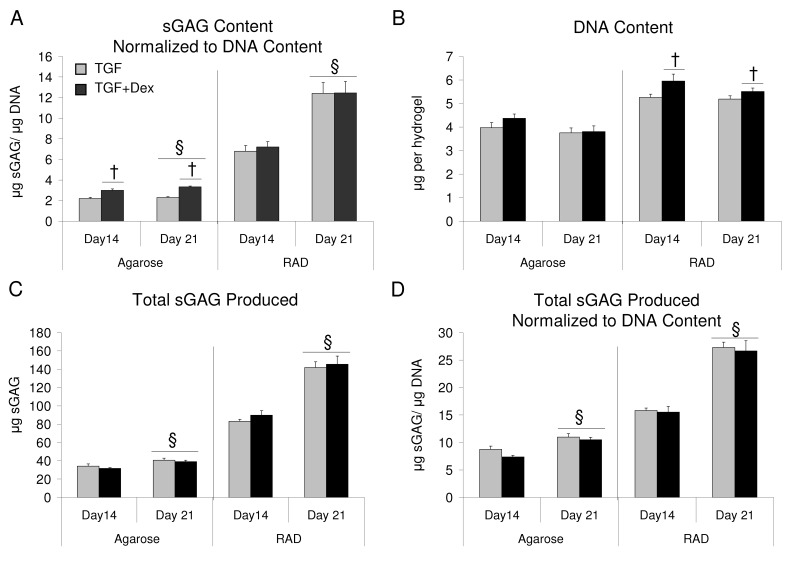
sGAG content increased with time in both scaffolds seeded with bovine BMSCs, though there was no statistical difference between days 14 and 21 in agarose hydrogels. By day 21, sGAG content of RAD hydrogels was nearly 150% of agarose hydrogels. Consistent with previous literature,11 TGF + Dex significantly increased sGAG accumulation over TGF alone in agarose hydrogels (Fig. 1A). In RAD, a decrease in sGAG accumulation was observed with Dex compared with TGF alone on days 14 and 21. DNA content did not change significantly between days 7 and 21 for either scaffold (Fig. 1B). In agarose, the addition of Dex increased DNA content at all time points. Whereas little or no proliferation was observed in agarose hydrogels, RAD hydrogels showed a 2.5-fold increase in DNA content over day 0 levels by day 7. DNA content and sGAG normalized to DNA are also reported in Supplementary Figure S1.
Figure 1.
Extracellular matrix and cellular content in agarose and RAD hydrogels seeded with bovine bone marrow stromal cells (BMSCs) and cultured in TGF or TGF + dexamethasone (Dex) medium. (A) Sulfated glycosaminoglycan (sGAG) content, (B) DNA content, (C) proteoglycan synthesis, (D) DNA synthesis, (E) percent sGAG retained, (F) hydroxyproline content. Values are mean ± standard error of the mean. n = 13-16 (3-4 hydrogels × 4 animals). †Versus TGF, *versus day 7, §versus day 14, P < 0.05.
Proteoglycan synthesis normalized to DNA content increased from day 7 to days 14 and 21 in RAD and remained elevated with TGF + Dex, whereas synthesis dropped at day 21 with TGF alone (Fig. 1C). Proteoglycan synthesis showed a trend of decreasing over time in agarose. TGF + Dex showed a significant increase compared with TGF alone for agarose at days 14 and 21, consistent with sGAG content. DNA synthesis rates normalized to DNA content were not statistically different over time for the TGF + Dex condition in RAD, whereas the TGF alone condition showed a significant decrease at day 21 (Fig. 1D). In agarose, DNA synthesis decreased with time in both conditions. Dex supplementation significantly increased DNA synthesis in agarose at day 7 and day 21.
Dex had a greater effect on sGAG retention in agarose than in RAD hydrogels (Fig. 1E). In agarose, sGAG retention decreased from day 14 to day 21, and the addition of Dex significantly increased retention at all time points. In contrast, sGAG retention was generally higher in RAD than in agarose at all times with or without Dex. Dex increased sGAG retention over TGF alone in RAD at day 7. Total sGAG produced and total sGAG normalized to DNA are also reported in Supplementary Figure S1.
