Abstract
Sporozoite vaccination of both humans and rodents elicits potent anti-malarial immunity, but the dose of sporozoites and the number of immunizations required varies with vaccination approach. Here we examine the immunological basis for superior protection afforded from single-dose vaccination with virulent sporozoites administered under prophylatic chloroquine-cover, referred to as infection-treatment-vaccination (ITV), compared to the well-studied approach of administering radiation-attenuated Plasmodium sporozoites (RAS). Earlier rodent studies utilizing ITV and RAS vaccination suggested a major role of CD8 T cells in reducing liver parasite burden after sporozoite challenge in a BALB/c mouse model. Consistent with this, we find that in C57Bl/6 mice ITV elicits substantially higher parasite-specific CD8 T cell responses than RAS vaccination and enhances immunity against P. yoelii infection. However, we show ITV-induced CD8 T cells are not necessary for protection following liver-stage sporozoite or blood-stage parasite challenge. Mechanistically, we found protection afforded from single-dose ITV is associated with low grade, transient parasitemia shortly following cessation of chloroquine treatment and generation of potent antibody responses to blood-stage parasites. Collectively, our data show the mechanistic basis for enhanced protective immunity against P. yoelli elicited by ITV in highly susceptible C57Bl/6 mice is independent of CD8 T cells. These studies may be relevant in understanding the potent immunity observed with ITV in humans.
Keywords: Plasmodium infections, CD8 T cells, vaccination, antibodies, subpatent infection
Introduction
Plasmodium infection exacts a significant toll on human public health with more than 375,000 malaria-related deaths reported in 2010 [1]. Anti-malarial vaccination represents an attractive intervention to break the cycle of disease transmission. Whole-parasite based approaches, specifically vaccination with radiation-attenuated sporozoites (RAS), have proven capable of generating immunity in humans [2]. Despite this success, RAS induced protection appears to require immunization with very large numbers of parasites (>1000 bites from mosquitoes harboring RAS [2]) and needle delivered RAS has yet to induce protection in humans [3]. Another approach first described in rodents (infection-treatment-vaccination, ITV) [4–7] also elicits protection against subsequent sporozoite exposure in human subjects [8, 9]. In this approach, human subjects receive mosquito bite inoculation of virulent P. falciparum sporozoites while concurrently undergoing chloroquine (CQ) chemoprophylaxis [8, 9]. Importantly, this ITV approach required fewer mosquito bites (~36–45 bites over 3 exposures) to elicit full protective immunity [8, 9]. Thus, in humans ITV appears to induce much more potent immunity compared to RAS vaccination.
Protection afforded from whole-sporozoite vaccinations, such as ITV and RAS, is reported to involve liver-stage directed CD8 T cells [4, 10–12]. For example, in a rodent model of ITV whereby BALB/c mice were given a single dose of 105 virulent P. yoelii 265BY sporozoites followed by 10 consecutive days of CQ chemoprophylaxis, reduction in liver parasite burden after challenge 15 days later involved CD8 T cells, IFN-γ and NO− as the primary immune effectors [4]. Similarly, ITV-induced protection in humans correlates with T cells producing effector cytokines [8]. In rodent models of RAS immunization, protection is critically linked to CD8 T cells exhibiting activity against the liver-stage of infection [13]. Collectively, these results highlight that CD8 T cell-mediated liver-stage protection can be achieved following whole-sporozoite vaccination approaches, such as ITV or RAS.
Although protection in rodents and humans receiving attenuated whole-sporozoite vaccination is associated with CD8 T cells against liver-stage antigens, it remains unclear how a single dose of ITV can afford immunity in rodents whereas multiple, high-doses of RAS are required [4]. These two whole-sporozoite vaccination approaches differ in that RAS vaccination results in only transient, non-replicative infection of hepatocytes, whereas ITV using chloroquine (CQ) allows for productive infection of hepatocytes, release of merozoites and infection of red blood cells (RBC). Due to the blood-stage specific inhibitory effects of CQ [7, 14], merozoites are unable to undergo further rounds of replication in RBC. Thus, critical differences in antigen load, and antigen targets may lead to differences in the protective T cell response and/or humoral responses, which may underlie the exceedingly potent immunity induced by ITV compared to RAS.
Although the widespread prevalence of CQ-resistant P. falciparum complicates direct clinical application of this approach, protection elicited by ITV platforms in human subjects further underscores the potential for whole-parasite approaches to elucidate the cellular and immunologic requirements for successful anti-malarial vaccination. At a minimum, experimental ITV may directly aid identification of both host and parasite-specific factors that determine high levels of protective anti-Plasmodial immunity. Thus, understanding the immunological mechanisms that underlie enhanced immunity following low-dose ITV would fill a critically important knowledge gap. Here, we analyzed the immunological basis of superior immunity induced by ITV compared to RAS vaccination in a stringent parasite-host model.
Materials and Methods
Mice and immunizations
Female 6–8 week old C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) and housed at the University of Iowa animal care unit at the appropriate biosafety level. C57BL/6 µS-AID−/− mice deficient in the immunoglobin heavy-chain µ;-chain secretory domain and activation-induced cytidine deaminase [15], were a gift from F. Lund (University of Alabama, Birmingham) and were bred at the University of Iowa. The Institution Animal Care and Use Committee approved animal experiments. P. yoelii 17XNL (Py) sporozoites were isolated from the salivary glands of infected A. stephensi mosquitoes obtained from New York University insectary and radiation attenuated by exposure to 200 Gy (20,000 rads). Mice were vaccinated intravenously (I.V.) with 10,000 radiation-attenuated or virulent sporozoites. Mice vaccinated with virulent sporozoites were given 10, 25, or 30 daily I.P. injections of 100 µ;l (8mg/mL) chloroquine (CQ) diphosphate salt (Sigma, St. Louis, MO) in PBS from d0–d9, d0–d24 or d0–d29, respectively.
Parasites for immunizations and challenges
Naïve and immunized mice were challenged I.V. >60 days post-immunization with the indicated number of virulent Py sporozoites or 106 Py-infected red blood cells. Low-grade, transient, parasitemia following CQ drug cessation was quantified by evaluating Giemsa stain of thin blood smear at least every two days from day 13–20 by examining at least 20 fields containing at least 150 red blood cells. Naïve mice used for challenge studies received ten daily injections of chloroquine >50 days prior to challenge to control for potential residual drug activity. Patent parasitemia following challenge was evaluated by Giemsa stain of thin blood smear at least every two days from day 4–10 post-challenge. Protection is defined as the absence of detectable blood-stage parasites at all time-points examined. At least 20 fields containing at least 150 red blood cells were examined for each mouse designated as exhibiting protection. Mice exhibiting less than 5% parasitemia at all time-points examined, and never for more than four consecutive days of detectable parasitemia were classified as resistant.
