Abstract
Prostate cancer (PCA) is the most commonly diagnosed cancer in men in the United States with growing worldwide incidence. Despite intensive investment in improving early detection, PCA often escapes timely detection and mortality remains high; this malignancy being the second highest cancer-associated mortality in American men. Collectively, health care costs of PCA results in an immense financial burden that is only expected to grow. Additionally, even in cases of successful treatment, PCA is associated with long-term and pervasive effects on patients. A proactive alternative to treating PCA is to prevent its occurrence and progression prior to symptomatic malignancy. This may serve to address the issue of burgeoning healthcare costs and increasing number of sufferers. One potential regimen in service of this alternative is PCA chemoprevention. Here, chemical compounds with cancer preventive efficacy are identified on the basis of their potential in a host of categories: their historical medicinal use, correlation with reduced risk in population studies, non-toxicity, their unique chemical properties, or their role in biological systems. PCA chemopreventive agents are drawn from multiple broad classes of chemicals, themselves further subdivided based on source or potential effect, with most derived from natural products. Many such compounds have shown efficacy, varying from inhibiting deregulated PCA cell signaling, proliferation, epithelial to mesenchymal transition (EMT), invasion, metastasis, tumor growth and angiogenesis and inducing apoptosis. Overall, these chemopreventive agents show great promise in PCA pre-clinical models, though additional work remains to be done in effectively translating these findings into clinical use.
Keywords: Prostate cancer, chemoprevention, natural products, grape seed extract, silibinin
Prostate Cancer
Prostate cancer (PCA) is the most commonly diagnosed cancer of American men, with an estimated 238,590 new cases of PCA along with 29,720 deaths in 2013 alone [1]. Studies have revealed that these numbers, as large as they are, may not be comprehensive despite intensive efforts to improve early detection of PCA. In an autopsy study, histological analysis of prostates revealed that prostatic intraepithelial neoplasia (PIN) was found in 9% of men still in their 20’s and carcinoma was found in 27% of the prostates from men in their 30’s [2]. In the PCA Prevention Trial, 2,950 men were found to have normal results in both annual prostate specific antigen (PSA) tests as well as digital rectal examinations, yet of these men, 449 (15.2%) were diagnosed with PCA [3]. Undiagnosed PCA is problematic, as PCA that has progressed to an advanced and metastatic stage is associated with high mortality, with 3/4th of patients dying within 5 years [1]. Even successful early detection and treatment of PCA is not without its burdens. In 2010, an estimated 11.85 billion dollars were spent in the United States on the direct health care costs of PCA alone [4]. This number is only expected to grow in the absence of alternative prevention and treatment modalities as a function of several factors. Diagnoses of PCA are expected to get better with time due to improved detection techniques and an increased life expectancy. New treatments have already served to increase the cost of treating PCA by more than 350 million dollars from 2002 to 2005 [5]. Thus the development of novel, but higher priced, interventions in combination with a predicted higher number of diagnosed cases may serve to greatly increase the financial costs of PCA. Furthermore, this does not include the personal burden of PCA, even successful treatment carrying with it years of urinary incontinence, bowel dysfunction, and sexual impotence. In a study following patients who claimed normal function prior to undergoing radical prostatectomy, it was found even 3 years later that 58% of them reported urinary incontinence, 27% reported bowel distress, and 94% reported minor to serious sexual dysfunction [6]. In another study following the effects of radical prostatectomy 2 years following surgery, patients reported urinary distress in 49% of cases and sexual disorders in 60% [7]. Radiation therapy carries similar risks even 3 years after treatment, with urinary issues being less of a concern, reported in only 17% of cases, but with a marked increase in the rate of cases of bowel dysfunction, rising to 66%, and again a high rate of sexual dysfunction, being reported by patients in 74% of cases [6]. In fact, the cost and consequences of screening and treatment are such that the United States Preventive Services Task Forces has recommended against regular PSA screening in asymptomatic men as it might contribute to unneeded and aggressive treatment that may not significantly protect the patient population [8, 9] though this conclusion is not universally accepted [10]. As a result of these factors, there is growing interest in developing novel prevention and treatment regimens intended to reduce the risk of PCA or even reverse its progression in a treated population without regard to symptomatic presentation.
Cancer Chemoprevention
Cancer chemoprevention has been conceived as one possible alternative/additional regimen, whereby well-tolerated and ideally inexpensive agents are taken to reduce cancer risk. This overarching philosophy for addressing cancer risk can be further categorized as primary, secondary, and tertiary cancer chemoprevention [11-13]. Primary chemoprevention addresses the cancer initiation or earliest dysfunction in initiated cells to reduce the risk of cancer development [13, 14]. Failing this, secondary chemoprevention is focused on the eradication or induction of stasis in premalignant lesions preventing the development of malignancy [13]. Finally, tertiary chemoprevention is intended to prevent the recurrence or second primary cancers in individuals who have earlier undergone successful intervention [13]. As chemopreventive compounds may be expected to be used long-term or even indefinitely as a counter to the lengthy multistage process by which cancers develop, these agents should ideally be nontoxic [15, 16] and thus there have been many chemoprevention studies focused on agents found in natural products.
Prostate Cancer Chemopreventive Agents
Fruits & Vegetables
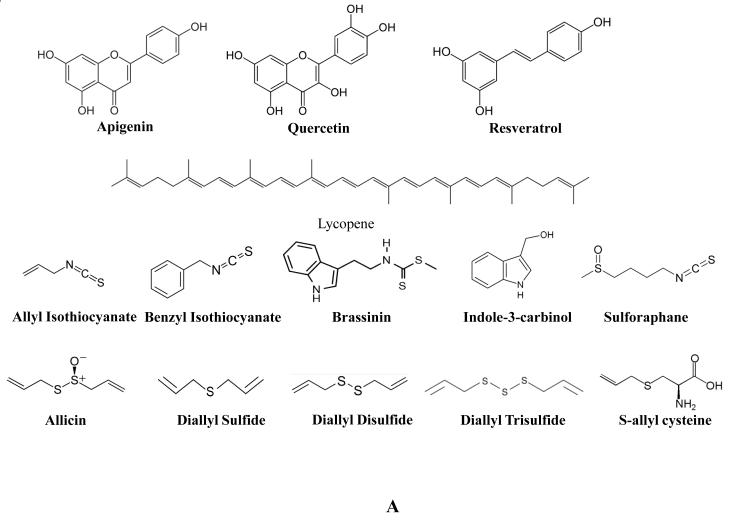
High intake of fruits and vegetables in general has not been correlated with a reduction in PCA risk, but agents contained in various fruits and vegetables have been shown to possess PCA chemopreventive potential [17, 18]. One agent, the flavone apigenin (Figure 1A), is found in many fruits and vegetables. In the prostate cell line, PC3-M, it was found that exposure to apigenin disrupted cytoskeletal alterations necessary for motility, resulting in morphological changes within the cell [19]. This appeared to be a result of a reduction in focal adhesion kinase (FAK)/Src signaling. Live cell imaging revealed that apigenin exposure reduced cell motion speed and directionality. In the PCA cell line 22Rv1, exposure to apigenin resulted in phosphorylation of p53, induction of p21/WAF-1, increase in Bax levels, and ROS generation, while inhibiting NF-κB/p65 transcriptional activity and down-regulating MDM2, Bcl-xL, and Bcl-2 [20]. These molecular alterations by apigenin together translated to a suppression of 22Rv1 growth and induction of apoptosis both in vitro as well as in an in vivo model [20]. Quercetin (Figure 1A) is a flavonoid found in a broad range of plant products and was found to enhance apoptosis and inhibit proliferative signaling, invasion, and metastasis in PCA cells [21, 22]. Resveratrol (Figure 1A) is a phytoalexin found in the skin of red grapes, wine, and peanuts. It was found to inhibit Akt and PI3K signaling in multiple PCA cell lines and in a mouse xenograft model [23, 24]. This translated to inhibition of PCA growth, metastasis, and induction of apoptosis. In addition, resveratrol was found to act as an anti-inflammatory agent through mitogen-activated protein kinase phosphatase-5 [25]. Pomegranates (Punica granatum) contain a complex mixture of antioxidant, polyphenolic compounds shown to inhibit androgen synthesizing genes [26]. Consumption by PCA patients was shown to delay disease progression [27]. Pomegranate extract enriched in naturally occurring polyphenolic ellagitannins, was found to inhibit LNCaP proliferation as well as markers of angiogenesis in vitro, and reduced PCA xenograft size and vessel density in a mouse model [28]. Tomatoes (Solanum lycopersicum) are rich in the carotenoid, lycopene (Figure 1A), a strong antioxidant. It has been shown that elevated lycopene consumption is strongly associated with decreased PCA risk as found in a study of 47,894 subjects [29, 30], though some evidence exists that other compounds within tomato may contribute to this effect as well [31].
Figure 1.
(A-E) Structural formula of compounds with chemopreventive efficacy against prostate cancer.
Cruciferous vegetables are of the family Brassicaceae, some examples being bok choy, broccoli, cabbage, mustard, and radish. In a multiethnic case-control study of 1619 men, intake of cruciferous vegetables correlated with reduced risk of prostate cancer [18], and consumption of cruciferous vegetables has been linked with reduced risk of PCA in several other epidemiological studies [32]. Thus compounds contained in them have been extensively investigated for recruitment as chemopreventive agents and Allyl isothiocyanate (Figure 1A) is a glucosinolate found in cruciferous vegetables that is responsible for the characteristic taste and effect of horseradish, mustard, and similar foods. Allyl isothiocyanate treatment significantly inhibited LNCaP and PC3 cell growth as a consequence of G2/M cell cycle arrest and induction of apoptosis [33]. These effects were associated with a decrease in cell cycle molecules Cdk1, Cdc25B, and Cdc25 as well as in the apoptosis regulatory molecules Bcl-2 and Bcl-xL. This reduction of cell cycle molecules was confirmed in vivo in a PC3 xenograft model where mice were i.p. injected with allyl isothiocyanate [34]. Treatments served to reduce tumor volume which was associated with an increase in apoptosis and reduction in mitosis by histological analysis. Importantly for the purposes of a chemopreventive agent, allyl isothiocyanate treatments were well tolerated by a normal prostate epithelial cell line [33]. Another isothiocyanate contained in cruciferous vegetables that has been investigated for chemopreventive potential is benzyl isothiocyanate (Figure 1A). In both 22Rv1 as well as PC3 PCA cell lines, benzyl isothiocyanate treatment was found to not only induce apoptosis but autophagy as well, as detected by aggregation of the autophagosomal marker, microtubule-associated protein 1 light chain 3 (LC3) and the formation of acidic organelles [35]. This last phenomenon was related to the dose-dependent inhibition of mammalian target of rapamycin (mTOR) kinase activity by benzyl isothiocyanate. Another compound found to affect mTOR activity is brassinin (Figure 1A), a phytoalexin found in many cruciferous vegetables and first identified in cabbage [36]. Consumption of cabbage was identified with lower risk of cancer and on this basis brassinin has been investigated as a potential chemopreventive agent [37]. Brassinin treatment inhibited constitutive activation of the PI3K/Akt/mTOR signaling pathway in PC3 cells [36]. This translated to increased apoptosis in treated cells as detected by positive annexin V binding, TUNEL staining, and disruption of mitochondrial membrane potential. This induction of apoptosis was associated with inhibition of Bcl-2, Bcl-xL, and survivin, activation of caspase, and cleavage of PARP. Indole-3-carbinol (Figure 1A) is the product of the degradation of glucobrassicin, a glucosinolate found at high levels in most cruciferous vegetables and thus a focus of interest regarding any chemopreventive potential found in these plants. Indole-3-carbinol treatment of LNCaP and PC3 cells was found to inhibit telomerase activity [38]. This translated to dose-dependent inhibition of PC3 proliferation [39] and induction of apoptosis [40]. Indole-3-carbinol-induced apoptosis in PC3 was associated with inhibition of Akt and PI3K and signaling through epidermal growth factor (EGF). Furthermore, expression of Bcl-xL and BAD was reduced by indole-3-carbinol treatment [40]. Sulforaphane (Figure 1A), an isothiocyanate, is the product of myrosinase activity on glucoraphanin in damaged cruciferous vegetables. Sulforaphane was found to dose-dependently inhibit proliferation [39] as well as protein synthesis [41] in treated PC3 cells. This effect was also found in vivo where broccoli sprout feeding of TRAMP mice reduced prostate tumor growth which was associated with decreased expression and activation of Akt and several downstream effectors [42]. Disruption of protein synthesis, as detected by incorporation of tritium labeled leucine, resulted in decreased PC3 viability as a consequence of the short half-life of the vital anti-apoptotic protein, survivin [41]. In addition, sulforaphane activated p38 MAPK and c-Jun N-terminal kinase (JNK) and reduced histone deacetylase (HDAC) activity in PC3 cells [43, 44]. In LNCaP cells, sulforaphane treatment reduced histone deacetylase activity and DNA methyltransferase (DNMT) expression [44, 45]. A potential mechanism for the anticancer properties of sulforaphane may be the release of H2S found to be induced when sulforaphane was placed into cell culture media or when placed in contact with mouse liver homogenates [43]. Importantly, the addition of two H2S scavengers, methemoglobin or oxidized glutathione, abrogated sulforaphane mediated inhibition of PC3 cells.