Hydroxyproline content increased over time in both scaffolds (Fig. 1F), similar to the trends in sGAG content. In RAD, addition of Dex decreased hydroxyproline content at all time points compared with TGF alone. In agarose, there was no statistical difference between TGF and TGF + Dex at any time point.
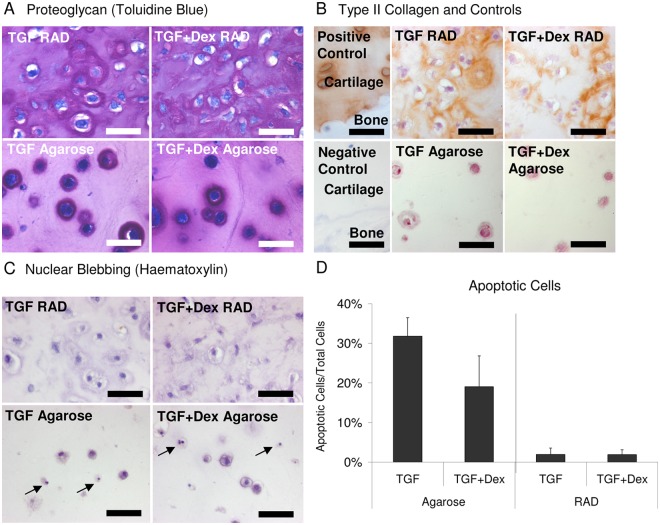
Histology and Immunohistochemistry
Toluidine blue staining of day 21 hydrogels (Fig. 2A) was consistent with the quantitative sGAG content (Fig. 1A), with TGF + Dex and TGF gels showing similar levels of staining in RAD and TGF + Dex agarose gels showing darker staining than TGF alone. Interestingly, addition of Dex led to more diffuse staining throughout the agarose scaffold compared with the largely pericellular staining for TGF alone.
Figure 2.
Representative staining of day 21 agarose and RAD hydrogels seeded with bovine bone marrow stromal cells (BMSCs) and cultured with TGF or TGF + dexamethasone (Dex) medium. (A) Toluidine blue staining for proteoglycans, (B) type II collagen immunohistochemistry, (C) hematoxylin staining for nuclear blebbing (arrows indicate apoptotic cells), and (D) percentage of apoptotic cells, n = 3; values are mean ± standard error of the mean. Controls performed on bovine osteochondral explants. Scale bar is 30 µm.
To investigate the types of collagen present in day 21 hydrogels, immunohistochemistry was performed. RAD hydrogels were positive for collagen type II staining, but agarose gels were not (Fig. 2B). After analyzing multiple sections of RAD gels, a trend of more collagen type II staining in TGF alone gels compared with TGF + Dex gels was seen in RAD, based on the distribution of matrix areas that were stained positively. This was consistent with higher levels of hydroxyproline in RAD gels with TGF alone compared with TGF + Dex (Fig. 1F). Collagen type I staining was not seen in either agarose or RAD hydrogels (data not shown).
Day 21 hydrogels were also analyzed for nuclear blebbing as an indicator of apoptosis by hematoxylin staining.33 Representative images showed little apoptosis in RAD gels with either medium condition, whereas agarose gels showed much higher levels of blebbing (Fig. 2C). After reviewing multiple sections, the percentage of apoptotic cells was calculated for each condition (Fig. 2D). Agarose hydrogels showed 20% to 30% of total cells being apoptotic, whereas RAD gels were near 2%. TGF + Dex showed a trend of less apoptosis than TGF alone in agarose (19.0% vs. 31.8%, P = 0.186).
Glucocorticoid Receptor Antagonist Studies
RU-486 was added to the culture medium in 10-fold excess of Dex to determine whether the previous responses were mediated by the glucocorticoid receptor. At day 21, TGF plus RU-486 (TGF + RU) was not significantly different from the TGF alone condition in either scaffold, as expected (Fig. 3A). Importantly, the addition of RU-486 to the TGF + Dex condition significantly reduced sGAG levels such that there was no difference between TGF + Dex + RU and TGF alone in agarose. In RAD, a decrease in sGAG content was observed for TGF + Dex + RU compared with TGF + RU and TGF alone. DNA content was not significantly different among media conditions in either scaffold (data not shown).