Detection of parasite-specific CD8 T cells in blood
Vaccine-induced CD8 T cell populations were identified by staining peripheral blood with anti-CD8α (53-6.7; Biolegend, San Diego, CA) and anti-CD11a (M17/4; Biolegend) antibodies as described [16, 17]. Cells were analyzed using a BD FACSCanto or BD LSR Fortessa and data were analyzed using FLOWJO Software (Tree Star, Inc, Ashland, OR). Animals were pre-bled prior to vaccination to establish individual background of circulating CD8αintermediate CD11ahi T cell frequencies.
T cell depletion
Mice were depleted of CD8 T cells or CD4 T cells by two I.P. injections of 50µ;g or 200µ;g anti-CD8 (2.43; BioXCell, West Lebanon, NH) or 200µ;g anti-CD4 (GK1.5; BioXCell). Depletion was confirmed in tissues for preliminary experiments or prior to challenge by staining PBMCs with anti-CD8 (53-6.7; Biolegend) and anti-CD4 (RM4-5; Biolegend).
Blood-stage reactive antibody detection
Mice were administered 10,000 Py sporozoites with 10 or 25 days of daily I.P. CQ injections, and serum was collected >40 days post immunization. Dilutions of serum were allowed to react to parasitized red blood cell lysate (prepared as described [18]) immobilized onto Maxisorb Immunoplates (Nunc-Immuno, Rockford, IL). Total IgG antibodies were detected with horseradish peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, Pike Grove, PA) and tetramethylbenzidine substrate (Sigma). Results are presented as absorbance (450 nm) as a function of serum dilution.
Liver parasite burden
Mice were administered 10,000 Py sporozoites with or without 10 days of daily I.P. CQ injections. Naïve mice were administered 10 days of daily I.P. CQ. Mice were challenged >60 days after immunization with 40,000 Py sporozoites. Liver RNA was extracted at 44 hours post challenge using TRIzol (Invitrogen) and one-step quantitative real-time reverse transcription PCR analysis for P. yoelii 18S rRNA was performed on 2µ;g of liver RNA. P. yoelii 18S rRNA was detected using 5′-GGGGATTGGTTTTGACGTTTTTGCG-3′ (forward primer), 5′-AAGCATTAAATAAAGCGAATACATCCTTAT-3′ (reverse primer), and 5′-FAM-CAATTGGTTTACCTTTTGCTCTTT-TAMRA-3′ (probe), which generates a 133-bp fragment [19]. Data were normalized to GAPDH control Ct value and infected naïve control Ct values by calculating 2^(−ΔΔCt). Data plotted as % parasite burden +/− S.E.M. with values normalized relative to infected naïve controls.
Detection of persisting parasites under CQ treatment
Mice were administered 10,000 Py sporozoites with or without 10 days of daily I.P. CQ injections. Naïve mice were administered 10 days of daily I.P. CQ. Mice were euthanized and spleens were excised on day 9 post-immunization (on last day of CQ treatment). Splenic RNA was extracted using TRIzol (Invitrogen) for quantitative real-time PCR analysis for P. yoelii 18S rRNA using primers described above. 18s rRNA copy numbers was estimated using a standard curve generated from known amounts of plasmid copy containing the identified 18s DNA insert.
Statistical Analysis
Data were analyzed using Prism4 software.
Results
ITV is associated with significantly larger anti-Plasmodial CD8 T cell responses and protection from challenge compared to RAS vaccination
Several whole-sporozoite vaccination models in rodents have been used to study the requirements for protective liver-stage immunity against stringent sporozoite challenge [20–26]. For example, RAS-vaccinated C57Bl/6 (B6) mice are more difficult to protect than RAS-vaccinated BALB/c against Plasmodium yoelii 17XNL (Py) infection, with the former requiring multiple sporozoite immunizations to elicit similar protection [10, 13]. Similarly, ITV was more potent in inducing protection than RAS in BALB/c mice [4]. B6 mice are more susceptible to Py than BALB/c mice and are harder to protect by RAS vaccination [10, 12, 13]. To understand the cellular basis of protective immunity in a stringent parasite-host model, we studied B6 mice vaccinated by single administration ITV or RAS. Naive B6 mice subjected to ITV or RAS vaccination were challenged >60 days after immunization by I.V. injection of 103 virulent Py sporozoites (~300× the ID50). Challenged mice were evaluated from 4–10 days post challenge for evidence of blood-stage parasites. All naive control mice, whether treated with CQ or not, exhibited blood-stage parasitemia, a single administration of ITV under 10 days of CQ treatment induced protection (no evidence of blood-stage parasites by evaluation of Giemsa stained thin blood smear) in 90% of mice, whereas mice receiving a single RAS vaccination exhibited no protection (Table 1). Thus, single administration ITV can induce protection against sporozoite challenge at a memory time point even in a stringent B6-Py model.
Table 1.
Single-dose infection-treatment vaccination (ITV) provides sterilizing protection from Py sporozoite challenge in B6 mice.
| Attenuated Sporozoite Vaccinationa |
Day challenged post-vaccination |
% Sterilizing protectionb |
|---|---|---|
| Naive+CQc | N/A | 0% (0/10) |
| 10,000 RAS | 62 days | 0% (0/10) |
| 10,000 ITV | 62 days | 90% (9/10) |
Age matched C57BL/6 female mice were vaccinated I.V. with 104 P. yoelii RAS or with virulent P. yoelii 17XNL sporozoites plus once daily I.P. injection of CQ for 10 ays, (ITV).
Sterilizing protection is defined as complete absence of detectable blood-stage parasitemia when assessed on days 3, 5, 7, 9 and 10 post-challenge with 103 P. yoelii sporozoites.
Naive C57Bl/6 female mice were administered CQ similarly as ITV group.