Seasonings
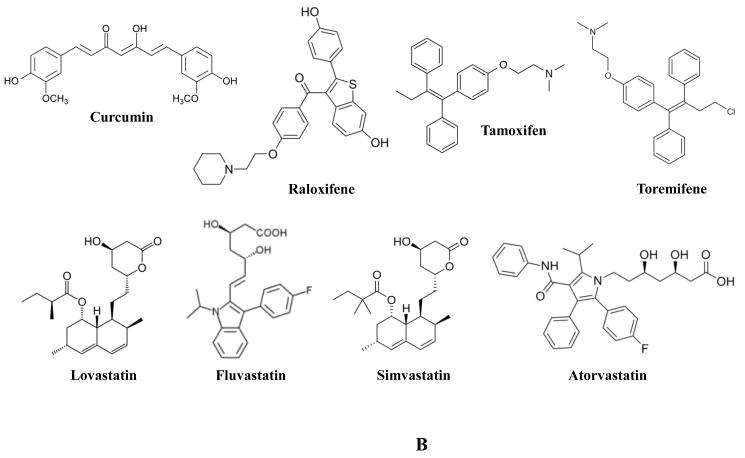
Garlic (Allium sativum) has been used for thousands of years as a staple food stuff, a potent seasoning, as well as a medicinal agent for a host of ailments [46]. It is associated with a pungent aroma and strong flavor as a result of the release and metabolism of reactive sulfur containing compounds. Alliin is an odorless sulfoxide derived from cysteine that is stored in vacuoles. It is rapidly converted into allicin (Figure 1A) when the cells of raw, fresh garlic are damaged. It is this compound that gives garlic its characteristic odor and flavor. Allicin in turn is metabolized into a host of compounds many of which have been investigated for efficacy in PCA chemoprevention. Among these, organosulfides diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) (Figure 1A) have shown varying potency against several PCA cell lines (LNCaP, DU-145, and PC3). These compounds were found to inhibit PCA cell growth, induce G2/M cell cycle arrest, and Akt-Bad mediated apoptosis [47-49]. Additionally, DATS inhibited the growth of PC3 xenografts in vivo [50]. Aged garlic contains S-allyl cysteine (SAC) (Figure 1A), that in treated PC3 cells, was found to suppress their proliferation and induce both G0/G1 cell cycle arrest and apoptosis, the latter associated with decreased Bcl-2 and increased Bax and caspase 8 [51]. In an in vivo model, SAC inhibited the growth of a human PCA xenograft, CWR22R. This correlated with a reduction in PSA levels, proliferation, and invasion, along with an increase in apoptosis and E-cadherin and γ-catenin expression [52]. Turmeric (Curcuma longa) is a plant of the ginger family that when processed into a powder exhibits a distinctive orange-yellow color [53]. As such, turmeric has been used as a spice, a medicinal agent, and a dye in South Asia and the Middle East for thousands of years. Turmeric’s color is partially a result of the phenolic active ingredient, curcumin (Figure 1B). Similar to other colored plant phenols, curcumin has been investigated as a cancer chemopreventive agent. Curcumin was found to modulate the Wnt/β-catenin pathway in LNCaP and C4-2 cells [54, 55], the NF-κB pathway in PC3 cells [56], and AR expression in LNCaP cells [55]. Curcumin treatment of PCA cells inhibited their capacity for proliferation, colony forming potential, and motility, while enhancing cellular contacts and apoptosis [54, 56] and in prostatic epithelial cells, it exhibited an anti-inflammatory activity [25]. In different mouse models of PCA, curcumin treatment inhibited xenograft growth [54], and lung metastases [56].
Pharmacological Agents
Several classes of agents have been brought forth as potential chemopreventive agents. Selective estrogen receptor modulators (SERMs) are compounds that have the capacity to be selective in their activity, serving to specifically target the estrogen receptor in selected tissues, and on this basis, their ability to alter the progression of PCA has been investigated. A critical issue with the long-term chemopreventive usage of SERMs is the various adverse effects, though many are less of a concern in male patients. The SERM raloxifene (Figure 1B), normally used to treat osteoporosis, was shown to induce apoptosis in LNCaP cells which was associated with caspase 9 activity [57]. Tamoxifen (Figure 1B), normally used in the treatment of breast cancer, was also found to induce apoptosis in LNCaP cells associated with DNA fragmentation and a reduction in Bcl-2 [58]. Growth inhibition of DU-145, PC3, and PC3-M by tamoxifen was also reportedly associated with inhibition of PKC. This corresponded to G1/S phase cell cycle arrest, induction of p21 and inactivation of Rb [59]. In a randomized, double-blind clinical study, toremifene (Figure 1B), used for the treatment of metastatic breast cancer, was found to decrease risk of PCA in men with high grade PIN with no significant difference in adverse events [60].
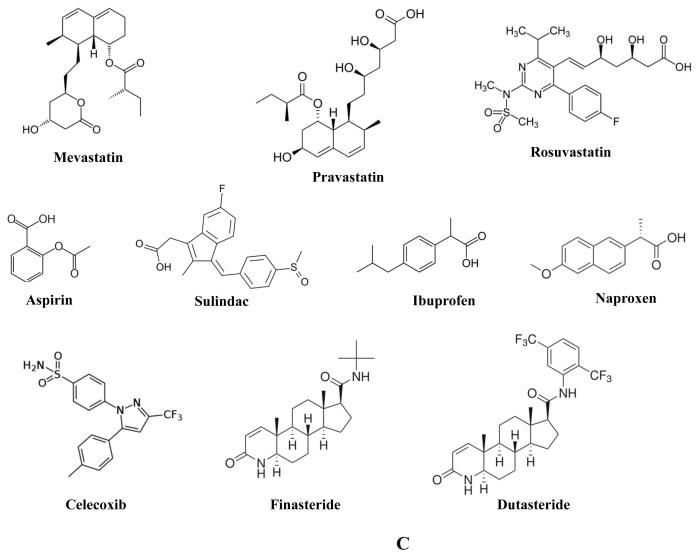
Statins are a set of drugs used to lower high levels of cholesterol in patients by inhibiting the activity of the HMG-CoA reductase enzyme. Statin use is generally well-tolerated though there are adverse effects that have been reported with their use, one being rhabdomyolysis. In a meta-analysis of 135 studies, statin use was found to be associated with a 9% increase in incidence of diabetes and concomitant increase in liver enzyme levels [61]. Since high cholesterol level has been linked with PCA risk, statins have been investigated for their potential to inhibit PCA by lowering cholesterol. Lovastatin (Figure 1B), found in several molds, was found to induce senescence, G1 cell cycle arrest, and apoptosis in LNCaP, DU-145, and PC3 cells. Induction of senescence was associated with inhibition of RhoA, G1 cell cycle arrest with reductions in Rb, phosphorylated Rb, c-myc, cyclin D1, cyclin A, cyclin B1, Cdc 2, and E2F-1, and apoptosis with activation of caspases [62-65]. Other statins have also been investigated for potential PCA chemopreventive activity. The statins fluvastatin and simvastatin (a synthetic derived from Aspergillus terreus) (Figure 1B) have been reported to induce similar inhibition of PCA cell signaling and proliferation in treated LNCaP and PC3 cells as lovastatin, though fluvastatin exhibited much less potency, requiring two orders higher concentrations to induce a similar response [66]. Simvastatin treatment of LNCaP cells was also found to lower lipid raft cholesterol content while inhibiting Akt activation, serving to induce apoptosis [67]. Atorvastatin (Figure 1B) treatment of PC3 cells resulted in extensive loss of cell viability, apoptosis, and increased expression of LC3-II as detected by immunoblot and immunofluorescent analyses [68, 69]. Mevastatin (Figure 1C), first identified from Penicillium citrinum, was found to elevate p21 levels while inhibiting Cdk2 activity in PC3 cells [70]. Treatment with pravastatin (Figure 1C), first identified from Nocardia autotrophica, elicited a modest inhibition of PC3 cell growth associated with clustering of cofilin and loss of p-caveolin [71]. In an assay where small molecules were examined for their potential to inhibit zebrafish angiogenesis, the statin rosuvastatin was identified as a potential anti-angiogenic compound. Rosuvastatin (Figure 1C) decreased microvessel density, induced tumor cell apoptosis, and suppressed growth of PPC-1 prostate tumor xenografts in non-obese diabetic severe combined immunodeficiency (NODSCID) mice [72]. In contrast to these experimental findings, the results of observational studies have been somewhat mixed. A meta-analysis of 27 (15 cohort and 12 case-control) studies revealed a significant decrease in both total as well as advanced PCA incidence [73]. This statin-mediated reduction in advanced PCA risk was found in several prospective cohort studies [74, 75] though perhaps as a result of long term use (>5 years) [75]. However, another population based study failed to identify any reduction in PCA risk with statin use, and in fact extended use in obese men was associated with increased PCA risk [76].