Figure 3.
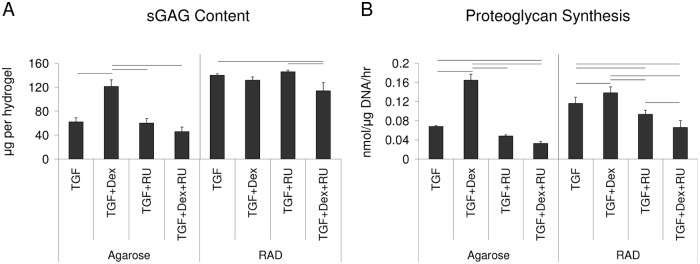
Effects of RU-486 on matrix production in agarose and RAD hydrogels seeded with bovine bone marrow stromal cells (BMSCs) and cultured in TGF medium ± RU-486 and TGF + Dex ± RU-486 medium for 21 days. (A) Sulfated glycosaminoglycan (sGAG) content and (B) proteoglycan synthesis. Values are mean ± standard error of the mean. n = 8 (4 hydrogels × 2 animals). Line indicates significant difference between two conditions, P < 0.05.
Addition of RU-486 to the TGF + Dex condition in agarose reduced proteoglycan synthesis levels to that below TGF alone levels (Fig. 3B), consistent with the results of total sGAG content analysis in Figure 3A. In RAD, addition of RU-486 lowered the TGF + Dex production of sGAG to that below the TGF alone level.
Bovine Aggrecan Western Blot
Western blots of aggrecan extracted from day 21 hydrogels were performed to analyze G1-NITEGE neoepitope fragments generated by ADAMTS-4/5 cleavage as well as all fragments containing a G1 domain. In agarose, both TGF and TGF + Dex showed NITEGE fragments (Fig. 4A), though the staining was reduced in the TGF + Dex condition. In RAD, NITEGE fragments were found in the TGF alone condition, but were dramatically reduced for TGF + Dex. Addition of the RU-486 GR antagonist resulted in the reappearance of full-intensity NITEGE bands when added to the TGF + Dex condition in both scaffolds. All conditions in both scaffolds showed full-length aggrecan as a dominant species, with NITEGE fragments also staining strongly in the agarose hydrogels (Fig. 4B). The ~140 kDa band is consistent with m-calpain activity,36,37 although the activity of this enzyme was not investigated specifically in this study. The bands between 40 and 50 kDa are consistent with link protein, which shares close homology to the aggrecan G1 domain.38,39
Figure 4.
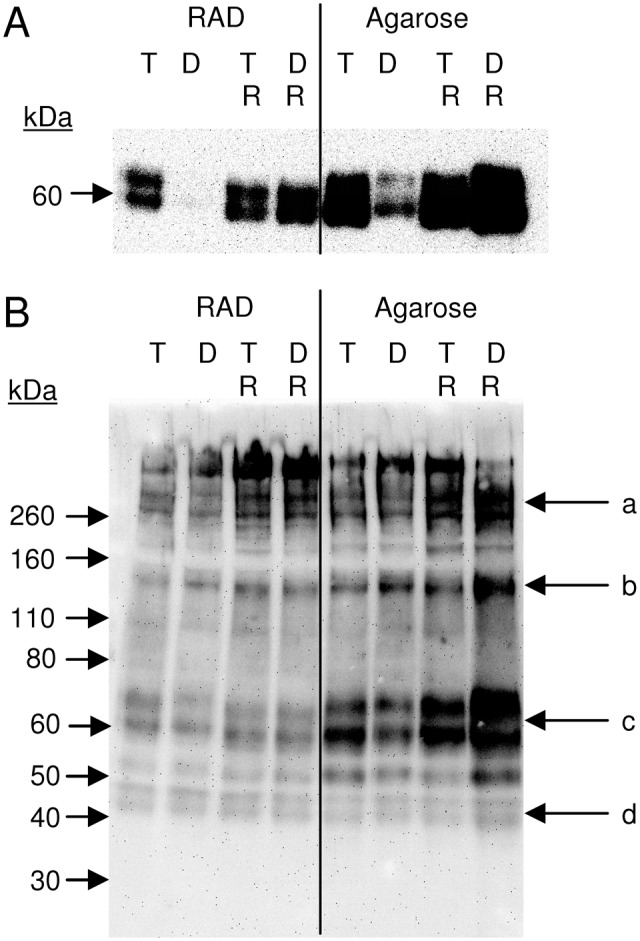
Analysis of aggrecan cleavage products extracted from day 21 agarose and RAD hydrogels seeded with bovine bone marrow stromal cells (BMSCs) and cultured in TGF medium (T) ± RU-486 (R) and TGF + Dex (D) ± RU-486 medium. Ten micrograms sGAG loaded per lane. (A) anti-NITEGE Western blot and (B) anti-G1 Western blot. Arrows in (B) correspond to (a) full-length aggrecan, (b) potential m-calpain cleavage fragment, (c) G1-NITEGE fragment, and (d) potential link protein. Anti-NITEGE blot was stripped and reprobed with anti-G1 antibody to obtain image B. Representative of two repeats for each experiment type.