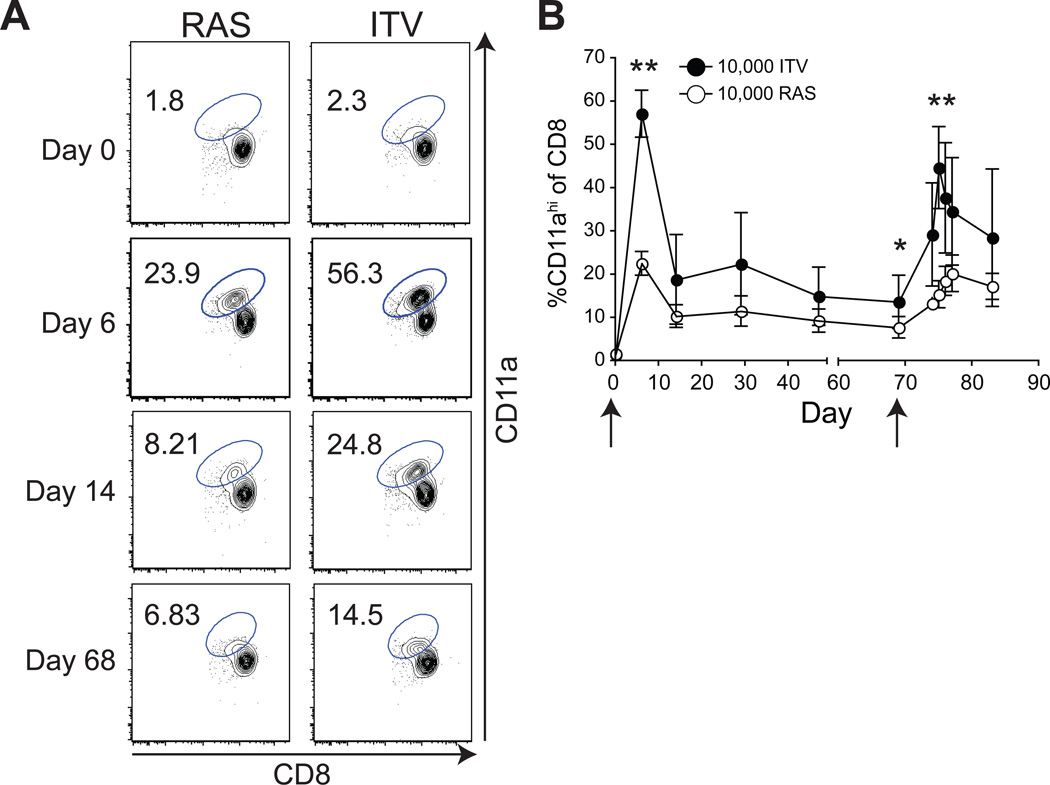
Memory CD8 T cells induced by whole-sporozoite vaccines (RAS, genetically attenuated parasites (GAP), and ITV) have been reported to mediate liver-stage protection against sporozoite challenge [4, 10, 12, 23, 26]. Thus, we hypothesized that quantitative and/or qualitative features of the CD8 T cell response would account for the enhanced protection following single administration ITV compared to RAS vaccination in B6 mice. To examine this, we used a surrogate activation marker approach, developed in our laboratory [16, 17], which allowed us to measure the magnitude and kinetics of the total CD8 T cell response to vaccination in the peripheral blood of individual mice without a priori knowledge of MHC-restriction, antigen-specificity, or precise epitopes. This method relies on the stable changes in surface marker expression of CD8α and CD11a on CD8 T cells in response to T cell receptor mediated activation following infection or immunization in vivo [17]. Thus, naïve CD8 T cells are CD8αhi, CD11aintermediate (int) whereas antigen-specific effector and memory CD8 T cells are CD8αint, CD11ahi [16, 17]. Naïve B6 mice have low frequencies of CD8αint, CD11ahi T cells at d0, prior to vaccination (Fig. 1A–B). Following ITV the total vaccine-induced CD8 T cell response was 2–3× larger at both effector (d6) and memory (d68) time points compared to RAS vaccination (Fig. 1A–B). Additionally, homologous challenge with 104 virulent Py sporozoites at a memory time point (day 69) led to >2× larger CD8 T cell secondary responses in ITV mice compared to secondary responses in RAS vaccinated mice (Fig. 1B, P < 0.00001, d76). These data show that superior protection afforded by single administration ITV compared to RAS vaccination correlates with the induction of significantly larger total effector (P <0.00001), memory (P = 0.00143) and secondary CD8 T cell responses.
Figure 1. ITV induces larger effector, memory, and permits higher secondary CD8 T cell responses than RAS vaccination in B6 mice.
B6 mice were immunized with 104 Py either as RAS or ITV with 10 days CQ chemoprophylaxis. Arrows indicate day of vaccination or rechallenge. (A), Representative CD8 T cell responses in blood at the indicated days post vaccination. Numbers indicate the frequency of CD8 T cells exhibiting an antigen-experienced (CD8αint, CD11ahi) phenotype. (B), Cumulative and kinetic analysis of ITV versus RAS vaccine-induced CD8 T cell responses. Secondary effector responses were measured after administration of 104 virulent sporozoites on day 68. Data are Mean +/− SD for 30 mice (through d68) and 10 mice (d68 to d88). **, P <0.0001; *, P = 0.00143.
ITV-induced CD8 T cells are not required for protection from liver-stage challenge
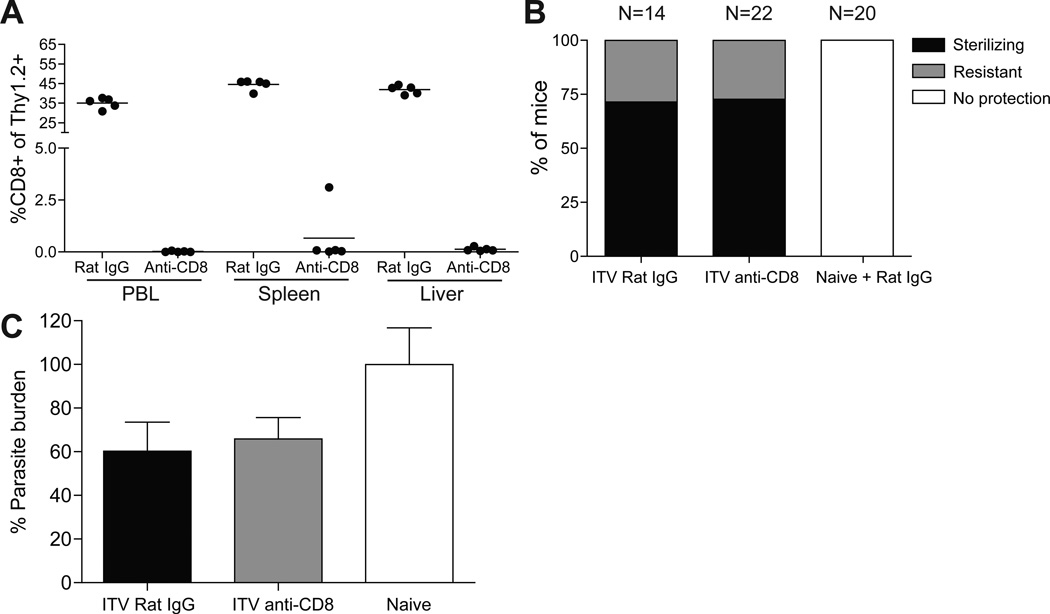
It has been argued that CD8 T cells mediate ITV-induced protection in humans and BALB/c mice [4, 8, 27]. We evaluated the requirement for ITV-induced CD8 T cells in mediating protection against a sporozoite (liver-stage) challenge in B6 mice by antibody-mediated depletion of these cells at a memory time point prior to challenge. Groups of mice were given a single administration of ITV and >60 days later were administered 50µ;g of anti-CD8 depleting antibody twice over three days. Successful depletion was verified by analyzing blood, spleen and liver. This treatment resulted in >98.5% CD8 T cell depletion in all tissues examined (Fig. 2A). A different group of mice received a single administration of ITV and >60 days later were administered 200µ;g of anti-CD8 depletion antibody one day prior and one day after sporozoite challenge to ensure depletion of CD8 T cells in peripheral tissues. For these mice, CD8 T cell depletion was verified by analyzing peripheral blood of individual mice one day after the last antibody treatment (>98% depletion, data not shown). CD8 T cell depleted and control rat IgG treated mice were challenged with 103 Py sporozoites. In contrast to previous studies [10, 12], CD8 T cell depletion did not decrease the frequency of protected mice as determined by evaluation of Giemsa stained thin blood smear, compared to non-depleted control mice (Fig. 2B). However, ITV mice that were not protected (~25% in each group) exhibited substantial control of infection compared to naïve control mice that develop parasitemia lasting >25 days. These ITV mice were categorized as “resistant” if parasitemia did not exceed 5% or was cleared below the level of detection (blood smear negative) by 10 days after challenge. Thus, in a stringent host-parasite model of protection, CD8 T cells induced by single administration ITV are not required to provide protection or substantial resistance against sporozoite challenge.
