Abstract
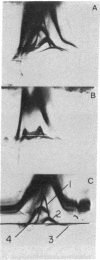
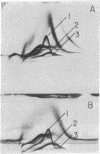
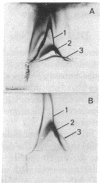
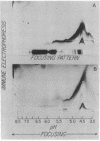
Highly purified plasma membranes from hamster lymphocytes transformed by simian virus 40 (GD 248) were compared with the membranes of normal cells by crossed immune electrophoresis, crossed-line immune electrophoresis, and bidimensional isoelectric focusing-immune electrophoresis. Antiserum raised by inoculation of guinea pigs with GD 248 membranes was used as serologic reagent, either directly or after absorption with membranes from normal cells. Bidimensional immune electrophoresis reveals the presence in the plasma membranes of GD 248 cells of at least three antigens not detectable in the membranes from the normal cell population. At least two of these are also present in the mitochondrial membranes of GD 248 cells, but none could be detected in membranes of embryonic fibroblasts. Bidimensional isoelectric focusing-immune electrophoresis indicates that the distinctive antigens of the GD 248 membranes are glycoproteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Ey P., Bhakdi-Lehnen B. Isolation of the terminal complement complex from target sheep erythrocyte membranes. Biochim Biophys Acta. 1976 Feb 6;419(3):445–457. doi: 10.1016/0005-2736(76)90258-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Knüfermann H., Fischer H., Wallach D. F. Interaction between erythrocyte membrane proteins and complement components. II. The identification and peptide composition of complement components C3 and C4 desorbed from erythrocyte membranes. Biochim Biophys Acta. 1974 Dec 10;373(2):295–307. doi: 10.1016/0005-2736(74)90153-9. [DOI] [PubMed] [Google Scholar]
- Bussell R. H., Robinson W. S. Membrane proteins of uninfected and Rous sarcoma virus- transformed avian cells. J Virol. 1973 Aug;12(2):320–327. doi: 10.1128/jvi.12.2.320-327.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. Surface carbohydrates of hamster fibroblasts. I. Chemical characterization of surface-labeled glycosphingolipids and aspecific ceramide tetrasaccharide for transformants. J Biol Chem. 1975 Apr 10;250(7):2438–2446. [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Verma S. P., Wallach D. F. Anomalous side chain amidation in plasma membrane proteins of simian virus 40-transformed lymphocytes indicated by isoelectric focussing and laser Raman spectroscopy. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1062–1069. doi: 10.1016/0006-291x(75)90782-2. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Wallach D. F., Davis F. D., 2nd Membranes of normal hamster lymphocytes and lymphoid cells neoplastically transformed by simian virus 40. I. High-yield purification of plasma membrane fragments. J Natl Cancer Inst. 1976 Nov;57(5):1107–1116. doi: 10.1093/jnci/57.5.1107. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Wallach D. F., Davis F. D., 2nd Membranes of normal hamster lymphocytes and lymphoid cells neoplastically transformed by simian virus 40. II. Plasma membrane proteins analyzed by dodecyl sulfate-polyacrylamide gel electrophoresis and two-dimensional immune electrophoresis. J Natl Cancer Inst. 1976 Nov;57(5):1117–1126. doi: 10.1093/jnci/57.5.1117. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- Wallach D. F. Cellular membranes and tumor behavior: a new hypothesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):868–874. doi: 10.1073/pnas.61.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickus G. G., Robbins P. W. Plasma membrane proteins of normal and Rous sarcoma virus-transformed chick-embryo fibroblasts. Nat New Biol. 1973 Sep 19;245(142):65–67. doi: 10.1038/newbio245065a0. [DOI] [PubMed] [Google Scholar]