Matrix Content and Aggrecan Western Blot for Human BMSC-Seeded Hydrogels
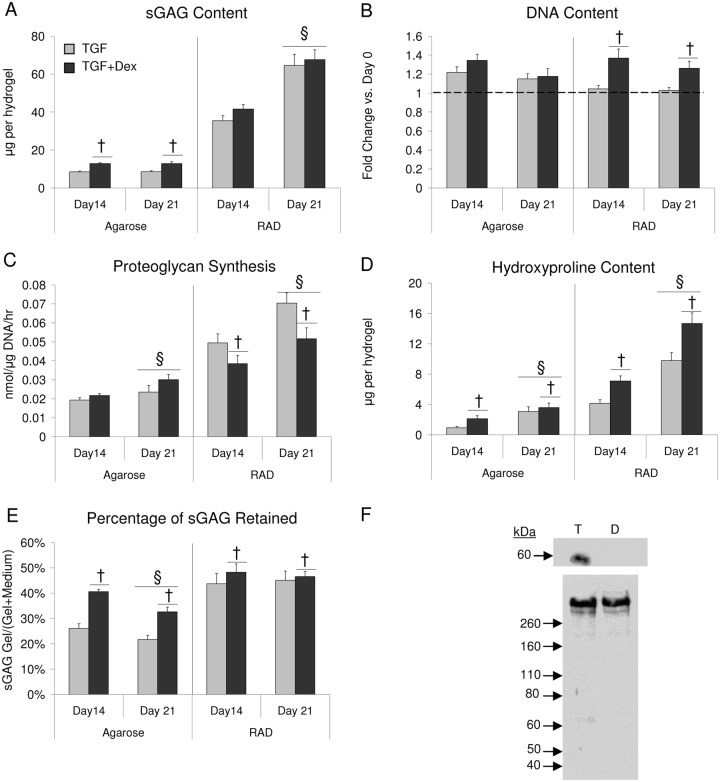
To investigate the applicability of these results to a more clinically relevant scenario, the response of human BMSCs (hBMSCs) to Dex when cultured in RAD and agarose scaffolds was also tested. hBMSCs produced minimal sGAG in agarose hydrogels (Fig. 5A), whereas sGAG accumulation in RAD hydrogels seeded with hBMSCs was ~50% of that of young bovine BMSCs in RAD gels. In agarose there was an increase in sGAG with Dex supplementation at both time points, whereas in RAD Dex had no significant effect. In agarose there was no significant increase in sGAG with time, whereas in RAD sGAG content increased significantly from day 14 to 21.
Figure 5.
Extracellular matrix and cellular content in agarose and RAD hydrogels seeded with human bone marrow stromal cells (BMSCs) and cultured in TGF or TGF + Dex medium. (A) Sulfated glycosaminoglycan (sGAG) content, (B) DNA content, (C) proteoglycan synthesis, (D) hydroxyproline content, (E) percent sGAG retained, (F) Western blot of aggrecan extracted from day 21 RAD gels seeded with human BMSCS and cultured in TGF (T) and TGF + Dex (D) medium. Top blot is anti-NITEGE, lower blot is anti-G1. Twenty micrograms sGAG loaded per lane. Values are mean ± standard error of the mean. n = 11-17 (3-6 hydrogels × 2-3 patients). †Versus TGF, §versus day 14, P < 0.05.