Figure 2. ITV-induced CD8 T cells are not required for protection from homologous sporozoite challenge.
(A) CD8 T cell depletion in blood, spleen and liver of ITV mice (day 60) given control Ig or anti-CD8 antibody (50 ug, day 57 and 59 post ITV). (B), Percent of ITV (day 60) or naïve B6 mice exhibiting protection (black shading), resistance (grey shading, defined in Materials and Methods), or no protection (open) to sporozoite challenge with or without depletion of CD8 T cells. Data are pooled from four independent experiments. P = 0.9324 for protection between rat IgG and anti-CD8 groups as assessed by Chi-square analysis. (C), Liver parasite burden 44 hours after 40,000 Py sporozoite challenge in ITV (day >50) or naïve mice depleted of CD8 T cells or treated with control Ig. Py-specific 18s ribosomal RNA transcript numbers were quantified via qRT-PCR. Data are normalized to GAPDH and infected naive control Ct values (set at 100%) +/− S.E.M. Data are pooled from two independent experiments.
To determine if a liver-stage specific protective role for ITV-induced CD8 T cells could be documented, we measured Py-specific 18s rRNA in the liver after a high dose sporozoite challenge in CD8-depleted and non-depleted ITV mice. Mice were given a single ITV administration and depleted of CD8 T cells or given rat IgG control prior to challenge with 40,000 Py sporozoites (Figure 2C). Parasite burdens were modestly reduced in ITV mice with this high-dose challenge, however, depletion of CD8 T cells did not significantly increase liver Py-specific 18s rRNA compared to non-depleted controls (Figure 2C). Collectively, these data demonstrate that single administration ITV-induced CD8 T cells are not required to achieve immunity against sporozoite challenge.
ITV-induced protection is associated with transient blood-stage parasite exposure
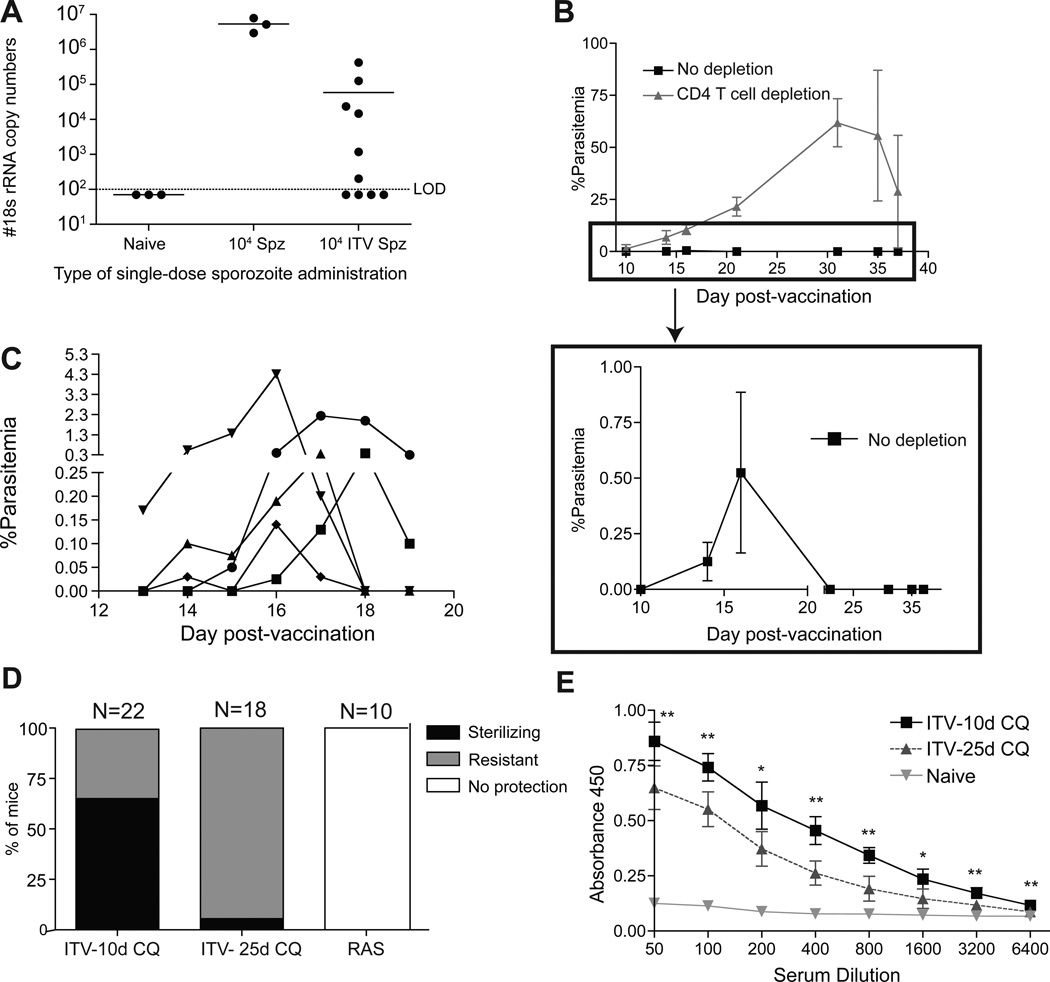
ITV allows for complete liver-stage development and merozoite entry into red blood cells before infection is truncated by the blood-stage-specific inhibitory effects of CQ [4, 7, 14]. In a human model trial, Plasmodium-specific nucleic acids were detectable for several days in the blood of all ten patients following the first administration of ITV with CQ chemoprophylaxis [8]. Further, when patients received a second and third ITV administration, 6 and 3 patients had detectable Plasmodium-specific nucleic acid in the blood, respectively [8]. Thus, it is possible that CQ chemoprophylaxis allows for a sustained subpatent (not detectable by blood smear) blood-stage infection, which may potentiate the generation of blood-stage-specific cellular and humoral immune responses that contribute to protection. We evaluated single administration ITV in B6 mice for evidence of sustained subpatent blood-stage infection by testing for Py-specific 18s rRNA in the spleen on the last day of CQ chemoprophylaxis [19]. Despite CQ treatment, Py-specific 18s rRNA was above the limit of detection in 60% of the ITV mice (Fig. 3A). The presence of Py-specific RNA suggests that metabolically active, viable blood-stage parasites may persist for the duration of the 10 day CQ chemoprophylaxis.