Non-steroidal anti-inflammatory drugs (NSAIDs) are generally known for their analgesic and anti-inflammatory properties. On the basis of their anti-inflammatory effects and the role of inflammation in the progression of PCA [77, 78], the potential of NSAID’s as chemopreventive agents has been investigated. Aspirin (Figure 1C) is an irreversible inhibitor of the cyclooxygenase enzymes generally used as an analgesic and in lower doses, as long-term protection against thrombotic events highlighting its suitability for sustained usage in older populations. Multiple epidemiological studies purport a reduction in PCA risk with aspirin usage though the evidence is not uniform. In a study involving 5,955 men treated for localized adenocarcinoma, aspirin use following treatment was associated with a significant decrease in PCA mortality [79]. In a much larger study of 70,144 men it was found current usage of aspirin was not correlated with reduction of PCA risk, but long-term usage was associated with inhibition of PCA [80]. In a large, diverse study involving 90,100 men followed up to 32 years, it was found that up to 6 aspirin a day were required to elicit a modest PCA protective effect [81]. In yet another study involving 47,882 patients, aspirin usage was not found to reduce total PCA incidence, but potentially reduced the occurrence of metastatic disease [82]. This may be related to the findings of another study of 74 men from families with the BRCA mutation where it was found that daily aspirin use conferred some protection against PCA [83]. Another study of 29,450 men also found PCA protective benefits of daily aspirin usage [84]. However, in a population-based case-control study of 1,001 PCA patients, while aspirin usage significantly reduced PCA risk, it was not related to disease aggressiveness [85]. Furthermore, a study involving 9,007 men failed to identify any protective benefits of aspirin intake [86]. Importantly, while aspirin treatment of LNCaP, DU-145, and PC3 cells markedly inhibited their proliferation, it was found to strongly induce P-glycoprotein expression in LNCaP cells [87] suggesting an increase in drug-resistance in PCA cells. This might be related to another study where high levels of aspirin failed to induce strong apoptosis in LNCaP cells [88]. Sulindac (Figure 1C) is an NSAID administered as a pro-drug that is metabolized into the active form, and derivatives of this drug have been investigated for PCA chemopreventive effect. Sulindac sulfide was found to induce apoptosis in multiple PCA cell lines as a result of up-regulating death receptor 5 [89] as well as inhibiting the growth of BPH-1, LNCaP, and PC3 cells [90]. Another sulindac derivative, sulindac sulfone exhibited similar growth inhibition and induction of apoptosis in BPH-1, LNCaP, and PC3 cells [90]. In addition, this inhibitory effect was confirmed in a mouse xenograft model of PCA using LNCaP cells. This response was associated with increased apoptosis as detected by TUNEL assay [91]. Ibuprofen (Figure 1C), derived from propanoic acid, has also been investigated for chemopreventive properties. Ibuprofen treatment of both LNCaP and DU-145 cells significantly impaired their cell viabilities. LNCaP cells exhibited cell cycle arrest as well with ibuprofen treatment [88]. In a study of 9,007 men, usage of ibuprofen was associated with a modest reduction of PCA risk [86]. However, in another study of 29,450 men, ibuprofen failed to confer a significant protective effect from PCA risk [84]. The NSAID, naproxen (Figure 1C) was found to inhibit cell viability of treated LNCaP cells [88]. In addition, usage of naproxen was found in a study of 9,007 men to be associated with a modest reduction of PCA risk [86]. Celecoxib (Figure 1C) is a selective COX-2 inhibitor and it was found to inhibit LNCaP and PC3 cell growth through G1 cell cycle arrest associated with reduction of cyclin D1 [92]. This result was verified in a PC3 xenograft model where celecoxib treatment reduced tumor size, cell proliferation, microvessel density, and cyclin D1 expression [92]. In a study focusing on PSA doubling time, there appeared to be significant decrease in PSA velocity induced by celecoxib treatment in a study of 78 men [93]. However, an international, open-label, randomized trial, STAMPEDE, of 2043 patients, found a lack of any benefit with celecoxib treatment and the trial involving celecoxib treatment was canceled [94].
Another class of agents being investigated for prostate cancer chemoprevention is 5-α-reductase inhibitors (5-ARI), currently used in the treatment of BPH and male pattern baldness [95, 96]. The 5-α-reductases, among other activities, convert testosterone into dihydrotestosterone. As dihydrotestosterone is a much higher affinity ligand for the androgen receptor (AR) [97], 5-ARI serve to indirectly inhibit AR signaling, a critical pathway for PCA [98]. One clinically used 5-ARI, finasteride (Figure 1C), which acts as a competitive inhibitor of testosterone metabolism has been investigated for chemopreventive efficacy [99]. In the PCPT trial of 18,882 men, finasteride treatment was found to reduce overall incidence of PCA, but was paradoxically associated with an increase in incidence of high grade cancers [100]. This was at first considered a possible mark against the use of finasteride. However, further analyses may possibly explain these findings. This paradox might be related to finasteride’s use in the treatment of BPH, in which it reduces prostate size [101]. This activity might in turn result in improvements in PCA detection rather than any unintended induction of high grade cancers [102]. In any case, further analysis remains to be done. Another 5-ARI, dutasteride (Figure 1C), in contrast to finasteride acts on two isoforms of 5-α-reductase, and partially on this basis, it has also been investigated for use as a possible cancer chemopreventive agent [99]. In the REDUCE trial of over 8,000 men, dutasteride treatment was also found to reduce incidence of prostate cancer, and similar to finasteride, also as a function of a reduction in lower grade prostate cancer. Unlike finasteride, there was no significant change in the incidence of higher grade prostate cancer [103, 104]; however, two-year follow-up revealed a higher number of low to mid-grade tumors in the dutasteride-treated group as compared to placebo [105]. Important to note in regards to any long-term usage of 5αR inhibitors is the potential for adverse effects, among these being reduced libido, impotence, cardiac events, and depression [106, 107].
Dietary Supplements
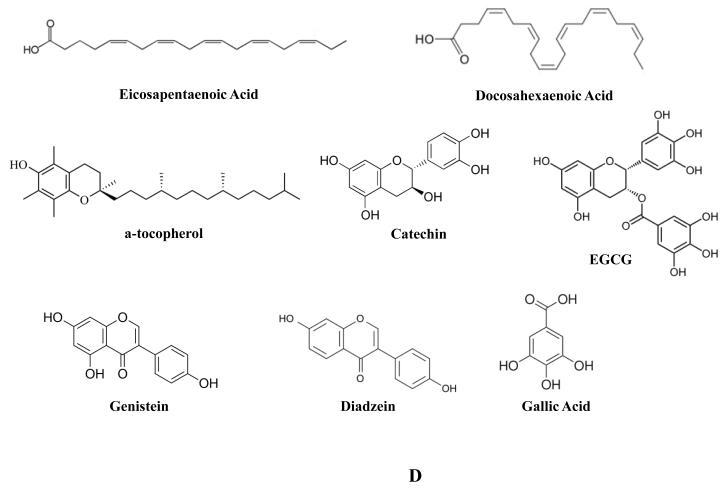
Fish oil is a heterogeneous mixture of a host of compounds. Among these are omega-3 fatty acids, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (Figure 1D). In a mouse xenograft model of PCA, mice fed only fish oil exhibited slower tumor growth and reduced mortality as compared to mice fed other fat sources [108]. This is consistent with a meta-analysis of case-control and cohort studies finding that incidence of PCA was not affected by consumption of fish, but mortality due to PCA was significantly reduced [109]. This association may be context dependent, as in a cohort of 2268 men in the AGES-Reykjavik study, consumption of fish that has been preserved (through salting or smoking) may increase PCA risk and fish oil may provide most benefit in elderly men [110]. Selenium is an essential micronutrient found in many plants, such as garlic and onions, as well as assorted sea life. It serves a critical role as a cofactor in the reduction of antioxidant enzymes and based on this biological activity it has been investigated as a cancer chemopreventive agent. Sodium selenite inhibited the growth of and induced apoptosis in several PCA cell lines (LNCaP, DU-145, and PC3) [111, 112]; in the case of LNCaP cells, this was associated with mitochondrial damage [112], where it also reduced expression of DNA methyltransferase I along with increased expression of tumor suppressor genes [113]. In multiple multi-year double-blind cancer prevention trials, patients given selenium supplements exhibited significantly reduced PCA incidence as compared to patients treated with placebo [114, 115] with a reduction in total cancer mortality in one study [115]. Vitamin E (Figure 1D) is a set of lipid soluble antioxidants found in many plant derived oils. The most investigated compound is α-tocopherol. Among several physiological roles, it serves to protect cellular membranes from oxidation and scavenges free radicals. Vitamin E succinate treatment of PC3 induced caspase-dependent apoptosis by disrupting Bcl-xL and Bcl-2 association with Bak [116]. In a randomized multi-year double-blind cancer chemoprevention trial involving Finnish smokers, α-tocopherol treatment resulted in fewer cases of PCA [117]. In addition, in the Lady transgenic mouse model of prostate cancer, feeding with a combination of lycopene, Vitamin E, and selenium resulted in a marked reduction of prostate tumor incidence [118]. On the basis of these findings, and to address the suitability of selenium and vitamin E as PCA chemopreventive agents, the phase III Selenium and Vitamin E Cancer Prevention Trial (SELECT) was initiated in 2001 [119]. It was to be an extensive investigation involving more than 35,000 patients followed for up to 12 years [120]. Unfortunately, no significant protective effect was found with either agent or in combination [120-122], though there remains the possibility that some aspect of dosing or formulation remains to be elucidated. Zinc is an essential mineral which is highly enriched in prostatic tissues. Zinc concentrations drop significantly in prostatic tumors with concomitant loss of the zinc transporter ZIP1 [123]. It was found that patients with diets rich in zinc exhibited reduced PCA mortality especially in patients with localized tumors [124]. Perhaps relatedly, exogenous zinc more potently inhibited the proliferation of benign prostatic hyperplasia cells (BPH-1) as compared to PC3 cells [125].
Green Tea & Soy
Green tea brewed from the leaves of the Camellia sinensis plant has been consumed for millennia throughout Asia. Green tea intake is inversely related to PCA risk and elevated consumption of green tea is associated with a lower incidence and degree of advancement of PCA [29, 126, 127]. Green tea is enriched in flavonoids, and of particular interest are the polyphenolic catechins (Figure 1D) exhibiting potent antioxidant potential [126, 128]. In vitro studies revealed that these catechins could variably induce apoptosis in several PCA cells lines (LNCaP, DU-145, and PC3) with (-)-epigallocatechin-3-gallate (EGCG) (Figure 1D) being the most potent compound [129, 130]. EGCG was found to act as a competitive inhibitor for DNA methyltransferase I in vitro, rescuing genes previously silenced as well as reducing expression of HDAC’s [131, 132]. In vivo, green tea catechins markedly reduced incidence of PCA in TRAMP mice [133]. Oral delivery of catechins were found to reduce PSA levels, and in patients with preexisting high grade PIN, it greatly reduced incidence of PCA [126, 134]. Soy, derived from soybeans (Glycine max), contains a mixture of isoflavones noted for their antioxidant properties. Soy is widely consumed as either fresh bean, as a processed foodstuff, or as a fermentation product. Elevated soy intake has been associated with decreased risk of PCA [135, 136]. According to USDA data, the isoflavones genistein and daidzein (Figure 1D) represent 57% and 37% of the total isoflavones in soybeans, respectively. Thus they have been the focus of studies regarding the cancer chemopreventive properties of soy consumption which has been reported to alter AR, Akt, NF-κB, MAPK, and Notch signaling [137, 138]. Genistein has been reported to inhibit PCA motility, induce apoptosis, and at higher levels in patient plasma is associated with lower risk of PCA [139-141]. It has also been reported to inhibit DNA methyltransferase activity abrogating silencing of tumor suppressor genes [142, 143]. In addition, genistein treatment of TRAMP mice resulted in a marked reduction in poorly differentiated tumors [144]. Confounding the suitability of genistein as a PCA chemopreventive agent are reports that in high concentrations, genistein induces metastasis in mouse xenograft models [145, 146], though the addition of diadzein (as in soybeans and various products) may prevent this response [147]. Anthocyanins are flavonoids found in many plants, identified as distinctive pigment molecules and like other related compounds are strong antioxidants. In a rat model of benign prostatic hyperplasia (BPH), rats treated with black soybean anthocyanin exhibited reduced prostate weight associated with increased apoptosis and reduced proliferation of prostate cells [148].
Grape Seed Extract
Grapes (fruit of the genus Vitis), have been cultivated for many thousands of years, often in wine, moderate consumption of which has been associated with various health benefits. As a consequence, components of grapes have been investigated for medicinal use. One such product, grape seed extract (GSE), has been found to be a potent antioxidant [149], with a high concentration of flavonoids, vitamin E, and proanthocyanidins. Evidence for the anti-PCA efficacy of GSE was provided in a large study with 35,239 men, the VITamins And Lifestyle (VITAL) study, where it was found that any use of grape seed supplements significantly lowered total risk of PCA [150]. In LNCaP cells, GSE treatment inhibited growth and induced cell detachment which was associated with a potent reduction in FAK levels, histone acetyltransferase activity (HAT), and AR-mediated transcription [151, 152]. In addition, GSE treatment mediated apoptosis in LNCaP cells which was associated with an increase in activated caspases, cleaved PARP, and cytosolic cytochrome c and apoptosis-inducing factor as well as inducing Chk2, p53, and ataxia telangiectasia mutated (ATM) kinase activity [151]. GSE treatment of DU-145 cells was found to inhibit EGFR and AP1 activation, Elk1 and IκBα phosphorylation, ERK1/2 and IKKα kinase activity, secretion of vascular endothelial growth factor (VEGF) and disrupted both constitutive as well as TNF-α induced NF-κB activity [153-155]. Additionally, GSE treatment induced phosphorylation of ERK 1/2, JNK1/2, and c-Jun and secretion of insulin-like growth factor binding protein (IGFBP)-3 [153]. Collectively, GSE potently induced apoptosis in treated DU-145 cells that was associated with multiple activated caspases and cleaved PARP and which appeared to be dependent on JNK activation [153, 155, 156]. These results were confirmed in a mouse xenograft model of PCA using DU-145 cells. Tumors in GSE fed mice exhibited significant inhibition of proliferation and induction of apoptosis [154]. This corresponded with a significant reduction in tumor size and microvessel formation and a modest inhibition of VEGF and induction of IGFBP-3 [154]. In PC3 cells, GSE treatment inhibited plasminogen activator (uPA) and NF-κB activity which translated to a reduction in PC3 migratory potential [157]. GSE feeding of TRAMP (transgenic adenocarcinoma of the mouse prostate) mice arrested the disease mostly at the PIN stage with a reduction in adenocarcinoma. This corresponded to a decrease in proliferating cells, and a marked increase in the presence of apoptotic ones which was associated with a reduction in regulatory cyclins A, B1, and E, CDK’s 2 and 6, and Cdc2 [158].
