Abstract
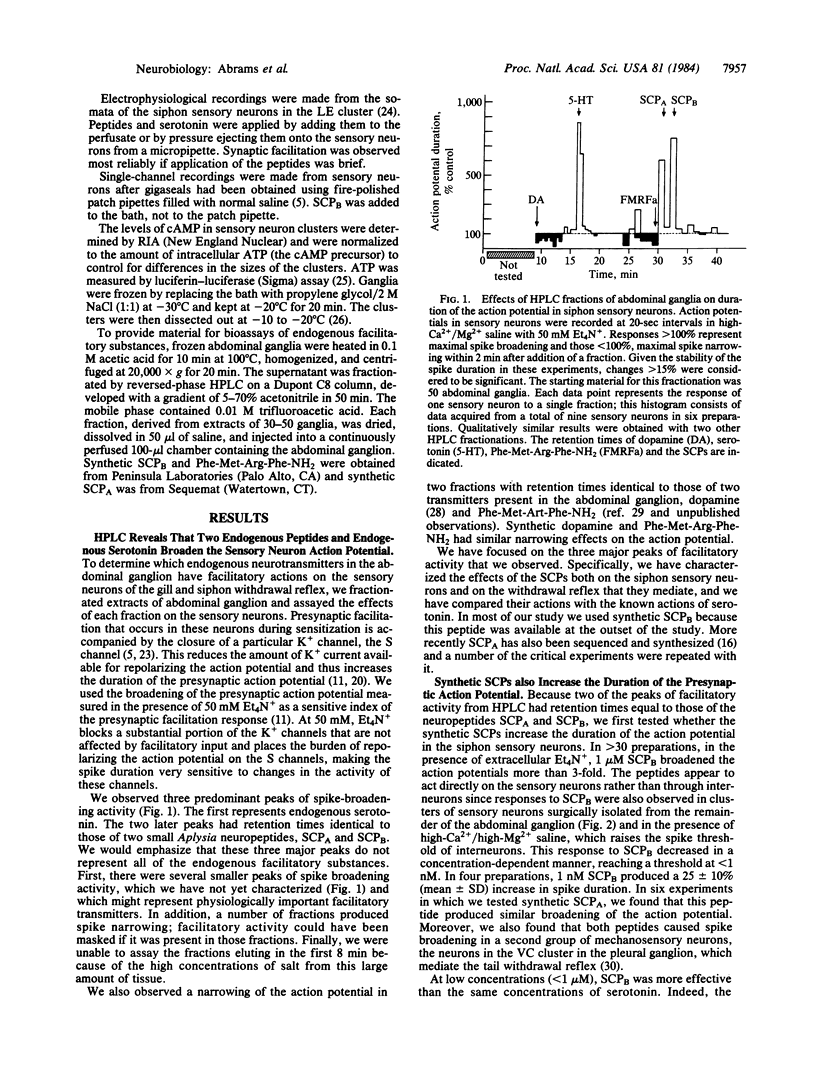
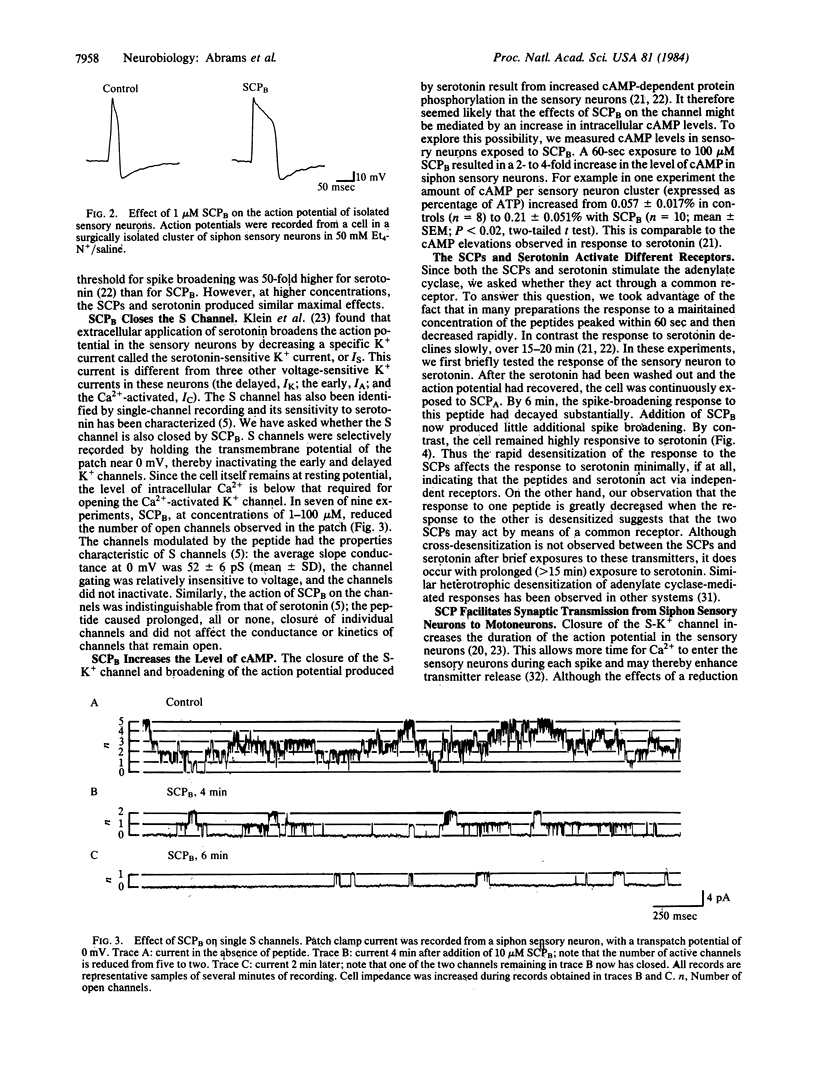
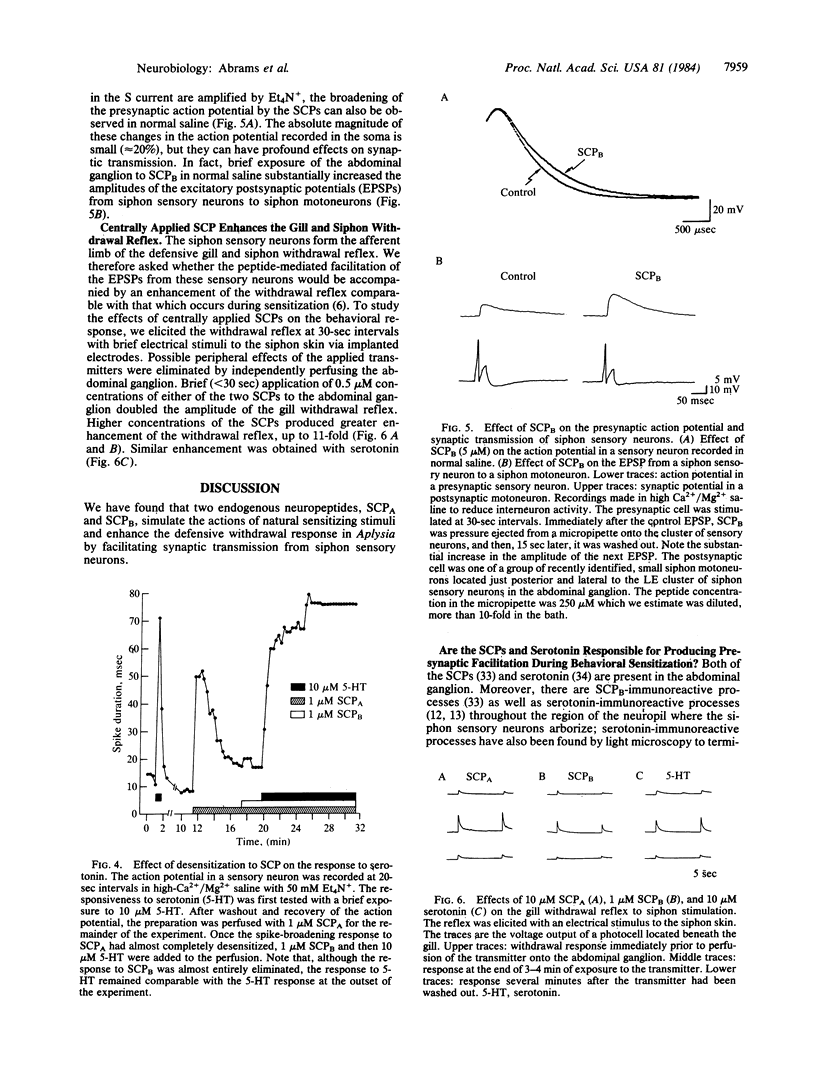
We have found that two endogenous neuropeptides in Aplysia, the small cardioactive peptides SCPA and SCPB, facilitate synaptic transmission from siphon mechano-sensory neurons and enhance the defensive withdrawal reflex that these sensory neurons mediate. Single-channel recording revealed that these peptides close a specific K+ channel, the S channel, which is sensitive to cAMP. Moreover, the peptides increase cAMP levels in these sensory neurons. This reduction in K+ current slows the repolarization of the action potential in these cells, which increases transmitter release. In these actions, the SCPs resemble both noxious sensitizing stimuli, which enhance the reflex, and serotonin. Bioassay of HPLC fractions of abdominal ganglion extracts and immunocytochemistry indicate that both the SCPs and serotonin are present in the ganglion and are found in processes close to the siphon sensory neurons, suggesting that these transmitters may be involved in behavioral sensitization. Recent evidence suggests that one group of identified facilitatory interneurons, the L29 cells, does not appear to contain either the SCPs or serotonin but may use yet another facilitatory transmitter. Thus, it appears that several transmitters can converge to produce presynaptic facilitation in the sensory neurons of the defensive withdrawal reflex. All of the transmitters studied here, the SCPs and serotonin, act via an identical molecular cascade: cAMP-dependent closure of the S-K+ channel, broadening of the presynaptic action potential, and facilitation of transmitter release.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Luteinizing hormone-releasing factor and muscarinic agonists act on the same voltage-sensitive K+-current in bullfrog sympathetic neurones. Br J Pharmacol. 1980 Mar;68(3):353–355. doi: 10.1111/j.1476-5381.1980.tb14547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier L., Castellucci V. F., Kandel E. R., Schwartz J. H. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3':5'-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982 Dec;2(12):1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Byrne J. H., Castellucci V. F., Kandel E. R. Contribution of individual mechanoreceptor sensory neurons to defensive gill-withdrawal reflex in Aplysia. J Neurophysiol. 