Figure 3. Transient parasitemia after CQ cessation correlates with protection and induction of anti-blood stage parasite antibodies during ITV.
(A), Py-specific ribosomal RNA copy number in the spleen of Py infected control (day 9) or ITV (day 9) B6 mice. Data represent individual mice and are pooled from two independent experiments. (B) Parasitemia in CD4 T cell depleted ITV mice after cessation of CQ (top panel). Low frequency parasitemia in control Ig treated mice after CQ cessation (bottom panel) in ITV B6 mice receiving 10 days of CQ chemoprophlyaxis. Error bars in bottom are mean +/− SD. (C), Parasitemia after CQ cessation in five individual ITV B6 mice receiving 10 days of CQ chemoprophylaxis. (D) Percent of B6 ITV mice, given 10 days or 25 days of CQ chemoprophylaxis, or RAS vaccinated mice exhibiting protection (black shading), resistance (grey shading), or no protection (no shading) after challenge with 103 virulent sporozoites >60 days after vaccination. Data are pooled from four independent experiments. P < 0.0001 by Chi-Square analysis comparing ITV-10d CQ to ITV-25d CQ for protection. (E), Total serum IgG recognizing parasitized red blood cell lysates from naïve or ITV B6 mice (ITV-10d CQ or ITV-25d CQ). The ITV-25d CQ group did not exhibit patent parasitemia after CQ cessation. Results are representative of two independent experiments. **, P <0.01; *, P <0.05.
CD4 T cells are a critical cellular component necessary for control of blood-stage parasite replication and persistence [28, 29]. To further address a role for persisting blood-stage parasites, naïve mice undergoing single administration ITV were immunocompromised by CD4 T cell depletion from day 0 through day 9, post sporozoite infection (Suppl. Fig. 1A), and subsequently monitored for the appearance of blood-stage parasites. Following cessation of CQ chemoprophylaxis, CD4 T cell depleted ITV mice had uncontrolled blood-stage infection (Fig. 3B). Strikingly, all ITV immunocompetent (no depletion) mice also exhibited detectable, albeit very low levels of blood-stage parasitemia following CQ cessation (Fig. 3B, lower panel). Higher resolution analyses revealed that approximately 3–5 days following cessation of CQ chemoprophylaxis all mice exhibited patent (albeit low-grade) parasitemia (Fig. 3C). The duration of low-grade, transient, parasitemia was between 3–6 days, and ranged from slightly above the limit of detection (greater or equal to 1 parasite out of 3000 red blood cells, 0.033% parasitemia) to 5% parasitemia. By day 7 after cessation of CQ, all ITV mice cleared the infection without requirement for further intervention. This low-grade, transient, parasitemia is not a consequence of insufficient CQ dosing: doubling the dose of CQ for the ten day time period still resulted in low-grade, transient parasitemia after drug withdrawal (data not shown), despite our observation that two low-dose CQ treatments can reduce patent blood-stage infection to undetectable frequency in heavily parasitized mice (Suppl. Fig. 1B). Taken together, these results indicate that after single administration ITV, viable blood-stage parasites persist in B6 mice following ten consecutive days of CQ chemoprophylaxis.
ITV-induced protection is associated with induction of blood-stage reactive antibodies
Low-grade, transient parasitemia after CQ cessation in single administration ITV mice may increase the duration or amount of exposure to blood-stage antigens and contribute to protective immunity. We next tested if abrogating low-grade patent parasitemia decreased protection in single administration ITV mice. Mice received ITV with 10 or 25 days of CQ chemoprophylaxis. All 10 day CQ mice exhibited low-grade, transient, parasitemia after CQ withdrawal, however 25 day treated CQ mice did not exhibit patency after cessation of drug treatment (data not shown). Both groups were challenged >60 days after start of immunization (>35 days after end of CQ treatment). ITV mice exhibiting parasitemia after CQ cessation exhibited greater than 65% protection as determined by evaluation of Giesma stained thin blood smear (Fig. 3D). Conversely, single administration ITV mice treated for 25 days with CQ, which did not exhibit low-grade, transient, parasitemia after CQ cessation, were not protected. Nevertheless, these mice still exhibited control of infection and were thus, relatively resistant to challenge (Fig. 3D).
It is well established that antibodies are elicited against asexual blood-stage parasites during Py infection [30, 31]. Since ITV-induced protection does not require CD8 T cells, but is associated with multi-day exposure to blood-stage infection (control of which depends on CD4 T cells), we hypothesized that ITV may elicit antibodies reactive against blood-stage antigens that overcomes specific requirements for CD8 T cells in protection. To evaluate this, we determined levels of total IgG against parasitized red blood cell lysate in serum from ITV mice experiencing low-grade, transient parasitemia compared to no parasitemia ITV mice. As expected, both vaccination groups had detectable levels of total IgG against blood-stage antigens. However, serum from mice that had experienced low-grade, transient parasitemia after ITV contained significantly higher titers of parasite-specific IgG (P < 0.01, Fig. 3D). Next, we determined if ITV-induced antibodies were required for control of blood-stage parasites. To address this question, we utilized µ;s-AID−/− mice on a C57Bl/6 background, as these mice lack the ability to secrete functional antibodies, but still maintain B cells [15]. Naïve µ;s-AID−/− mice and wild-type B6 mice were vaccinated with 104 virulent Py sporozoites with 10 days of CQ chemoprophylaxis. After cessation of CQ chemoprophylaxis, µ;s-AID−/− mice exhibited uncontrolled parasitemia and all eventually succumbed to hyperparasitemia (data not shown). Thus, we administered 25–30 days of CQ to µ;s-AID−/− mice in an attempt to prevent the occurrence of parasitemia. In 7 out of 15 of the µ;s-AID−/− mice administered ITV with 25–30 days of CQ, parasitemia still occurred after CQ cessation, and these mice also all eventually succumbed to hyperparasitemia (Suppl. Fig. 2A). These results demonstrate the ability of Py blood-stage parasites to persist for a long duration under CQ chemoprophylaxis specifically in the absence of secreted antibodies. Further, as an extension for the requirement for CD4 T cells to control low-grade, transient parasitemia (Fig. 3B), antibodies appear to be necessary to control the low-grade, transient parasitemia that occurs after CQ treatment cessation. We challenged the surviving vaccinated µ;s-AID−/− mice with 103 virulent Py sporozoites. All 8 challenged ITV µ;s-AID−/− mice developed patent parasitemia and 7 out of 8 succumbed following the challenge (Suppl. Fig. 2B). Taken together, these data suggest ITV elicits antibodies reactive against blood-stage antigens, which are critical for the control of low-grade, transient parasitemia after CQ cessation, and protection following sporozoite challenge.