In light of these findings, and the fact that GSE is an incompletely defined mixture of many compounds, identifying the specific agents that mediate GSE’s anticancer effects are of great interest. A polyphenolic fraction of GSE enriched in antioxidant procyanidins was found to significantly inhibit growth and induce G1 cell cycle arrest and apoptosis in DU-145 cells [159]. This was associated with a reduction of cyclin E, and CDK’s 2 and 4, as well as phosphorylated ERK1/2 along with an increase in Cip1/p21. The capacity of this polyphenolic fraction of GSE to inhibit PCA cell growth and disrupt cell viability was further confirmed in the LNCaP cell line [159]. Further partitioning of GSE by reverse-phase high-performance liquid chromatography (HPLC) identified multiple fractions exhibiting potent PCA inhibitory capacity [160]. Contained in these fractions, along with other compounds, was gallic acid (GA) (Figure 1D), a potent antioxidant. On this basis, it has been investigated as a potential anti-cancer agent. GA was found to potently inhibit cell growth and induce cell cycle arrest and apoptosis in treated DU-145 cells [160-162]. This was associated with time dependent inhibition of regulatory cyclins and CDK’s, phosphorylation of Cdc2, Cdc25A, Cdc25C, histone 2AX, ATM, and Chk2 [161], induction of Cip1/p21, activation of caspases, and PARP cleavage [160]. Loss of cell viability by GA treatment was further reported in the 22Rv1 cell line [162]. GA mediated inhibition of PCA growth and viability was confirmed in vivo in both xenograft as well as TRAMP mouse models. GA treatment of mice inhibited the growth of both DU-145 as well as 22Rv1 xenografts which were associated with inhibition of PCA cell proliferation and angiogenesis as well as induction of apoptosis as detected by PCNA, CD31, and TUNEL immunostaining respectively [162]. Similar to results obtained with GSE, GA fed TRAMP mice exhibited a reduction in the rate of disease progression with a decrease in incidence of advanced prostatic tumors [163]. Consistent with xenograft models, tumors in TRAMP mice fed GA exhibited decreased proliferation and increased apoptosis, which were associated with a decrease in cyclins B1 and E, CDK’s 2, 4, and 6, and Cdc2.
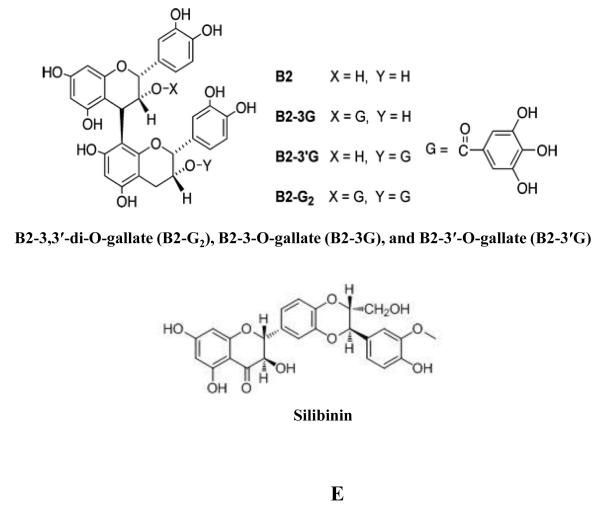
Other compounds contained within GSE have also been found to possess anti-PCA potential. GSE fractions containing procyanidin trimers and esterified dimeric and trimeric GA were found to significantly inhibit DU-145 cell growth and induce apoptosis [160]. Gallate esters B2-3,3′-di-O-gallate (B2-G2), B2-3-O-gallate (B2-3G), and B2-3′-O-gallate (B2-3′G) (Figure 1E) were all found to reduce LNCaP viability, though GA did not and only B2-G2 was found to significantly reduce LNCaP cell growth [164]. This loss of viability was associated with induction of apoptosis: activation of caspases, PARP cleavage, and decreased Bcl-2, Bcl-xL, and AR. Similarly, in DU-145 cells, B2-G2 treatment potently induced growth inhibition and apoptosis [165].
Milk Thistle
The milk thistle (Silybum marianum) has been used for millennia as a panacea to a host of ailments, especially of the liver, gall bladder, and kidney. It has shown efficacy in the treatment of hepatic injury along several vectors: bile duct inflammation, cirrhosis, fatty liver, mushroom poisoning, and viral hepatitis [166]. In contrast to its historical usage, medicinal interest in milk thistle currently focuses on the derivative, silymarin, which is the standardized extract of milk thistle seeds and is composed of many related flavonolignans and other compounds. The flavonoid silibinin (Figure 1E) is the most abundant compound and is the principal active ingredient of silymarin. Silibinin has demonstrated broad spectrum efficacy against PCA as well as several other cancers as detailed elsewhere [167]. Silibinin was reported to disrupt a host of signaling pathways across multiple PCA cell lines. Silibinin treatments disrupted EGFR signaling in LNCaP and DU-145 cells [168, 169] which was associated with a decrease in secreted transforming growth factor-α (TGF-α) and inhibition of both ERK1/2 and JNK1/2 phosphorylation [169]. In PC3 cells, silibinin targeted insulin-like growth factor receptor (IGFR) signaling resulting in higher concentrations of IGFBP-3 in PC3 conditioned media [170]. This effect was supported with in vivo studies where silibinin fed mice were found to have elevated levels of both circulating and tumor specific IGFBP-3, which was associated with improved differentiation in tumor cells [171-174]. In LNCaP cells, silibinin disrupted AR signaling through multiple modalities [175-177] and in DU-145 and PC3 cells, silibinin targeted the Wnt/β-catenin pathway [178]. Furthermore, DU-145 cells lost their constitutively activated STAT-3 and NF-κB when treated with silibinin [179-181]. Silibinin has been shown in multiple studies to inhibit the proliferative capacity of PCA cells both in vitro [182-185] as well as in vivo [172-174, 186, 187] where silibinin fed mice exhibited decreased tumor volume. This is partially a consequence of silibinin robustly inducing both G1 cell-cycle arrest in PCA cells [182] by modulating a myriad elements of the cyclin–CDK–CDKI pathway [173, 182, 184, 188, 189] and transcription activating factors [184, 188] as well as G2-M arrest [184] by modulating the Chk2– Cdc25C–Cdc2/cyclin B1 pathway [182, 190, 191]. Furthermore, the antiproliferative effect exerted by silibinin was associated with an inhibition of telomerase in LNCaP cells as well as DNA topoisomerase IIα in DU-145 cells [176, 192]. Beyond inhibiting proliferation, silibinin has also been shown to decrease Bcl-2 and survivin levels, activate caspases (caspases 3, 9, and 7), induce release of cytochrome c, and ultimately initiating apoptosis in multiple PCA cell lines both in vitro as well as in vivo [172, 173, 179, 193, 194]. Silibinin has also been shown to disrupt the mesenchymal nature of PCA cell lines, shifting them back into a more epithelial phenotype [181, 186, 187, 195]. Silibinin treatment of the PCA cell lines, PC3, PC3MM2, and C4-2B was found to down-regulate their levels of Slug, Snail, phospho-Akt (ser473), nuclear β-catenin, phospho-Src (tyr419) and Hakai, which are all important regulators of EMT (epithelial to mesenchymal transition). In turn, silibinin treatment upregulated E-cadherin levels which potently disrupted the capacity of PC3, PC3MM2 and C4-2B for migration and invasion [195]. Silibinin-mediated upregulation of E-cadherin was confirmed in the TRAMP mouse model wherein silibinin treatment decreased matrix metalloproteinases (MMPs), Snail, fibronectin, and vimentin levels which together served to inhibit metastasis [186, 187]. Similarly, in ARCaPM cells, silibinin treatment was found to depress levels of ZEB1, Slug, vimentin, and MMP-2 which was associated with a reduction in motility, migration, and invasion [181, 196]. We recently reviewed in detail the anti-metastatic efficacy of silibinin including its effect on tumor microenvironment components [197, 198]. Silibinin has also been reported to inhibit MMP-9 expression in human prostate carcinoma cell lines [186, 187]. Furthermore, silibinin treatment has been reported to inhibit angiogenesis, reducing tumor microvessel density which was associated with decreased expression of platelet endothelial cell adhesion molecule-1 (PECAM1)/CD-31, VEGF, VEGFR2, HIF-1α, and iNOS in prostate tumors of mice fed silibinin in both xenograft and TRAMP models [172, 173, 186, 187], as well as altering the metabolic profile of the tumor microenvironment [199]. HIF-1α inhibition by silibinin treatment was also found in LNCaP and PC3 cell lines under both normoxic and hypoxic conditions [200]. Collectively, in vivo work conducted in both xenograft as well as TRAMP models reveals that mice fed silibinin exhibit significantly reduced prostate tumor growth, mesenchymal phenotype, and angiogenesis, along with a concomitant increase in apoptosis serving to inhibit prostate tumor development consistent with foundational in vitro work. On the basis of these findings, and to further investigate the potential of silibinin as a chemopreventive, human studies were undertaken. In these trials silybin-phytosome was the test agent as it is a commercial formulation of silibinin and phosphatidylcholine intended to improve bioavailability. In the first trial consisting of thirteen patients with advanced PCA, silybin-phytosome was taken orally 3 times daily. The study was initiated at 2.5g of silybin-phytosome ingested daily, gradually escalating to 20g, with each given dose maintained for 4 weeks prior to a change. Observed toxicity was relatively low, principally consisting of grade 1-2 hyperbilirubinemia (9 of 13), with one instance of grade 3 toxicity. For future studies, it was determined that 13g daily of silybin-phytosome divided into 3 doses a day, were well tolerated [201]. Thus, in the next trial consisting of 12 patients with localized PCA scheduled for prostatectomy, 6 were selected to be given 3 doses of silybin-phytosome daily (in total 13g), and 6 were selected as controls. The length of treatment varied between patients from 14-31 days with a mean of 20. Serum blood levels of silibinin reached a mean value of 19.7 μM. Observed toxicity was similar to the previous trial, though one grade 4 thromboembolic event following surgery was found [202].