Neither scaffold showed high levels of proliferation as measured by a fold change in DNA compared with day 0 (Fig. 5B). In RAD, TGF + Dex showed more proliferation than TGF alone at both time points. In agarose there was no significant effect of Dex. Neither scaffold showed a significant change in DNA content from day 14 to 21. DNA content and sGAG normalized to DNA are also reported in Supplementary Figure S2.
Proteoglycan synthesis normalized to DNA content in RAD increased with time and was higher in the TGF alone condition than TGF + Dex at both time points (Fig. 5C). Proteoglycan synthesis in agarose increased with time and showed no significant effect of Dex.
Hydroxyproline content in RAD hydrogels increased with TGF + Dex compared with TGF alone at both time points (Fig. 5D). Hydroxyproline content increased with time in RAD for both media conditions. Agarose showed identical statistical differences between conditions, though the levels of hydroxyproline content were less than ~33% of those found in RAD.
sGAG retention levels were ~50% in RAD with a significant increase for TGF + Dex compared with TGF alone (Fig. 5E). sGAG retention levels did not change significantly over time in RAD. In agarose, sGAG retention decreased with time and was increased by the addition of Dex. Total sGAG produced and total sGAG normalized to DNA are also reported in Supplementary Figure S2.
Consistent with Western blots performed on hydrogels seeded with bovine BMSCs, aggrecan extracted from day 21 hBMSC-seeded RAD hydrogels showed NITEGE fragments with TGF alone, but a dramatic decrease in NITEGE fragments with TGF + Dex (Fig. 5F). Both conditions showed a strong full-length aggrecan band in the G1 blot. Aggrecan from hBMSCs cultured in agarose gels was not analyzed by Western blotting because of the limited quantity of sGAG produced.
Discussion
We hypothesized that Dex would improve matrix production and reduce ADAMTS-4/5 activity in agarose and RAD hydrogels seeded with adult human and young bovine BMSCs. We tested this hypothesis by comparing sGAG, DNA, and hydroxyproline accumulation, apoptosis, and ADAMTS-4/5-generated NITEGE fragments for self-assembling peptide and agarose hydrogels cultured in TGF-β1 ± Dex. To our knowledge, this is the first study to report that Dex affects ADAMTS-4/5 activity in a tissue engineering system. We found that Dex reduced ADAMTS-4/5 activity across both hydrogel types and cell sources, but the overall effects of Dex on chondrogenesis depended on the donor species/age and the type of hydrogel. We have demonstrated chondrogenic differentiation through cartilage-like matrix production, including aggrecan and type II collagen. Others have investigated the effects of Dex on TGF-β-induced chondrogenesis at the gene expression level for both bovine and human BMSCs and have found evidence of the pro-anabolic effects of Dex that are consistent with our results.3,11,16,40
For young bovine BMSCs in agarose, Dex caused a twofold increase in sGAG content, consistent with previous literature.11 This increase was because of the increase in proteoglycan synthesis per cell caused by Dex. Although statistical comparisons were not made between agarose and RAD hydrogel results, as this was the focus of our previous work,3 it is interesting to note one additional finding. Bovine BMSCs proliferated to a greater extent in RAD than agarose, which ultimately resulted in the accumulation of more total sGAG even though sGAG per cell (as measured by sGAG normalized to DNA) was lower in RAD than agarose for the TGF + Dex condition (Fig. S1A). sGAG was better retained in bovine BMSC-seeded agarose hydrogels with TGF + Dex compared with TGF alone. A trend of decreasing apoptosis was found in the presence of Dex for young bovine BMSCs in agarose at day 21, whereas RAD hydrogels with bovine BMSCs showed very little apoptosis in either condition. RAD gels seeded with young bovine BMSCs had reduced sGAG and hydroxyproline content in the presence of Dex compared with TGF-β1 alone, but the overall levels of these matrix components were still higher than in agarose hydrogels in the presence of Dex at day 21. An increase in proteoglycan synthesis with Dex was observed on day 21; therefore, it is possible that longer culture duration with TGF + Dex could have resulted in increased sGAG accumulation. The effects of Dex on sGAG accumulation and synthesis in both scaffolds were mediated by the signaling of Dex through the glucocorticoid receptor, which correlates well with the ability of RU-486 to reverse Dex-induced aggrecan gene expression and type II collagen production by human mesenchymal progenitor cells derived from bone.16