1978 Mar;41(2):418–431. doi: 10.1152/jn.1978.41.2.418. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Walters E. T., Kandel E. R. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981 Dec;1(12):1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D., Breese G., Schanberg S., Kopin I. Serotonin and dopamine: distribution and accumulation in Aplysia nervous and non-nervous tissues. Int J Neurosci. 1971 Jul;2(1):49–56. doi: 10.3109/00207457109146992. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Nairn A., Greengard P., Schwartz J. H., Kandel E. R. Inhibitor of adenosine 3':5'-monophosphate-dependent protein kinase blocks presynaptic facilitation in Aplysia. J Neurosci. 1982 Dec;2(12):1673–1681. doi: 10.1523/JNEUROSCI.02-12-01673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Kandel E. R. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976 Dec 10;194(4270):1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Cheer S., Gentile J. H., Hegre C. S. Improved methods for ATP analysis. Anal Biochem. 1974 Jul;60(1):102–114. doi: 10.1016/0003-2697(74)90134-1. [DOI] [PubMed] [Google Scholar]
- Greenberg M. J., Painter S. D., Doble K. E., Nagle G. T., Price D. A., Lehman H. K. The molluscan neurosecretory peptide FMRFamide: comparative pharmacology and relationship to the enkephalins. Fed Proc. 1983 Jan;42(1):82–86. [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Castellucci V. F., Kandel E. R. Interneurons involved in mediation and modulation of gill-withdrawal reflex in Aplysia. II. Identified neurons produce heterosynaptic facilitation contributing to behavioral sensitization. J Neurophysiol. 1981 Feb;45(2):315–328. doi: 10.1152/jn.1981.45.2.315. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., North R. A., Williams J. T. The action of substance P on neurons of the myenteric plexus of the guinea-pig small intestine. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1163):191–208. doi: 10.1098/rspb.1979.0101. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd P. E. Cardioactive neuropeptides in gastropods. Fed Proc. 1982 Nov;41(13):2948–2952. [PubMed] [Google Scholar]