Conclusions
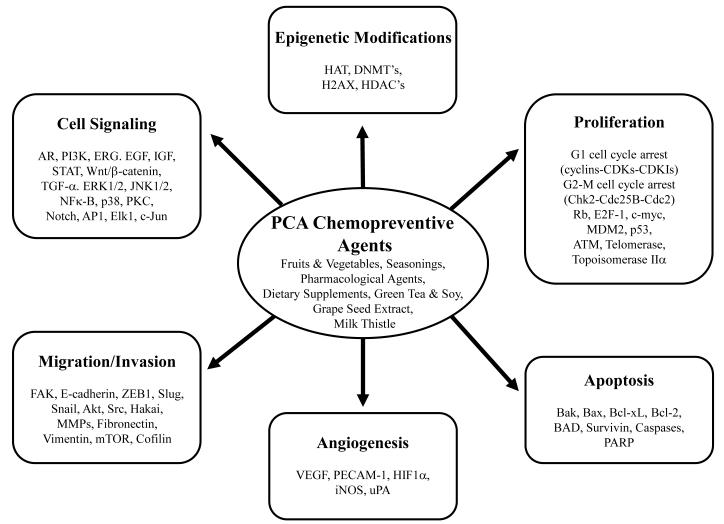
In general, some practical strategies for reducing PCA risk might include reducing intake of alcohol, red meat, saturated fats, and excessive carbohydrates (particularly of sugary drinks) while increasing intake of fruits, vegetables and ω-3 fatty acids along with exercise [203-205], though further work investigating these correlations remains to be done. In addition, numerous chemical agents with identified anti-PCA efficacy have been investigated for their potential as PCA chemopreventive agents (Figure 1A-E). This may be of particular use in secondary and tertiary chemoprevention where initiating events have already occurred and anti-PCA properties may then be useful. These agents have been selected based on their historical medicinal use, correlation with reduced risk in population studies, their unique chemical properties, or their role in biological systems. They are often derived from natural products, and ideally all of them must be well tolerated for long-term, potentially indefinite usage. Many show promise, collectively inhibiting a host of deregulated PCA cell signaling and regulation [206] cell proliferation, EMT, invasion and metastasis, and angiogenesis, and inducing apoptosis (Figure 2). Dysfunctions in these cellular properties have been recognized as hallmark as well as critically necessary events for the progression of cancer [207]. Thus, while the presentation of metastatic cancer remains the line demarking poor prognosis [1], addressing any other targets may also be expected to mitigate PCA-related mortality. Important to note in the context of chemoprevention is the possibility of identifying genetic backgrounds that might be susceptible to these dysfunctions and that may be more amenable to (and in greater need of) these chemopreventive strategies. An extensive genomic analysis of 218 prostate tumors and 12 PCA cell lines revealed a preponderance of mutations in molecules involved in the Rb, PI3K, and Ras/Raf pathways, particularly in metastatic prostate tumors [208], which along with other mutations, were associated with genomic clusters that correlated with increased relapse risk following prostatectomy. Another study identified single nucleotide polymorphisms in five chromosomal regions that along with familial history strongly correlated with risk of PCA, though not the progression of the disease [209]. These findings may thus serve to identify individuals that could benefit most from chemopreventive interventions.
Figure 2.
Schematic representation of the molecular mechanisms for prostate cancer chemoprevention by natural and pharmacological agents.
The context by which many of these strategies are taken (as well as the agents discussed in this work) may be critical for any possible protective effects. This might contribute to the gap in efficacy between retroactive studies that have identified potentially chemopreventive agents and randomized clinical trials investigating these agents. The context in how these agents are consumed might be critical to any protective effects. As discussed earlier, the protection induced by an elevated intake of fish might be predicated on avoiding preservative techniques [110]. Alternatively, lycopene has been found to be more bioavailable when tomato products are consumed with oil [210], and in tomato paste versus the fresh fruit [211]. So recording and subsequently comparing the amount or frequency of intake of an agent, but not the context in which it is consumed, may not be enough to identify any protective properties of a chemopreventive agent. This is especially important as in this review many identified agents were investigated on the basis of an inverse correlation between PCA risk and elevated intake of a particular food. This food might contain hundreds of other compounds which together provide some subtle contribution that may need to be replicated to properly recapitulate any protective properties. In addition, high intake of specific agents (as might be found in supplements and often used in clinical trials) vs. the more modest levels found in dietary intake may obfuscate otherwise protective properties. As previously mentioned, high levels of genistein was found to induce metastasis in mice [145, 146]. Furthermore, a meta-analysis of 19 clinical studies involving Vitamin E found a correlation between increased mortality in studies using high doses of Vitamin E [212]. This might be related to a pro-oxidant effect as a result of high-dose Vitamin E supplementation [213], which was further identified in other vitamin antioxidants [214]. These findings all together point to the need to remain cognizant of context while investigating chemopreventive agents.
Additional work remains to be done in mechanistic studies, confirming these findings in large scale observational studies, especially in the cases of agents where evidence remains inconclusive, refining bioavailability, efficacy, and consistency of these agents for clinical usage, while assessing drug-drug interaction. Collectively, the body of evidence provides a framework of investigation for the potential of chemopreventive compounds to inhibit PCA progression at all stages of cancer development over the course of a lifetime to reduce PCA incidence, and to thus systematically reduce the heavy burden, both financial and personal, of this disease throughout the population.
Acknowledgements
Original studies in our laboratories related to prostate cancer chemoprevention by natural agents are supported by the NCI R01 grants CA91883 (to CA) and CA102514 (to RA). This article is dedicated to the fond memory of Victor Fung, Ph.D., a former Program Officer at NCI and former Scientific Review Officer of the Cancer Etiology study section of CSR, NIH, for his wisdom, compassion, integrity, his love of sciences and the arts, his incredible culinary skills, and above all, his contributions to the career development of so many investigators during his own distinguished career.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors declare that there are no conflicts of interest.
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- [2].Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. The Journal of urology. 1993;150:379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- [3].Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. The New England journal of medicine. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- [4].Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nguyen PL, Gu X, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, Hoffman KE, Hu JC. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009;27:3916–3922. doi: 10.1200/JCO.2008.18.6486. [DOI] [PubMed] [Google Scholar]
- [7].Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, Albertsen PC, Harlan LC, Potosky AL. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–360. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- [8].Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, Gleitsmann K, Koenig HC, Lam C, Maltz A, Rugge JB, Lin K. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Annals of internal medicine. 2011;155:762–771. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- [9].Collins MM, Barry MJ. Controversies in prostate cancer screening. Analogies to the early lung cancer screening debate. JAMA. 1996;276:1976–1979. [PubMed] [Google Scholar]
- [10].Carroll PR, Whitson JM, Cooperberg MR. Serum prostate-specific antigen for the early detection of prostate cancer: always, never, or only sometimes? J Clin Oncol. 2011;29:345–347. doi: 10.1200/JCO.2010.32.5308. [DOI] [PubMed] [Google Scholar]
- [11].Hong WK, Spitz MR, Lippman SM. Cancer chemoprevention in the 21st century: genetics, risk modeling, and molecular targets. J Clin Oncol. 2000;18:9S–18S. [PubMed] [Google Scholar]
- [12].Sandhu GS, Nepple KG, Tanagho YS, Andriole GL. Prostate cancer chemoprevention. Seminars in oncology. 2013;40:276–285. doi: 10.1053/j.seminoncol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- [13].Steward WP, Brown K. Cancer chemoprevention: a rapidly evolving field. British journal of cancer. 2013;109:1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rittmaster RS. Chemoprevention of prostate cancer. Acta Oncol. 2011;50(Suppl 1):127–136. doi: 10.3109/0284186X.2010.527367. [DOI] [PubMed] [Google Scholar]
- [15].Kucuk O. Cancer chemoprevention. Cancer metastasis reviews. 2002;21:189–197. doi: 10.1023/a:1021298508095. [DOI] [PubMed] [Google Scholar]
- [16].Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) International journal of oncology. 2003;23:17–28. [PubMed] [Google Scholar]
- [17].Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- [18].Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS., Jr. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2000;9:795–804. [PubMed] [Google Scholar]
- [19].Franzen CA, Amargo E, Todorovic V, Desai BV, Huda S, Mirzoeva S, Chiu K, Grzybowski BA, Chew TL, Green KJ, Pelling JC. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev Res (Phila) 2009;2:830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- [20].Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free radical biology & medicine. 2008;44:1833–1845. doi: 10.1016/j.freeradbiomed.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jung YH, Heo J, Lee YJ, Kwon TK, Kim YH. Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life sciences. 2010;86:351–357. doi: 10.1016/j.lfs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Senthilkumar K, Arunkumar R, Elumalai P, Sharmila G, Gunadharini DN, Banudevi S, Krishnamoorthy G, Benson CS, Arunakaran J. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3) Cell biochemistry and function. 2011;29:87–95. doi: 10.1002/cbf.1725. [DOI] [PubMed] [Google Scholar]
- [23].Chen Q, Ganapathy S, Singh KP, Shankar S, Srivastava RK. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PloS one. 2010;5:e15288. doi: 10.1371/journal.pone.0015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sheth S, Jajoo S, Kaur T, Mukherjea D, Sheehan K, Rybak LP, Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PloS one. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28:1188–1196. doi: 10.1093/carcin/bgl241. [DOI] [PubMed] [Google Scholar]
- [26].Hong MY, Seeram NP, Heber D. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. The Journal of nutritional biochemistry. 2008;19:848–855. doi: 10.1016/j.jnutbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, Seeram N, Liker H, Wang H, Elashoff R, Heber D, Aviram M, Ignarro L, Belldegrun A. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- [28].Sartippour MR, Seeram NP, Rao JY, Moro A, Harris DM, Henning SM, Firouzi A, Rettig MB, Aronson WJ, Pantuck AJ, Heber D. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. International journal of oncology. 2008;32:475–480. [PubMed] [Google Scholar]
- [29].Jian L, Lee AH, Binns CW. Tea and lycopene protect against prostate cancer. Asia Pac J Clin Nutr. 2007;16(Suppl 1):453–457. [PubMed] [Google Scholar]
- [30].Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- [31].Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr., Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- [32].Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutrition and cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- [33].Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- [34].Srivastava SK, Xiao D, Lew KL, Hershberger P, Kokkinakis DM, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- [35].Lin JF, Tsai TF, Liao PC, Lin YH, Lin YC, Chen HE, Chou KY, Hwang TI. Benzyl isothiocyanate induces protective autophagy in human prostate cancer cells via inhibition of mTOR signaling. Carcinogenesis. 2013;34:406–414. doi: 10.1093/carcin/bgs359. [DOI] [PubMed] [Google Scholar]
- [36].Kim SM, Park JH, Kim KD, Nam D, Shim BS, Kim SH, Ahn KS, Choi SH. Brassinin Induces Apoptosis in PC-3 Human Prostate Cancer Cells through the Suppression of PI3K/Akt/mTOR/S6K1 Signaling Cascades. Phytotherapy research : PTR. 2013 doi: 10.1002/ptr.5010. [DOI] [PubMed] [Google Scholar]
- [37].Mehta RG, Liu J, Constantinou A, Thomas CF, Hawthorne M, You M, Gerhuser C, Pezzuto JM, Moon RC, Moriarty RM. Cancer chemopreventive activity of brassinin, a phytoalexin from cabbage. Carcinogenesis. 1995;16:399–404. doi: 10.1093/carcin/16.2.399. [DOI] [PubMed] [Google Scholar]
- [38].Adler S, Rashid G, Klein A. Indole-3-carbinol inhibits telomerase activity and gene expression in prostate cancer cell lines. Anticancer research. 2011;31:3733–3737. [PubMed] [Google Scholar]