ITV confers protection against blood-stage challenge
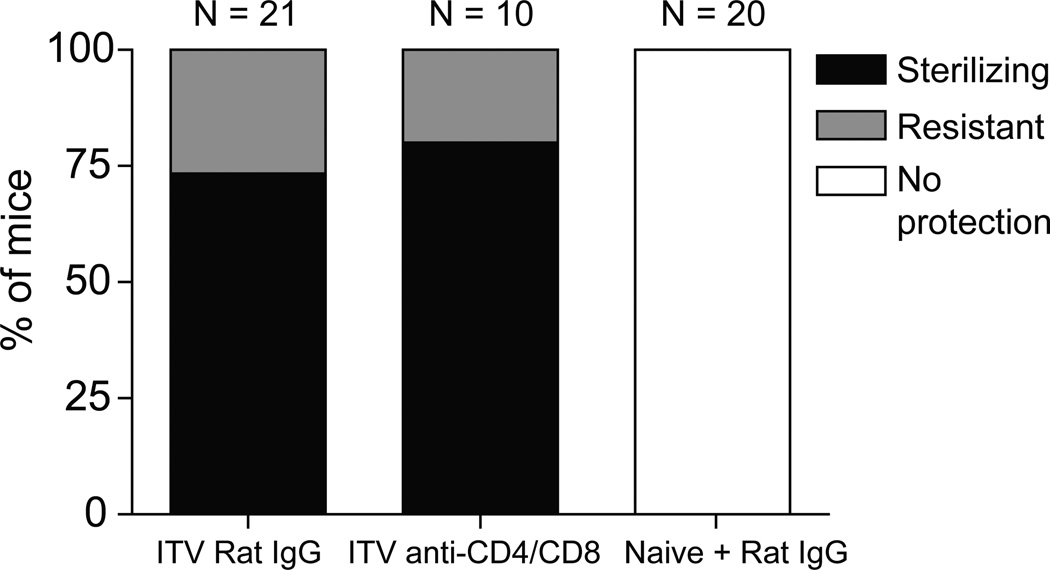
Our results demonstrate that ITV induces antibodies reactive against blood-stage antigens and does not require CD8 T cells for protection against liver-stage, sporozoite challenge in the Py model of infection in B6 mice. To further define the role of antibodies in ITV-induced protection, mice were administered 103 sporozoites with 10 days of CQ chemoprophylaxis, to permit low-grade, transient parasitemia after CQ cessation and induction of higher blood-stage reactive antibody titers. One day prior to challenge at a memory time point, some mice were depleted of both CD8 and CD4 T cells. T cell depleted and control mice were then challenged with 106 Py parasitized red blood cells, which resulted in patent parasitemia in all non-depleted, naïve mice. Strikingly, T cell depletion did not reduce the fraction of ITV mice exhibiting protection or resistance to this blood-stage challenge compared to IgG treated ITV mice (Fig. 4). Taken together, these data indicate that neither CD4 nor CD8 T cells are required for protection against blood-stage challenge in this model of single administration ITV in B6 mice. Collectively, our results suggest that ITV-induced antibodies are the primary mediator of protection from blood-stage infection.
Figure 4. T cells are not required for protection from blood-stage challenge in single administration ITV mice.
Percent of ITV or naïve B6 mice exhibiting protection (black shading), resistance (grey shading), or no protection (no shading) with or without depletion of CD4 and CD8 T cells prior to challenge with 106 blood-stage Py parasites. Data are pooled from three independent experiments.
Discussion
ITV elicits superior protection compared to RAS vaccination in human and rodents [2, 4, 8]. However, the precise mechanisms of this superior protection are unknown. We addressed this knowledge gap using the B6-Py 17XNL model because of the more stringent requirements to achieve protection (no evidence of blood-stage parasites after evaluation of Giemsa stained thin blood smear) after challenge in B6 mice given RAS and GAP vaccinations compared to BALB/c mice challenged with Py or P. berghei [10, 12, 13]. Unexpectedly, we found evidence for patent blood-stage infection after cessation of CQ chemoprophylaxis in the ITV mice, permitting not only liver-stage immunity but also protective T cell-independent blood-stage immunity.
Whole-sporozoite vaccine-induced, liver-stage directed CD8 T cells are required for protection in multiple rodent models. This requirement applies to both RAS [10, 11, 13] and genetically attenuated parasite (GAP) vaccination [12]. Thus, as expected, ITV also induces CD8 T cells that are capable of reducing the liver parasite burden. Indeed, in a BALB/c-P. yoelii 265BY model of ITV, CD8 T cells, IFN-γ and NO− were the primary immune effectors of protection [4, 11]. Similarly, in a B6 model using P. berghei ANKA sporozoites, protection from challenge was correlated with the amount of IFN-γ produced by memory CD8 T cells [11]. In accordance with rodent studies, ITV-induced protection in humans correlates with the presence of T cells producing effector cytokines [8]. Collectively, these results highlight that significant CD8 T cell-mediated liver-stage protection can be achieved following whole-sporozoite vaccination approaches, such as ITV, GAP or RAS.
Previously, we have shown that protection against sporozoite challenge correlates with larger vaccine-induced effector and memory CD8 T cell responses [12]. The larger magnitude of CD8 T cell response was a result of additional liver-stage antigens expressed during GAP vaccination compared to RAS (reviewed in [32]). Surprisingly, we found that despite significantly larger effector and memory CD8 T cells induced from ITV compared to RAS vaccination, CD8 T cells were not required for protection from sporozoite challenge in the B6 model of Py 17XNL ITV. RAS and GAP vaccination require multiple immunizations to achieve CD8 T cell mediated protection from challenge in the B6-Py17XNL model [12]. For instance, in Py fabb/f−/− GAP vaccination, wherein the parasite is blocked late in liver-stage differentiation, a single immunization with 2 × 104 attenuated sporozoites failed to protect B6 mice from sporozoite challenge [12]. These results highlight the requirement for numerically boosting RAS- and GAP-induced CD8 T cell responses to provide protective immunity against sporozoite challenge. In the B6-Py 17XNL ITV model, a single immunization dose elicited protective immunity, but this was dependent on low-grade, transient parasitemia after cessation of CQ treatment. Thus, an explanation for the larger CD8 T cell response from ITV may be exposure to blood-stage antigens. Several studies suggest that CD8 T cells are induced from exposure to blood-stage parasites [33, 34]. The specificity of the ITV-induced effector and memory CD8 T cell responses is unknown since both liver- and blood-stage antigens are expressed during immunization. However, our data indicate in the B6-Py 17XNL model of ITV, CD8 T cells are not required for immunity against liver-stage or blood-stage challenge. Therefore, our results suggest other component(s) of the immune response elicited by ITV may be primarily responsible for immunity. It is possible the ITV-induced CD4 T cells could contribute to reducing liver-stage parasites [4]. However, neither memory CD4 nor CD8 T cells were required for protection against blood-stage challenge, highlighting that the potent blood-stage immunity we observe is independent of memory T cells.