Hydrogels seeded with adult human BMSCs showed less matrix production overall compared with young bovine-BMSC-seeded gels. In RAD hydrogels with hBMSCs, the addition of Dex increased DNA and hydroxyproline content, but not sGAG content. We were surprised to find very little evidence of chondrogenic differentiation by hBMSCs in agarose. A previous study of hBMSCs in agarose showed evidence of proteoglycan and type II collagen production at the protein level, but only one cell cluster was shown.25 hBMSCs have been successfully cultured in other scaffold materials, though pellet culture remains one of the most common culture methods for these cells.10,16,41-44
ADAMTS-4/5 activity, as measured by anti-NITEGE Western blotting, was decreased by Dex in RAD and agarose hydrogels seeded with young bovine BMSCs and for RAD gels seeded with adult human BMSCs. It is exciting that this finding was consistent across scaffold type and cell species/age. A recent study has shown that Dex does not regulate ADAMTS-4/5 activity at the gene expression level15 but suggests the possibility that Dex could be involved in regulating the activation of latent ADAMTS-4/5 enzymes. This is an exciting research area that is the focus of ongoing studies. Use of the glucocorticoid receptor antagonist RU-486 confirmed that the decrease in ADAMTS-4/5-generated NITEGE fragments was mediated by the glucocorticoid receptor. This is an interesting finding that should be investigated further.
There were several limitations to this study. First, apoptosis was only investigated at early time points to ensure high viability, and at day 21 to compare with other histology samples. Further study of cell death over the entire culture duration comparing across conditions could aid in optimizing tissue engineering systems. Second, proteoglycan and DNA synthesis data were normalized to DNA content (Figs. 1, 3, and 5), which underestimates the synthesis levels in agarose, as the DNA levels include live and dead cells. Third, hydroxyproline content reflects multiple collagen types and the type present in agarose gels seeded with bovine BMSCs (Fig. 1F) was not determined in this study. As the matrix in agarose was largely pericellular (Fig. 2A) and the pericellular matrix (PCM) of primary chondrocytes cultured for 21 days in agarose was found to be rich in type VI collagen,45 we believe this is an abundant constituent of the PCM. Finally, our results encompassing young bovine and adult human BMSCs do not allow us to conclude whether differences in outcomes were associated with donor age or species. Both of these factors are important, and given the conflicting evidence about the importance of donor age for BMSC therapies, further studies are warranted.46 Recent work by Erickson et al. has shown that BMSCs from young bovine tissue produce cell aggregates with higher sGAG and collagen content than BMSCs from skeletally mature adult donors, bringing forth the hypothesis that the difference in cell donor ages could be a contributing factor in this study.47 Despite the limited number of human donors used in this study, we did find reproducible trends, which allowed us to find the statistically significant differences between conditions presented here. The differences we found between the cell types used here highlight the importance of considering the effects of species and age differences when translating in vitro studies into a clinical setting.
Several questions for future work remain, including how cell interactions with the scaffold microenvironment affect differentiation, proliferation, matrix production and remodeling, and cell death. This question is especially interesting given the lack of cartilage-like matrix produced by human BMSCs in agarose. Variables that may affect the response of BMSCs to Dex in different scaffolds include scaffold mechanical properties, scaffold interactions with newly synthesized matrix, and cell-mediated scaffold compaction. We previously reported that BMSCs maintain a rounded morphology in agarose throughout the culture duration, whereas BMSCs in RAD spread and elongate to achieve a networked morphology with extensive cell–cell contact early in culture.3 This is likely an important factor because cell–cell contact is an essential aspect of chondrogenesis in limb bud formation.48
Dex does affect matrix production by BMSCs in agarose and RAD peptide scaffolds, but the specific results depend on the cell source and the scaffold type. These findings highlight the importance of choosing a scaffold, cell type, and growth factor combination carefully since the interactions between these variables can change the outcome. Dex reduced ADAMTS-4/5 activity in both types of hydrogels and both cell sources, suggesting an exciting new avenue for investigating interactions between Dex and ADAMTS-4/5.