- [39].Frydoonfar HR, McGrath DR, Spigelman AD. The effect of indole-3-carbinol and sulforaphane on a prostate cancer cell line. ANZ journal of surgery. 2003;73:154–156. doi: 10.1046/j.1445-2197.2003.02652.x. [DOI] [PubMed] [Google Scholar]
- [40].Chinni SR, Sarkar FH. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin Cancer Res. 2002;8:1228–1236. [PubMed] [Google Scholar]
- [41].Wiczk A, Hofman D, Konopa G, Herman-Antosiewicz A. Sulforaphane, a cruciferous vegetable-derived isothiocyanate, inhibits protein synthesis in human prostate cancer cells. Biochimica et biophysica acta. 2012;1823:1295–1305. doi: 10.1016/j.bbamcr.2012.05.020. [DOI] [PubMed] [Google Scholar]
- [42].Keum YS, Khor TO, Lin W, Shen G, Kwon KH, Barve A, Li W, Kong AN. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharmaceutical research. 2009;26:2324–2331. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- [43].Pei Y, Wu B, Cao Q, Wu L, Yang G. Hydrogen sulfide mediates the anti-survival effect of sulforaphane on human prostate cancer cells. Toxicology and applied pharmacology. 2011;257:420–428. doi: 10.1016/j.taap.2011.09.026. [DOI] [PubMed] [Google Scholar]
- [44].Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27:811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hsu A, Wong CP, Yu Z, Williams DE, Dashwood RH, Ho E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clinical epigenetics. 2011;3:3. doi: 10.1186/1868-7083-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bostock M.D. John, Riley FRSHT, Esq. BA. Pliny the Elder. Taylor and Francis; Fleet Street, Red Lion Court, London: 1855. The Natural History. [Google Scholar]
- [47].Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, Lee YJ, Singh SV. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- [48].Xiao D, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Brisson M, Lazo JS, Singh SV. Diallyl trisulfide-induced G(2)-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc 25 C. Oncogene. 2005;24:6256–6268. doi: 10.1038/sj.onc.1208759. [DOI] [PubMed] [Google Scholar]
- [49].Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Molecular cancer therapeutics. 2007;6:1599–1609. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xiao D, Lew KL, Kim YA, Zeng Y, Hahm ER, Dhir R, Singh SV. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;12:6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- [51].Liu Z, Li M, Chen K, Yang J, Chen R, Wang T, Liu J, Yang W, Ye Z. S-allylcysteine induces cell cycle arrest and apoptosis in androgen-independent human prostate cancer cells. Molecular medicine reports. 2012;5:439–443. doi: 10.3892/mmr.2011.658. [DOI] [PubMed] [Google Scholar]
- [52].Chu Q, Lee DT, Tsao SW, Wang X, Wong YC. S-allylcysteine, a water-soluble garlic derivative, suppresses the growth of a human androgen-independent prostate cancer xenograft, CWR22R, under in vivo conditions. BJU international. 2007;99:925–932. doi: 10.1111/j.1464-410X.2006.06639.x. [DOI] [PubMed] [Google Scholar]
- [53].N.C.f.C.a.A.M. (NCCAM) Herbs at a Glance: Turmeric, Science & Safety. National Institutes of Health; 2012. [Google Scholar]
- [54].Sundram V, Chauhan SC, Ebeling M, Jaggi M. Curcumin attenuates beta-catenin signaling in prostate cancer cells through activation of protein kinase D1. PloS one. 2012;7:e35368. doi: 10.1371/journal.pone.0035368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/beta-catenin signaling pathway in LNCaP prostate cancer cells. Prostate cancer and prostatic diseases. 2010;13:343–349. doi: 10.1038/pcan.2010.26. [DOI] [PubMed] [Google Scholar]
- [56].Killian PH, Kronski E, Michalik KM, Barbieri O, Astigiano S, Sommerhoff CP, Pfeffer U, Nerlich AG, Bachmeier BE. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis. 2012;33:2507–2519. doi: 10.1093/carcin/bgs312. [DOI] [PubMed] [Google Scholar]
- [57].Kim IY, Seong DH, Kim BC, Lee DK, Remaley AT, Leach F, Morton RA, Kim SJ. Raloxifene, a selective estrogen receptor modulator, induces apoptosis in androgen-responsive human prostate cancer cell line LNCaP through an androgen-independent pathway. Cancer research. 2002;62:3649–3653. [PubMed] [Google Scholar]
- [58].El Etreby MF, Liang Y, Lewis RW. Induction of apoptosis by mifepristone and tamoxifen in human LNCaP prostate cancer cells in culture. The Prostate. 2000;43:31–42. doi: 10.1002/(sici)1097-0045(20000401)43:1<31::aid-pros5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- [59].Rohlff C, Blagosklonny MV, Kyle E, Kesari A, Kim IY, Zelner DJ, Hakim F, Trepel J, Bergan RC. Prostate cancer cell growth inhibition by tamoxifen is associated with inhibition of protein kinase C and induction of p21(waf1/cip1) The Prostate. 1998;37:51–59. doi: 10.1002/(sici)1097-0045(19980915)37:1<51::aid-pros8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [60].Price D, Stein B, Sieber P, Tutrone R, Bailen J, Goluboff E, Burzon D, Bostwick D, Steiner M. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. The Journal of urology. 2006;176:965–970. doi: 10.1016/j.juro.2006.04.011. discussion 970-961. [DOI] [PubMed] [Google Scholar]
- [61].Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circulation. Cardiovascular quality and outcomes. 2013;6:390–399. doi: 10.1161/CIRCOUTCOMES.111.000071. [DOI] [PubMed] [Google Scholar]
- [62].Lee J, Lee I, Park C, Kang WK. Lovastatin-induced RhoA modulation and its effect on senescence in prostate cancer cells. Biochemical and biophysical research communications. 2006;339:748–754. doi: 10.1016/j.bbrc.2005.11.075. [DOI] [PubMed] [Google Scholar]
- [63].Park C, Lee I, Kang WK. Lovastatin-induced E2F-1 modulation and its effect on prostate cancer cell death. Carcinogenesis. 2001;22:1727–1731. doi: 10.1093/carcin/22.10.1727. [DOI] [PubMed] [Google Scholar]
- [64].Hoque A, Chen H, Xu XC. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:88–94. doi: 10.1158/1055-9965.EPI-07-0531. [DOI] [PubMed] [Google Scholar]
- [65].Marcelli M, Cunningham GR, Haidacher SJ, Padayatty SJ, Sturgis L, Kagan C, Denner L. Caspase-7 is activated during lovastatin-induced apoptosis of the prostate cancer cell line LNCaP. Cancer research. 1998;58:76–83. [PubMed] [Google Scholar]
- [66].Sivaprasad U, Abbas T, Dutta A. Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase inhibitors on the cell cycle of prostate cancer cells. Molecular cancer therapeutics. 2006;5:2310–2316. doi: 10.1158/1535-7163.MCT-06-0175. [DOI] [PubMed] [Google Scholar]
- [67].Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. The Journal of clinical investigation. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Parikh A, Childress C, Deitrick K, Lin Q, Rukstalis D, Yang W. Statin-induced autophagy by inhibition of geranylgeranyl biosynthesis in prostate cancer PC3 cells. The Prostate. 2010;70:971–981. doi: 10.1002/pros.21131. [DOI] [PubMed] [Google Scholar]
- [69].He Z, Mangala LS, Theriot CA, Rohde LH, Wu H, Zhang Y. Cell killing and radiosensitizing effects of atorvastatin in PC3 prostate cancer cells. Journal of radiation research. 2012;53:225–233. doi: 10.1269/jrr.11114. [DOI] [PubMed] [Google Scholar]
- [70].Ukomadu C, Dutta A. Inhibition of cdk2 activating phosphorylation by mevastatin. The Journal of biological chemistry. 2003;278:4840–4846. doi: 10.1074/jbc.M208658200. [DOI] [PubMed] [Google Scholar]
- [71].Menter DG, Ramsauer VP, Harirforoosh S, Chakraborty K, Yang P, Hsi L, Newman RA, Krishnan K. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PloS one. 2011;6:e28813. doi: 10.1371/journal.pone.0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang C, Tao W, Wang Y, Bikow J, Lu B, Keating A, Verma S, Parker TG, Han R, Wen XY. Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. European urology. 2010;58:418–426. doi: 10.1016/j.eururo.2010.05.024. [DOI] [PubMed] [Google Scholar]
- [73].Bansal D, Undela K, D’Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PloS one. 2012;7:e46691. doi: 10.1371/journal.pone.0046691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- [75].Flick ED, Habel LA, Chan KA, Eeden S.K. Van Den, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry CP, Jr., Sternfeld B, Jacobsen SJ, Whitmer RA, Caan BJ. Statin use and risk of prostate cancer in the California Men’s Health Study cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:2218–2225. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- [76].Agalliu I, Salinas CA, Hansten PD, Ostrander EA, Stanford JL. Statin use and risk of prostate cancer: results from a population-based epidemiologic study. American journal of epidemiology. 2008;168:250–260. doi: 10.1093/aje/kwn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. The Journal of urology. 2004;172:S6–11. doi: 10.1097/01.ju.0000142058.99614.ff. discussion S11-12. [DOI] [PubMed] [Google Scholar]
- [78].De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schroder F, Sciarra A, Tubaro A. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. European urology. 2011;60:106–117. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- [79].Choe KS, Cowan JE, Chan JM, Carroll PR, D’Amico AV, Liauw SL. Aspirin use and the risk of prostate cancer mortality in men treated with prostatectomy or radiotherapy. J Clin Oncol. 2012;30:3540–3544. doi: 10.1200/JCO.2011.41.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005;97:975–980. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- [81].Habel LA, Zhao W, Stanford JL. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer causes & control : CCC. 2002;13:427–434. doi: 10.1023/a:1015788502099. [DOI] [PubMed] [Google Scholar]
- [82].Leitzmann MF, Stampfer MJ, Ma J, Chan JM, Colditz GA, Willett WC, Giovannucci E. Aspirin use in relation to risk of prostate cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:1108–1111. [PubMed] [Google Scholar]
- [83].Cossack M, Ghaffary C, Watson P, Snyder C, Lynch H. Aspirin Use is Associated with Lower Prostate Cancer Risk in Male Carriers of BRCA Mutations. Journal of genetic counseling. 2013 doi: 10.1007/s10897-013-9629-8. [DOI] [PubMed] [Google Scholar]
- [84].Shebl FM, Sakoda LC, Black A, Koshiol J, Andriole GL, Grubb R, Church TR, Chia D, Zhou C, Chu LW, Huang WY, Peters U, Kirsh VA, Chatterjee N, Leitzmann MF, Hayes RB, Hsing AW. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. British journal of cancer. 2012;107:207–214. doi: 10.1038/bjc.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Salinas CA, Kwon EM, FitzGerald LM, Feng Z, Nelson PS, Ostrander EA, Peters U, Stanford JL. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. American journal of epidemiology. 2010;172:578–590. doi: 10.1093/aje/kwq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mahmud SM, Franco EL, Turner D, Platt RW, Beck P, Skarsgard D, Tonita J, Sharpe C, Aprikian AG. Use of non-steroidal anti-inflammatory drugs and prostate cancer risk: a population-based nested case-control study. PloS one. 2011;6:e16412. doi: 10.1371/journal.pone.0016412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rotem R, Tzivony Y, Flescher E. Contrasting effects of aspirin on prostate cancer cells: suppression of proliferation and induction of drug resistance. The Prostate. 2000;42:172–180. doi: 10.1002/(sici)1097-0045(20000215)42:3<172::aid-pros2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [88].Andrews J, Djakiew D, Krygier S, Andrews P. Superior effectiveness of ibuprofen compared with other NSAIDs for reducing the survival of human prostate cancer cells. Cancer chemotherapy and pharmacology. 2002;50:277–284. doi: 10.1007/s00280-002-0485-8. [DOI] [PubMed] [Google Scholar]
- [89].Huang Y, He Q, Hillman MJ, Rong R, Sheikh MS. Sulindac sulfide-induced apoptosis involves death receptor 5 and the caspase 8-dependent pathway in human colon and prostate cancer cells. Cancer research. 2001;61:6918–6924. [PubMed] [Google Scholar]
- [90].Lim JT, Piazza GA, Han EK, Delohery TM, Li H, Finn TS, Buttyan R, Yamamoto H, Sperl GJ, Brendel K, Gross PH, Pamukcu R, Weinstein IB. Sulindac derivatives inhibit growth and induce apoptosis in human prostate cancer cell lines. Biochemical pharmacology. 1999;58:1097–1107. doi: 10.1016/s0006-2952(99)00200-2. [DOI] [PubMed] [Google Scholar]
- [91].Goluboff ET, Shabsigh A, Saidi JA, Weinstein IB, Mitra N, Heitjan D, Piazza GA, Pamukcu R, Buttyan R, Olsson CA. Exisulind (sulindac sulfone) suppresses growth of human prostate cancer in a nude mouse xenograft model by increasing apoptosis. Urology. 1999;53:440–445. doi: 10.1016/s0090-4295(98)00513-5. [DOI] [PubMed] [Google Scholar]
- [92].Patel MI, Subbaramaiah K, Du B, Chang M, Yang P, Newman RA, Cordon-Cardo C, Thaler HT, Dannenberg AJ. Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin Cancer Res. 2005;11:1999–2007. doi: 10.1158/1078-0432.CCR-04-1877. [DOI] [PubMed] [Google Scholar]