Sporozoites administered with CQ chemoprophylaxis are competent to undergo a full program of amplification and differentiation in the liver and are released from the liver to undergo a round of infection in the blood as blood-stage merozoites. Due to the inhibitory effects of CQ, further replication is prevented by newly released blood-stage merozoites [7, 14]. In the B6-Py 17XNL ITV model, we found that CQ chemoprophylaxis does not completely eliminate the parasites during the 10 day course of treatment. Unexpectedly, we show subpatent parasites persist during CQ chemoprophylaxis, which can replicate to patent levels once CQ treatment ends. Further, multiple days (3–6 days) of patent, low-grade parasitemia were required for the induction of immunity. Moreover, depletion of CD8 and CD4 T cells prior to blood-stage challenge did not abrogate ITV-induced protection. Low-grade, transient parasitemia during ITV with CQ chemoprophylaxis is not limited to rodent studies, but has also been observed in human ITV studies [8, 9]. Indeed, in these two studies, qPCR analysis of the blood revealed the majority of patients had subpatent infection following the first administration of ITV. Interestingly, in one study, 2 of the 15 human subjects undergoing ITV were reported to have a positive thick blood smear on day 7 and received atovaquone/proguanil treatment before continuation in the study [9]. The mechanism of blood-stage parasite persistence during CQ chemoprophylaxis in humans and rodents is unknown. CQ interrupts the capacity of merozoites to detoxify heme by preventing the polymerization of heme into hemazoin in red blood cells [35]. Recently Wykes et al. provided evidence that rodent blood-stage parasites can be found to persist in plasmocytoid dendritic cells (pDCs) [36]. Persistence in a non-red blood cell, where heme is not present and thus not utilized by the parasites, is a potential mechanism whereby Plasmodia may avoid the impact of CQ chemoprophylaxis. It remains to be determined whether human Plasmodium blood-stage parasites can similarly persist in pDCs, but such persistence could provide an explanation for patent parasitemia observed following the cessation of CQ treatment in rodents.
In the B6-Py 17XNL model of ITV, we demonstrate the requirement of low-grade, transient parasitemia in induction of protective blood-stage directed antibody responses to provide protection. The role of subpatent, or low-grade, transient parasitemia, in the human studies was not further investigated, but it is possible low-grade blood-stage infections could elicit blood-stage immunity. Indeed, Pombo et al. demonstrated the induction of blood-stage immunity from low-grade blood-stage infection through the vaccination of human subjects with three administrations of ~30 P. falciparum-infected red blood cells followed by drug treatment 8–14 days later [28]. This vaccination strategy resulted in complete protection in 3 of the 4 human subjects when challenged with ~30 P. falciparum-infected red blood cells. The delayed treatment, compared to immediate treatment in human studies of ITV [8, 9], could allow sufficient replication and host exposure to blood-stage specific antigens during subpatent parasitemia to induce blood-stage immunity. However, the authors could not link protection to measureable IgG responses against blood-stage antigens. Rather, they correlated protection with induction of a Th1 response [28]. Conversely, when the human subjects receiving ITV were challenged with ~2,000 viable P. falciparum-infected erythrocytes I.V., the subjects were not protected and did not differ from naïve controls in the pre-patent period as detected by thick blood smear or PCR [9]. From these results, the authors concluded that ITV in human subjects did not elicit functional blood-stage immunity. However, it is important to note the human model precludes the ability to determine if vaccine-induced blood-stage immunity can control parasitemia, because these patients must be treated when blood-stage parasites are first detected to prevent symptoms of malaria. Indeed, we found that the pre-patent period did not dramatically differ in mice containing antibodies reactive to blood-stage antigens compared to naïve mice. Nevertheless, blood-stage specific antibodies ultimately contribute to demonstrable control and earlier clearance of parasitemia [34]. Thus, it is possible the restricted analysis of antibodies produced against only one liver-stage and two blood-stage antigens [9], combined with the inability to directly test whether blood-stage immunity can control parasitemia, may explain the lack of support for blood stage immunity in humans receiving ITV.
Based on such ethical constraints in human studies, rodent models are essential to understand the immunological mechanism underlying immunity elicited by whole-sporozoite and blood-stage parasite vaccinations. Indeed, the scalability of production, potential for drug resistance, and requirement of multiple immunizations complicates the application of whole-parasite vaccination approaches in the field, whereas a subunit vaccination approach could circumvent all these issues. The rodent model of RAS vaccination has predicted a strategy that provides protection in humans [2, 37, 38]. Therefore, continued studies in rodent models, and the analysis of the targeted antigens responsible for cellular and antibody-dependent immunity from sporozoite and blood-stage challenges, may reveal potent components for eliciting protection which can be translated for use in a human subunit vaccine.
In conclusion, our data provide insight into the cellular and immunological basis for enhanced protective immunity following vaccination with virulent sporozoites under chloroquine chemoprophylaxis. Our data reveal ITV-induced immunity against homologous challenge is not solely mediated by memory CD8 T cells, but is also mediated by T-cell independent blood-stage immune responses induced following exposure of the host to low-grade blood-stage parasitemia. A major issue in protection against Plasmodium in the field is the antigenic diversity of human malaria strains. Thus, it will be of major interest to determine whether ITV can provide heterologous immunity. In sum, these data further strengthen the rationale for mechanistic studies focused on identifying the cellular and humoral antigenic targets of whole parasite vaccine-induced, protective anti-Plasmodial immunity.
Supplementary Material
Highlights.
P. yoelii ITV induces a larger CD8 T cell response than RAS vaccination in B6 mice
Depletion of CD8 T cells does not abrogate ITV-induced sterilizing immunity
ITV-induced immunity correlates with blood-stage resistance and antibodies
Acknowledgements
The authors would like to thank members of the Harty laboratory for comments. We also thank Sandra Gonzalez at the NYU insectary for coordinating shipment of Py-infected mosquitoes. We thank Drs. Amanda Kalen and Michael McCormick of the radiation core facility and personnel at the University of Iowa for help with radiation attenuation of sporozoites. Work in the J.T.H. laboratory was supported by grants from the National Institutes of Health (AI85515, AI95178, AI100527). Work in the laboratory of N.S.B. is supported by a grant from the National Institutes of Health (AI099070).