Supplementary Material
Supplementary Material
Footnotes
Acknowledgments and Funding: The authors would like to thank Rita Kirsch for her excellent technical support. Grant support provided by National Science Foundation Graduate Research Fellowship, National Institutes of Health Grant EB003805.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The three patients whose cells were used in this study granted informed consent, and surgical procedures were preapproved by the local institutional review board at either Brigham and Women’s Hospital or Massachusetts General Hospital (Boston, MA).
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-7. [DOI] [PubMed] [Google Scholar]
- 2. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-72. [DOI] [PubMed] [Google Scholar]
- 3. Kopesky PW, Vanderploeg EJ, Sandy JS, Kurz B, Grodzinsky AJ. Self-assembling peptide hydrogels modulate in vitro chondrogenesis of bovine bone marrow stromal cells. Tissue Eng Part A. 2010;16(2):465-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dickhut A, Gottwald E, Steck E, Heisel C, Richter W. Chondrogenesis of mesenchymal stem cells in gel-like biomaterials in vitro and in vivo. Front Biosci. 2008;13:4517-28. [DOI] [PubMed] [Google Scholar]
- 5. Noth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4(7):371-80. [DOI] [PubMed] [Google Scholar]
- 6. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568-84. [DOI] [PubMed] [Google Scholar]
- 7. Kopesky PW, Lee HY, Vanderploeg EJ, Kisiday JD, Frisbie DD, Plaas AH, et al. Adult equine bone marrow stromal cells produce a cartilage-like ECM mechanically superior to animal-matched adult chondrocytes. Matrix Biol. 2010;29(5):427-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10(5):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan IM, Gilbert SJ, Singhrao SK, Duance VC, Archer CW. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater. 2008;16:26-39. [DOI] [PubMed] [Google Scholar]
- 10. Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80(12):1745-57. [DOI] [PubMed] [Google Scholar]
- 11. Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25(3):655-63. [DOI] [PubMed] [Google Scholar]
- 12. Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ, Blake SM, Hung HH, et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009;60(10):2985-96. [DOI] [PubMed] [Google Scholar]
- 13. Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358(Pt 3):615-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277(25):22201-8. [DOI] [PubMed] [Google Scholar]
- 15. Lu YC, Evans CH, Grodzinsky AJ. Effects of short-term glucocorticoid treatment on changes in cartilage matrix degradation and chondrocyte gene expression induced by mechanical injury and inflammatory cytokines. Arthritis Res Ther. 2011;13(5):R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Derfoul A, Perkins CL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24(6):1487-95. [DOI] [PubMed] [Google Scholar]
- 17. Miller RE, Grodzinsky AJ, Vanderploeg EJ, Lee C, Ferris DJ, Barrett MF, et al. Effect of self-assembling peptide, chondrogenic factors, and bone marrow-derived stromal cells on osteochondral repair. Osteoarthritis Cartilage. 2010;18(12):1608-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah RN, Shah NA, Del Rosario Lim MM, Hsieh C, Nuber G, Stupp SI. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci U S A. 2010;107(8):3293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103(21):8155-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kopesky PW, Vanderploeg EJ, Kisiday JD, Frisbie DD, Sandy JD, Grodzinsky AJ. Controlled delivery of transforming growth factor beta1 by self-assembling peptide hydrogels induces chondrogenesis of bone marrow stromal cells and modulates Smad2/3 signaling. Tissue Eng Part A. 2011;17(1-2):83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller RE, Kopesky PW, Grodzinsky AJ. Growth factor delivery through self-assembling peptide scaffolds. Clin Orthop Relat Res. 2011;469(10):2716-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porter RM, Liu F, Pilapil C, Betz OB, Vrahas MS, Harris MB, et al. Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J Orthop Res. 2009;27(1):42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Muller PE, et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60(3):801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14(2):179-89. [DOI] [PubMed] [Google Scholar]