- [93].Smith MR, Manola J, Kaufman DS, Oh WK, Bubley GJ, Kantoff PW. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006;24:2723–2728. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- [94].James ND, Sydes MR, Mason MD, Clarke NW, Anderson J, Dearnaley DP, Dwyer J, Jovic G, Ritchie AW, Russell JM, Sanders K, Thalmann GN, Bertelli G, Birtle AJ, O’Sullivan JM, Protheroe A, Sheehan D, Srihari N, Parmar MK. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: first results from the STAMPEDE multiarm, multistage, randomised controlled trial. The lancet oncology. 2012;13:549–558. doi: 10.1016/S1470-2045(12)70088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. Journal of the American Academy of Dermatology. 2006;55:1014–1023. doi: 10.1016/j.jaad.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [96].Tarter TH, Vaughan ED., Jr. Inhibitors of 5alpha-reductase in the treatment of benign prostatic hyperplasia. Current pharmaceutical design. 2006;12:775–783. doi: 10.2174/138161206776056010. [DOI] [PubMed] [Google Scholar]
- [97].Allan GF, Sui Z. Therapeutic androgen receptor ligands. Nuclear receptor signaling. 2003;1:e009. doi: 10.1621/nrs.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- [99].Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M. An overview on 5alpha-reductase inhibitors. Steroids. 2010;75:109–153. doi: 10.1016/j.steroids.2009.10.005. [DOI] [PubMed] [Google Scholar]
- [100].Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr. The influence of finasteride on the development of prostate cancer. The New England journal of medicine. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- [101].Chiu KY, Yong CR. Effects of finasteride on prostate volume and prostate-specific antigen. Journal of the Chinese Medical Association : JCMA. 2004;67:571–574. [PubMed] [Google Scholar]
- [102].Cohen YC, Liu KS, Heyden NL, Carides AD, Anderson KM, Daifotis AG, Gann PH. Detection bias due to the effect of finasteride on prostate volume: a modeling approach for analysis of the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007;99:1366–1374. doi: 10.1093/jnci/djm130. [DOI] [PubMed] [Google Scholar]
- [103].Musquera M, Fleshner NE, Finelli A, Zlotta AR. The REDUCE trial: chemoprevention in prostate cancer using a dual 5alpha-reductase inhibitor, dutasteride. Expert review of anticancer therapy. 2008;8:1073–1079. doi: 10.1586/14737140.8.7.1073. [DOI] [PubMed] [Google Scholar]
- [104].Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. Effect of dutasteride on the risk of prostate cancer. The New England journal of medicine. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- [105].Grubb RL, Andriole GL, Somerville MC, Mahoney C, Manyak MJ, Castro R. The REDUCE Follow-Up Study: low rate of new prostate cancer diagnoses observed during a 2-year, observational, followup study of men who participated in the REDUCE trial. The Journal of urology. 2013;189:871–877. doi: 10.1016/j.juro.2012.09.099. [DOI] [PubMed] [Google Scholar]
- [106].Erdemir F, Harbin A, Hellstrom WJ. 5-alpha reductase inhibitors and erectile dysfunction: the connection. The journal of sexual medicine. 2008;5:2917–2924. doi: 10.1111/j.1743-6109.2008.01001.x. [DOI] [PubMed] [Google Scholar]
- [107].Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. The journal of sexual medicine. 2011;8:872–884. doi: 10.1111/j.1743-6109.2010.02157.x. [DOI] [PubMed] [Google Scholar]
- [108].Lloyd JC, Masko EM, Wu C, Keenan MM, Pilla DM, Aronson WJ, Chi JT, Freedland SJ. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate cancer and prostatic diseases. 2013 doi: 10.1038/pcan.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. The American journal of clinical nutrition. 2010;92:1223–1233. doi: 10.3945/ajcn.2010.29530. [DOI] [PubMed] [Google Scholar]
- [110].Torfadottir JE, Valdimarsdottir UA, Mucci LA, Kasperzyk JL, Fall K, Tryggvadottir L, Aspelund T, Olafsson O, Harris TB, Jonsson E, Tulinius H, Gudnason V, Adami HO, Stampfer M, Steingrimsdottir L. Consumption of fish products across the lifespan and prostate cancer risk. PloS one. 2013;8:e59799. doi: 10.1371/journal.pone.0059799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2000;9:1171–1182. [PubMed] [Google Scholar]
- [112].Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer research. 2001;61:7071–7078. [PubMed] [Google Scholar]
- [113].Xiang N, Zhao R, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29:2175–2181. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Clark LC, Dalkin B, Krongrad A, Combs GF, Jr., Turnbull BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, Borosso C, Falk S, Rounder J. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. British journal of urology. 1998;81:730–734. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- [115].Clark LC, Combs GF, Jr., Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr., Park HK, Sanders BB, Jr., Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- [116].Shiau CW, Huang JW, Wang DS, Weng JR, Yang CC, Lin CH, Li C, Chen CS. alpha-Tocopheryl succinate induces apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 function. The Journal of biological chemistry. 2006;281:11819–11825. doi: 10.1074/jbc.M511015200. [DOI] [PubMed] [Google Scholar]
- [117].The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The New England journal of medicine. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- [118].Venkateswaran V, Fleshner NE, Sugar LM, Klotz LH. Antioxidants block prostate cancer in lady transgenic mice. Cancer research. 2004;64:5891–5896. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- [119].Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, Coltman C. SELECT: the next prostate cancer prevention trial. Selenum and Vitamin E Cancer Prevention Trial. The Journal of urology. 2001;166:1311–1315. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- [120].Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr., Baker LH, Coltman CA., Jr. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Dunn BK, Richmond ES, Minasian LM, Ryan AM, Ford LG. A nutrient approach to prostate cancer prevention: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) Nutrition and cancer. 2010;62:896–918. doi: 10.1080/01635581.2010.509833. [DOI] [PubMed] [Google Scholar]
- [122].Ledesma MC, Jung-Hynes B, Schmit TL, Kumar R, Mukhtar H, Ahmad N. Selenium and vitamin E for prostate cancer: post-SELECT (Selenium and Vitamin E Cancer Prevention Trial) status. Mol Med. 2011;17:134–143. doi: 10.2119/molmed.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Molecular cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Epstein MM, Kasperzyk JL, Andren O, Giovannucci EL, Wolk A, Hakansson N, Andersson SO, Johansson JE, Fall K, Mucci LA. Dietary zinc and prostate cancer survival in a Swedish cohort. The American journal of clinical nutrition. 2011;93:586–593. doi: 10.3945/ajcn.110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yan M, Hardin K, Ho E. Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. The Journal of nutritional biochemistry. 2010;21:687–694. doi: 10.1016/j.jnutbio.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Khan N, Adhami VM, Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutrition and cancer. 2009;61:836–841. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. American journal of epidemiology. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- [128].Fujiki H. Green tea: Health benefits as cancer preventive for humans. Chem Rec. 2005;5:119–132. doi: 10.1002/tcr.20039. [DOI] [PubMed] [Google Scholar]
- [129].Paschka AG, Butler R, Young CY. Induction of apoptosis in prostate cancer cell lines by the green tea component, (-)-epigallocatechin-3-gallate. Cancer letters. 1998;130:1–7. doi: 10.1016/s0304-3835(98)00084-6. [DOI] [PubMed] [Google Scholar]
- [130].Chung LY, Cheung TC, Kong SK, Fung KP, Choy YM, Chan ZY, Kwok TT. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life sciences. 2001;68:1207–1214. doi: 10.1016/s0024-3205(00)01020-1. [DOI] [PubMed] [Google Scholar]
- [131].Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–7041. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- [132].Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer. 2010;126:2520–2533. doi: 10.1002/ijc.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Caporali A, Davalli P, Astancolle S, D’Arca D, Brausi M, Bettuzzi S, Corti A. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis. 2004;25:2217–2224. doi: 10.1093/carcin/bgh235. [DOI] [PubMed] [Google Scholar]
- [134].Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer research. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- [135].Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. International journal of cancer. Journal international du cancer. 2005;117:667–669. doi: 10.1002/ijc.21266. [DOI] [PubMed] [Google Scholar]
- [136].Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. The American journal of clinical nutrition. 2009;89:1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- [137].Hsu A, Bray TM, Ho E. Anti-inflammatory activity of soy and tea in prostate cancer prevention. Exp Biol Med (Maywood) 235:659–667. doi: 10.1258/ebm.2010.009335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. The Journal of biological chemistry. 2008;283:27707–27716. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, Johnsen NF, Olsen A, Kaaks R, Linseisen J, Boeing H, Nothlings U, Bueno-de-Mesquita HB, Ros MM, Sacerdote C, Palli D, Tumino R, Berrino F, Trichopoulou A, Dilis V, Trichopoulos D, Chirlaque MD, Ardanaz E, Larranaga N, Gonzalez C, Suarez LR, Sanchez MJ, Bingham S, Khaw KT, Hallmans G, Stattin P, Rinaldi S, Slimani N, Jenab M, Riboli E, Key TJ. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. British journal of cancer. 2009;100:1817–1823. doi: 10.1038/sj.bjc.6605073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Wojewoda C, Miele L, Sarkar FH. Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. Journal of cellular biochemistry. 2011;112:78–88. doi: 10.1002/jcb.22770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [141].Xu L, Ding Y, Catalona WJ, Yang XJ, Anderson WF, Jovanovic B, Wellman K, Killmer J, Huang X, Scheidt KA, Montgomery RB, Bergan RC. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J Natl Cancer Inst. 2009;101:1141–1155. doi: 10.1093/jnci/djp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Majid S, Dar AA, Shahryari V, Hirata H, Ahmad A, Saini S, Tanaka Y, Dahiya AV, Dahiya R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer. 2010;116:66–76. doi: 10.1002/cncr.24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Vardi A, Bosviel R, Rabiau N, Adjakly M, Satih S, Dechelotte P, Boiteux JP, Fontana L, Bignon YJ, Guy L, Bernard-Gallon DJ. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells, In Vivo. 2010;24:393–400. [PubMed] [Google Scholar]
- [144].Wang J, Eltoum IE, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. Journal of carcinogenesis. 2007;6:3. doi: 10.1186/1477-3163-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer research. 2009;69:3695–3703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PloS one. 2011;6:e20034. doi: 10.1371/journal.pone.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Singh-Gupta V, Zhang H, Yunker CK, Ahmad Z, Zwier D, Sarkar FH, Hillman GG. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: potentiation of radiotherapy. Pharmaceutical research. 2010;27:1115–1127. doi: 10.1007/s11095-010-0107-9. [DOI] [PubMed] [Google Scholar]
- [148].Jang H, Ha US, Kim SJ, Yoon BI, Han DS, Yuk SM, Kim SW. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. Journal of agricultural and food chemistry. 2010;58:12686–12691. doi: 10.1021/jf102688g. [DOI] [PubMed] [Google Scholar]
- [149].de la Iglesia R, Milagro FI, Campion J, Boque N, Martinez JA. Healthy properties of proanthocyanidins. Biofactors. 2010;36:159–168. doi: 10.1002/biof.79. [DOI] [PubMed] [Google Scholar]
- [150].Brasky TM, Kristal AR, Navarro SL, Lampe JW, Peters U, Patterson RE, White E. Specialty supplements and prostate cancer risk in the VITamins and Lifestyle (VITAL) cohort. Nutrition and cancer. 2011;63:573–582. doi: 10.1080/01635581.2011.553022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kaur M, Agarwal R, Agarwal C. Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Molecular cancer therapeutics. 2006;5:1265–1274. doi: 10.1158/1535-7163.MCT-06-0014. [DOI] [PubMed] [Google Scholar]