K.L.D. and N.S.B. designed the experiments, performed the work, analyzed the data, and wrote the manuscript. J.T.H. designed the experiments, analyzed the data, and wrote the manuscript.
Abbreviations
- B6
C57Bl/6
- CQ
chloroquine
- GAP
genetically attenuated parasites
- ITV
infection-treatment vaccination
- Py
Plasmodium yoelii 17XNL
- RAS
radiation-attenuated sporozoites
- RBC
red blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no financial conflicts of interest.
References
- 1.W.M.R. World Malaria Report 2010. 2010:2010. [Google Scholar]
- 2.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002 Apr 15;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 3.Roestenberg M, Bijker EM, Sim BK, Billingsley PF, James ER, Bastiaens GJ, et al. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2013 Jan;88(1):5–13. doi: 10.4269/ajtmh.2012.12-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belnoue E, Costa FT, Frankenberg T, Vigario AM, Voza T, Leroy N, et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004 Feb 15;172(4):2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 5.Beaudoin RL, Strome CP, Mitchell F, Tubergen TA. Plasmodium berghei: immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp Parasitol. 1977 Jun;42(1):1–5. doi: 10.1016/0014-4894(77)90054-6. [DOI] [PubMed] [Google Scholar]
- 6.Golenser J, Heeren J, Verhave JP, Kaay HJ, Meuwissen JH. Crossreactivity with sporozoites, exoerythrocytic forms and blood schizonts of Plasmodium berghei in indirect fluorescent antibody tests with sera of rats immunized with sporozoites or infected blood. Clin Exp Immunol. 1977 Jul;29(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- 7.Orjih AU, Cochrane AH, Nussenzweig RS. Comparative studies on the immunogenicity of infective and attenuated sporozoites of Plasmodium berghei. Trans R Soc Trop Med Hyg. 1982;76(1):57–61. doi: 10.1016/0035-9203(82)90019-0. [DOI] [PubMed] [Google Scholar]
- 8.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009 Jul 30;361(5):468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 9.Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A. 2013 Apr 18; doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6(7):e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nganou-Makamdop K, van Gemert GJ, Arens T, Hermsen CC, Sauerwein RW. Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNgamma responses of hepatic CD8+ memory T cells. PLoS One. 2012;7(5):e36508. doi: 10.1371/journal.pone.0036508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011 Jun 16;9(6):451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000 Aug 1;165(3):1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 14.Fink E. Assessment of causal prophylactic activity in Plasmodium berghei yoelii and its value for the development of new antimalarial drugs. Bull World Health Organ. 1974;50(3–4):213–222. [PMC free article] [PubMed] [Google Scholar]
- 15.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009 Mar 20;30(3):421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doll KL, Butler NS, Harty JT. Tracking the total CD8 T cell response following whole Plasmodium vaccination. Methods Mol Biol. 2013;923:493–504. doi: 10.1007/978-1-62703-026-7_34. [DOI] [PubMed] [Google Scholar]
- 17.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009 Dec 15;183(12):7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amante FH, Engwerda CR, Good MF. Experimental asexual blood stage malaria immunity. Curr Protoc Immunol. 2011 Apr;Chapter 19(Unit 19):4. doi: 10.1002/0471142735.im1904s93. [DOI] [PubMed] [Google Scholar]
- 19.Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol. 2001 Nov;31(13):1499–1502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 20.Chattopadhyay R, Conteh S, Li M, James ER, Epstein JE, Hoffman SL. The Effects of radiation on the safety and protective efficacy of an attenuated Plasmodium yoelii sporozoite malaria vaccine. Vaccine. 2009 Jun 2;27(27):3675–3680. doi: 10.1016/j.vaccine.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 21.Doolan DL, Hoffman SL. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999 Jul 15;163(2):884–892. [PubMed] [Google Scholar]
- 22.Khan ZM, Vanderberg JP. Specific inflammatory cell infiltration of hepatic schizonts in BALB/c mice immunized with attenuated Plasmodium yoelii sporozoites. Int Immunol. 1992 Jul;4(7):711–718. doi: 10.1093/intimm/4.7.711. [DOI] [PubMed] [Google Scholar]
- 23.Kumar KA, Baxter P, Tarun AS, Kappe SH, Nussenzweig V. Conserved protective mechanisms in radiation and genetically attenuated uis3(−) and uis4(−) Plasmodium sporozoites. PLoS One. 2009;4(2):e4480. doi: 10.1371/journal.pone.0004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun. 2007 Aug;75(8):3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedegah M, Weiss WW, Hoffman SL. Cross-protection between attenuated Plasmodium berghei and P. yoelii sporozoites. Parasite Immunol. 2007 Nov;29(11):559–565. doi: 10.1111/j.1365-3024.2007.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarun AS, Dumpit RF, Camargo N, Labaied M, Liu P, Takagi A, et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J Infect Dis. 2007 Aug 15;196(4):608–616. doi: 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- 27.Friesen J, Silvie O, Putrianti ED, Hafalla JC, Matuschewski K, Borrmann S. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci Transl Med. 2010 Jul 14;2(40):40ra9. doi: 10.1126/scitranslmed.3001058. [DOI] [PubMed] [Google Scholar]
- 28.Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002 Aug 24;360(9333):610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 29.Elliott SR, Kuns RD, Good MF. Heterologous immunity in the absence of variant-specific antibodies after exposure to subpatent infection with blood-stage malaria. Infect Immun. 2005 Apr;73(4):2478–2485. doi: 10.1128/IAI.73.4.2478-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 31.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991 Sep;45(3):297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 32.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012 May;33(5):247–254. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Lau LS, Fernandez Ruiz D, Davey GM, de Koning-Ward TF, Papenfuss AT, Carbone FR, et al. Blood-stage Plasmodium berghei infection generates a potent, specific CD8+ T-cell response despite residence largely in cells lacking MHC I processing machinery. J Infect Dis. 2011 Dec 15;204(12):1989–1996. doi: 10.1093/infdis/jir656. [DOI] [PubMed] [Google Scholar]
- 34.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012 Feb;13(2):188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinappi M, Via A, Marcatili P, Tramontano A. On the mechanism of chloroquine resistance in Plasmodium falciparum. PLoS One. 2010;5(11):e14064. doi: 10.1371/journal.pone.0014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wykes MN, Kay JG, Manderson A, Liu XQ, Brown DL, Richard DJ, et al. Rodent blood-stage Plasmodium survive in dendritic cells that infect naive mice. Proc Natl Acad Sci U S A. 2011 Jul 5;108(27):11205–11210. doi: 10.1073/pnas.1108579108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975 May;24(3):397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- 38.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967 Oct 14;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.