- Lloyd P. E., Kupfermann I., Weiss K. R. Evidence for parallel actions of a molluscan neuropeptide and serotonin in mediating arousal in Aplysia. Proc Natl Acad Sci U S A. 1984 May;81(9):2934–2937. doi: 10.1073/pnas.81.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeri E., Brownell P., Branton W. D. Multiple, prolonged actions of neuroendocrine bag cells on neurons in Aplysia. II. Effects on beating pacemaker and silent neurons. J Neurophysiol. 1979 Jul;42(4):1185–1197. doi: 10.1152/jn.1979.42.4.1185. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Panico M., Karplus A., Lloyd P. E., Riniker B. Elucidation by FAB-MS of the structure of a new cardioactive peptide from Aplysia. Nature. 1982 Dec 16;300(5893):643–645. doi: 10.1038/300643a0. [DOI] [PubMed] [Google Scholar]
- Mudge A. W., Leeman S. E., Fischbach G. D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci U S A. 1979 Jan;76(1):526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono J. K., McCaman R. E. Identification of additional histaminergic neurons in Aplysia: improvement of single cell isolation techniques for in tandem physiological and chemical studies. Neuroscience. 1980;5(5):835–840. doi: 10.1016/0306-4522(80)90152-9. [DOI] [PubMed] [Google Scholar]
- Ono J. K., McCaman R. E. Immunocytochemical localization and direct assays of serotonin-containing neurons in Aplysia. Neuroscience. 1984 Mar;11(3):549–560. doi: 10.1016/0306-4522(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Pinsker H., Kupfermann I., Castellucci V., Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H., Carew T. J., Kandel E. R. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol. 1983 Dec;50(6):1522–1542. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- Weinreich D., McCaman M. W., McCaman R. E., Vaughn J. E. Chemical, enzymatic and ultrastructural characterization of 5-hydroxytryptamine-containing neurons from the ganglia of Aplysia californica and Tritionia diomedia. J Neurochem. 1973 Apr;20(4):969–976. doi: 10.1111/j.1471-4159.1973.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Jessell T. M., Gamse R., Mudge A. W., Leeman S. E. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980 Jul 10;286(5769):155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., North R. A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983 Oct 6;305(5934):529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]