- [152].Park SY, Lee YH, Choi KC, Seong AR, Choi HK, Lee OH, Hwang HJ, Yoon HG. Grape seed extract regulates androgen receptor-mediated transcription in prostate cancer cells through potent anti-histone acetyltransferase activity. Journal of medicinal food. 2011;14:9–16. doi: 10.1089/jmf.2010.1264. [DOI] [PubMed] [Google Scholar]
- [153].Tyagi A, Agarwal R, Agarwal C. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene. 2003;22:1302–1316. doi: 10.1038/sj.onc.1206265. [DOI] [PubMed] [Google Scholar]
- [154].Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. International journal of cancer. Journal international du cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- [155].Dhanalakshmi S, Agarwal R, Agarwal C. Inhibition of NF-kappaB pathway in grape seed extract-induced apoptotic death of human prostate carcinoma DU145 cells. International journal of oncology. 2003;23:721–727. [PubMed] [Google Scholar]
- [156].Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–1876. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- [157].Uchino R, Madhyastha R, Madhyastha H, Dhungana S, Nakajima Y, Omura S, Maruyama M. NFkappaB-dependent regulation of urokinase plasminogen activator by proanthocyanidin-rich grape seed extract: effect on invasion by prostate cancer cells. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2010;21:528–533. doi: 10.1097/MBC.0b013e32833a9b61. [DOI] [PubMed] [Google Scholar]
- [158].Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer research. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- [159].Agarwal C, Sharma Y, Agarwal R. Anticarcinogenic effect of a polyphenolic fraction isolated from grape seeds in human prostate carcinoma DU145 cells: modulation of mitogenic signaling and cell-cycle regulators and induction of G1 arrest and apoptosis. Molecular carcinogenesis. 2000;28:129–138. doi: 10.1002/1098-2744(200007)28:3<129::aid-mc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [160].Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]
- [161].Agarwal C, Tyagi A, Agarwal R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Molecular cancer therapeutics. 2006;5:3294–3302. doi: 10.1158/1535-7163.MCT-06-0483. [DOI] [PubMed] [Google Scholar]
- [162].Kaur M, Velmurugan B, Rajamanickam S, Agarwal R, Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm Res. 2009;26:2133–2140. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R, Agarwal C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Molecular cancer therapeutics. 2008;7:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- [164].Chou SC, Kaur M, Thompson JA, Agarwal R, Agarwal C. Influence of gallate esterification on the activity of procyanidin B2 in androgen-dependent human prostate carcinoma LNCaP cells. Pharmaceutical research. 2010;27:619–627. doi: 10.1007/s11095-009-0037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Agarwal C, Veluri R, Kaur M, Chou SC, Thompson JA, Agarwal R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3′-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2007;28:1478–1484. doi: 10.1093/carcin/bgm045. [DOI] [PubMed] [Google Scholar]
- [166].Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- [167].Ting H, Deep G, Agarwal R. Molecular mechanisms of silibinin-mediated cancer chemoprevention with major emphasis on prostate cancer. The AAPS journal. 2013;15:707–716. doi: 10.1208/s12248-013-9486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Sharma Y, Agarwal C, Singh AK, Agarwal R. Inhibitory effect of silibinin on ligand binding to erbB1 and associated mitogenic signaling, growth, and DNA synthesis in advanced human prostate carcinoma cells. Molecular carcinogenesis. 2001;30:224–236. doi: 10.1002/mc.1032. [DOI] [PubMed] [Google Scholar]
- [169].Tyagi A, Sharma Y, Agarwal C, Agarwal R. Silibinin impairs constitutively active TGFalpha-EGFR autocrine loop in advanced human prostate carcinoma cells. Pharm Res. 2008;25:2143–2150. doi: 10.1007/s11095-008-9545-z. [DOI] [PubMed] [Google Scholar]
- [170].Zi X, Zhang J, Agarwal R, Pollak M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer research. 2000;60:5617–5620. [PubMed] [Google Scholar]
- [171].Verschoyle RD, Greaves P, Patel K, Marsden DA, Brown K, Steward WP, Gescher AJ. Evaluation of the cancer chemopreventive efficacy of silibinin in genetic mouse models of prostate and intestinal carcinogenesis: Relationship with silibinin levels. Eur J Cancer. 2008;44:898–906. doi: 10.1016/j.ejca.2008.02.020. [DOI] [PubMed] [Google Scholar]
- [172].Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomarkers Prev. 2003;12:933–939. [PubMed] [Google Scholar]
- [173].Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis. 2007;28:2567–2574. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- [174].Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer research. 2002;62:3063–3069. [PubMed] [Google Scholar]
- [175].Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22:1399–1403. doi: 10.1093/carcin/22.9.1399. [DOI] [PubMed] [Google Scholar]
- [176].Thelen P, Wuttke W, Jarry H, Grzmil M, Ringert RH. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. The Journal of urology. 2004;171:1934–1938. doi: 10.1097/01.ju.0000121329.37206.1b. [DOI] [PubMed] [Google Scholar]
- [177].Thelen P, Jarry H, Ringert RH, Wuttke W. Silibinin down-regulates prostate epithelium-derived Ets transcription factor in LNCaP prostate cancer cells. Planta medica. 2004;70:397–400. doi: 10.1055/s-2004-818965. [DOI] [PubMed] [Google Scholar]
- [178].Lu W, Lin C, King TD, Chen H, Reynolds RC, Li Y. Silibinin inhibits Wnt/beta-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell Signal. 24:2291–2296. doi: 10.1016/j.cellsig.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Agarwal C, Tyagi A, Kaur M, Agarwal R. Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis. 2007;28:1463–1470. doi: 10.1093/carcin/bgm042. [DOI] [PubMed] [Google Scholar]
- [180].Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits constitutive and TNFalpha-induced activation of NF-kappaB and sensitizes human prostate carcinoma DU145 cells to TNFalpha-induced apoptosis. Oncogene. 2002;21:1759–1767. doi: 10.1038/sj.onc.1205240. [DOI] [PubMed] [Google Scholar]
- [181].Wu K, Zeng J, Li L, Fan J, Zhang D, Xue Y, Zhu G, Yang L, Wang X, He D. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol Rep. 23:1545–1552. [PubMed] [Google Scholar]
- [182].Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- [183].Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci U S A. 1999;96:7490–7495. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Tyagi A, Agarwal C, Agarwal R. Inhibition of retinoblastoma protein (Rb) phosphorylation at serine sites and an increase in Rb-E2F complex formation by silibinin in androgen-dependent human prostate carcinoma LNCaP cells: role in prostate cancer prevention. Molecular cancer therapeutics. 2002;1:525–532. [PubMed] [Google Scholar]
- [185].Bhatia N, Zhao J, Wolf DM, Agarwal R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin. Cancer letters. 1999;147:77–84. doi: 10.1016/s0304-3835(99)00276-1. [DOI] [PubMed] [Google Scholar]
- [186].Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;14:7773–7780. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer research. 2008;68:6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Tyagi A, Agarwal C, Agarwal R. The cancer preventive flavonoid silibinin causes hypophosphorylation of Rb/p107 and Rb2/p130 via modulation of cell cycle regulators in human prostate carcinoma DU145 cells. Cell Cycle. 2002;1:137–142. [PubMed] [Google Scholar]
- [189].Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Molecular cancer therapeutics. 2007;6:2696–2707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- [190].Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:3512–3519. [PubMed] [Google Scholar]
- [191].Deep G, Oberlies NH, Kroll DJ, Agarwal R. Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells. International journal of cancer. Journal international du cancer. 2008;123:41–50. doi: 10.1002/ijc.23485. [DOI] [PubMed] [Google Scholar]
- [192].Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer research. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- [193].Dhanalakshmi S, Agarwal P, Glode LM, Agarwal R. Silibinin sensitizes human prostate carcinoma DU145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptotic death. International journal of cancer. Journal international du cancer. 2003;106:699–705. doi: 10.1002/ijc.11299. [DOI] [PubMed] [Google Scholar]
- [194].Tyagi A, Bhatia N, Condon MS, Bosland MC, Agarwal C, Agarwal R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. The Prostate. 2002;53:211–217. doi: 10.1002/pros.10146. [DOI] [PubMed] [Google Scholar]
- [195].Deep G, Gangar SC, Agarwal C, Agarwal R. Role of E-cadherin in antimigratory and antiinvasive efficacy of silibinin in prostate cancer cells. Cancer Prev Res (Phila) 4:1222–1232. doi: 10.1158/1940-6207.CAPR-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D, Li L, Fan JH, Wang XY, He DL. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin. 2009;30:1162–1168. doi: 10.1038/aps.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer metastasis reviews. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Deep G, Agarwal R. Targeting Tumor Micro environment with Silibinin: Promise and Potential for a Translational Cancer Chemopreventive Strategy. Current cancer drug targets. 2013 doi: 10.2174/15680096113139990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Raina K, Serkova NJ, Agarwal R. Silibinin feeding alters the metabolic profile in TRAMP prostatic tumors: 1H-NMRS-based metabolomics study. Cancer research. 2009;69:3731–3735. doi: 10.1158/0008-5472.CAN-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Jung HJ, Park JW, Lee JS, Lee SR, Jang BC, Suh SI, Suh MH, Baek WK. Silibinin inhibits expression of HIF-1alpha through suppression of protein translation in prostate cancer cells. Biochemical and biophysical research communications. 2009;390:71–76. doi: 10.1016/j.bbrc.2009.09.068. [DOI] [PubMed] [Google Scholar]
- [201].Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glode LM. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- [202].Flaig TW, Glode M, Gustafson D, Bokhoven A. van, Tao Y, Wilson S, Su LJ, Li Y, Harrison G, Agarwal R, Crawford ED, Lucia MS, Pollak M. A study of high-dose oral silybin phytosome followed by prostatectomy in patients with localized prostate cancer. The Prostate. 2010;70:848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- [203].Torti DC, Matheson GO. Exercise and prostate cancer. Sports Med. 2004;34:363–369. doi: 10.2165/00007256-200434060-00003. [DOI] [PubMed] [Google Scholar]
- [204].Masko EM, Allott EH, Freedland SJ. The relationship between nutrition and prostate cancer: is more always better? European urology. 2013;63:810–820. doi: 10.1016/j.eururo.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Arab L, Su J, Steck SE, Ang A, Fontham ET, Bensen JT, Mohler JL. Adherence to World Cancer Research Fund/American Institute for Cancer Research lifestyle recommendations reduces prostate cancer aggressiveness among African and Caucasian Americans. Nutrition and cancer. 2013;65:633–643. doi: 10.1080/01635581.2013.789540. [DOI] [PubMed] [Google Scholar]
- [206].Ho E, Beaver LM, Williams DE, Dashwood RH. Dietary factors and epigenetic regulation for prostate cancer prevention. Adv Nutr. 2011;2:497–510. doi: 10.3945/an.111.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [208].Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Zheng SL, Sun J, Wiklund F, Smith S, Stattin P, Li G, Adami HO, Hsu FC, Zhu Y, Balter K, Kader AK, Turner AR, Liu W, Bleecker ER, Meyers DA, Duggan D, Carpten JD, Chang BL, Isaacs WB, Xu J, Gronberg H. Cumulative association of five genetic variants with prostate cancer. The New England journal of medicine. 2008;358:910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- [210].Perdomo F, Franquiz F. Cabrera, Cabrera J, Serra-Majem L. [Influence of cooking procedure on the bioavailability of lycopene in tomatoes] Nutricion hospitalaria. 2012;27:1542–1546. doi: 10.3305/nh.2012.27.5.5908. [DOI] [PubMed] [Google Scholar]
- [211].Gartner C, Stahl W, Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. The American journal of clinical nutrition. 1997;66:116–122. doi: 10.1093/ajcn/66.1.116. [DOI] [PubMed] [Google Scholar]
- [212].Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Annals of internal medicine. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- [213].Pearson P, Lewis SA, Britton J, Young IS, Fogarty A. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2006;20:271–273. doi: 10.2165/00063030-200620050-00002. [DOI] [PubMed] [Google Scholar]
- [214].Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]