- 25. Huang CY, Reuben PM, D’Ippolito G, Schiller PC, Cheung HS. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Biol. 2004;278(1):428-36. [DOI] [PubMed] [Google Scholar]
- 26. Honer C, Nam K, Fink C, Marshall P, Ksander G, Chatelain RE, et al. Glucocorticoid receptor antagonism by cyproterone acetate and RU486. Mol Pharmacol. 2003;63(5):1012-20. [DOI] [PubMed] [Google Scholar]
- 27. Pandit S, Geissler W, Harris G, Sitlani A. Allosteric effects of dexamethasone and RU486 on glucocorticoid receptor-DNA interactions. J Biol Chem. 2002;277(2):1538-43. [DOI] [PubMed] [Google Scholar]
- 28. Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247-8. [DOI] [PubMed] [Google Scholar]
- 29. Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174(1):168-76. [DOI] [PubMed] [Google Scholar]
- 30. Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267-73. [DOI] [PubMed] [Google Scholar]
- 31. Domm C, Schunke M, Christesen K, Kurz B. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthritis Cartilage. 2002;10(1):13-22. [DOI] [PubMed] [Google Scholar]
- 32. Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99(15):9996-10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurz B, Lemke A, Kehn M, Domm C, Patwari P, Frank EH, et al. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50(1):123-30. [DOI] [PubMed] [Google Scholar]
- 34. Chockalingam PS, Zeng W, Morris EA, Flannery CR. Release of hyaluronan and hyaladherins (aggrecan G1 domain and link proteins) from articular cartilage exposed to ADAMTS-4 (aggrecanase 1) or ADAMTS-5 (aggrecanase 2). Arthritis Rheum. 2004;50(9):2839-48. [DOI] [PubMed] [Google Scholar]
- 35. Sandy JD, Thompson V, Doege K, Verscharen C. The intermediates of aggrecanase-dependent cleavage of aggrecan in rat chondrosarcoma cells treated with interleukin-1. Biochem J. 2000;351(Pt 1):161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maehara H, Suzuki K, Sasaki T, Oshita H, Wada E, Inoue T, et al. G1-G2 aggrecan product that can be generated by M-calpain on truncation at Ala709-Ala710 is present abundantly in human articular cartilage. J Biochem. 2007;141(4):469-77. [DOI] [PubMed] [Google Scholar]
- 37. Wilson CG, Nishimuta JF, Levenston ME. Chondrocytes and meniscal fibrochondrocytes differentially process aggrecan during de novo extracellular matrix assembly. Tissue Eng Part A. 2009;15(7):1513-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neame PJ, Barry FP. The link proteins. Experientia. 1993;49(5):393-402. [DOI] [PubMed] [Google Scholar]
- 39. Kim YJ, Grodzinsky AJ, Plaas AH. Compression of cartilage results in differential effects on biosynthetic pathways for aggrecan, link protein, and hyaluronan. Arch Biochem Biophys. 1996;328(2):331-40. [DOI] [PubMed] [Google Scholar]
- 40. Shintani N, Hunziker EB. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cell Mater. 2011;22:302-19. [DOI] [PubMed] [Google Scholar]
- 41. Kavalkovich KW, Boynton RE, Murphy JM, Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38(8):457-66. [DOI] [PubMed] [Google Scholar]
- 42. Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52(2):246-55. [DOI] [PubMed] [Google Scholar]
- 43. Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26(6):599-609. [DOI] [PubMed] [Google Scholar]
- 44. Radice M, Brun P, Cortivo R, Scapinelli R, Battaliard C, Abatangelo G. Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J Biomed Mater Res. 2000;50(2):101-9. [DOI] [PubMed] [Google Scholar]
- 45. Dimicco MA, Kisiday JD, Gong H, Grodzinsky AJ. Structure of pericellular matrix around agarose-embedded chondrocytes. Osteoarthritis Cartilage. 2007;15(10):1207-16. [DOI] [PubMed] [Google Scholar]
- 46. De Bari C, Kurth TB, Augello A. Mesenchymal stem cells from development to postnatal joint homeostasis, aging, and disease. Birth Defects Res C Embryo Today. 2010;90(4):257-71. [DOI] [PubMed] [Google Scholar]
- 47. Erickson IE, van Veen SC, Sengupta S, Kestle SR, Mauck RL. Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent. Clin Orthop Relat Res. 2011;469(10):2744-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8(5):309-34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.