Abstract
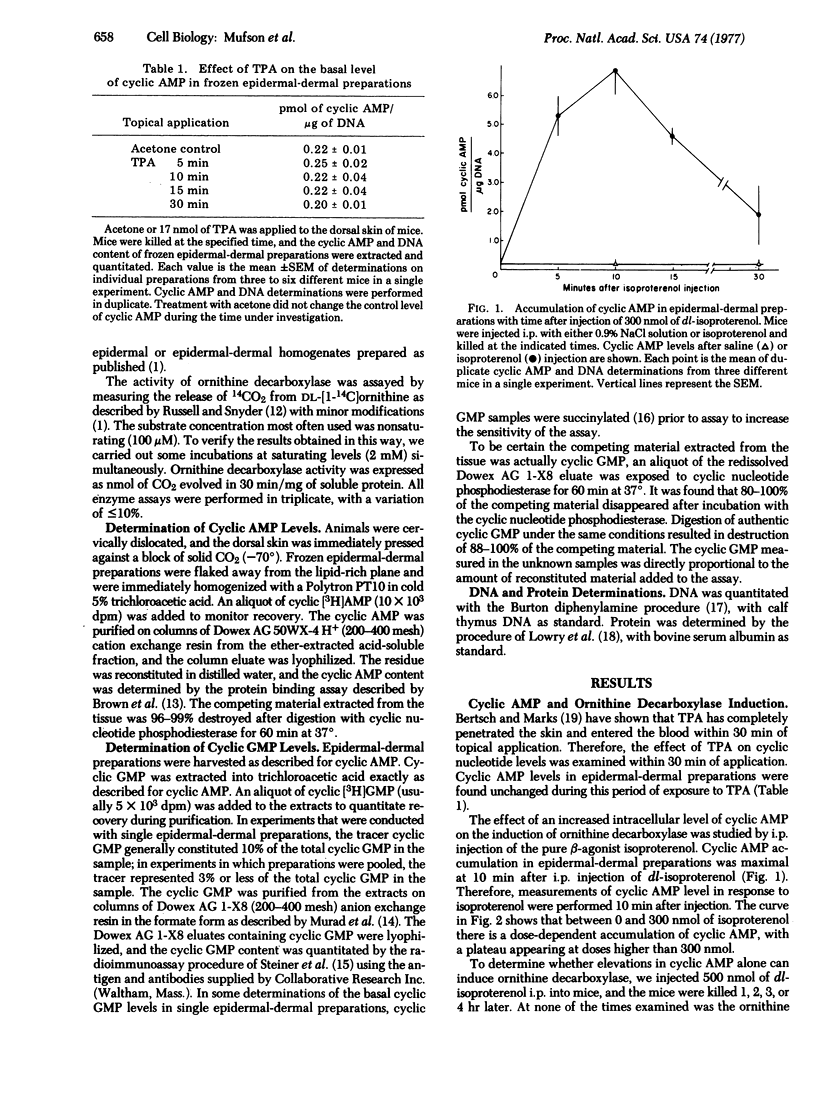
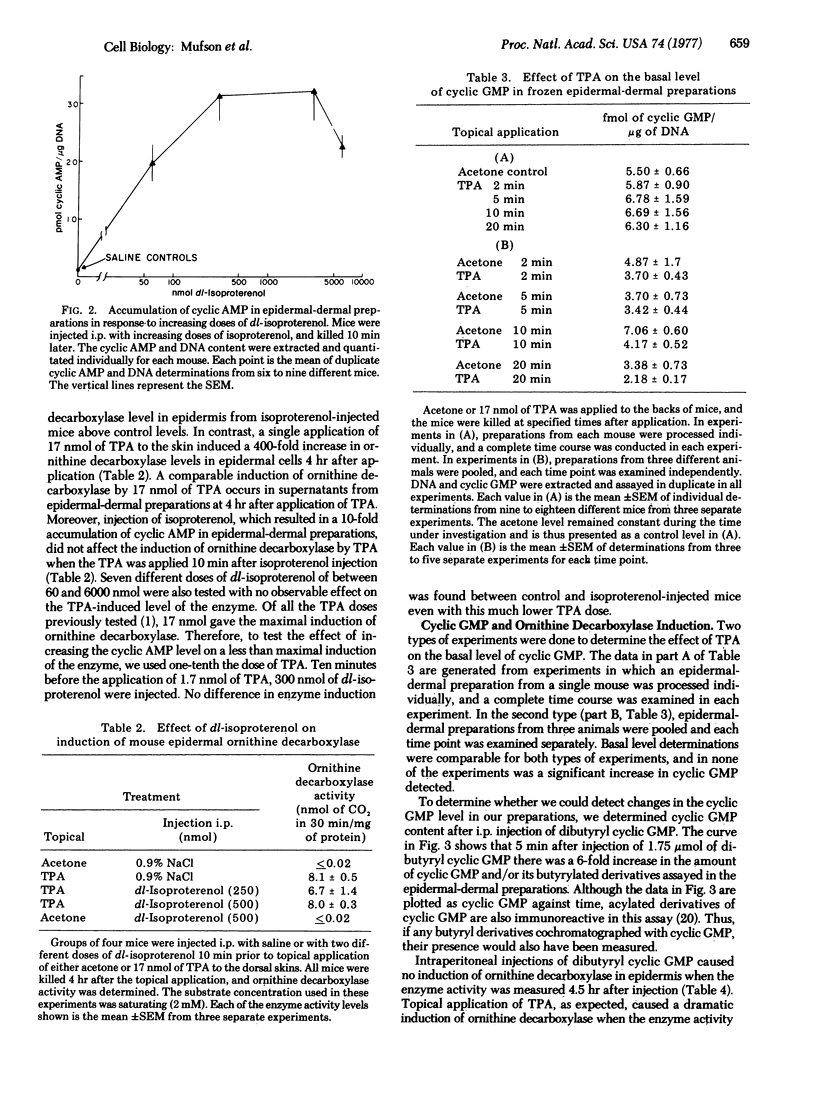
A single application of 17 nmol of 12-O-tetradecanoyl phorbol-13-acetate (TPA) to mouse skin caused a marked (200- to 400-fold) induction of ornithine decarboxylase (EC 4.1.1.17, L-ornithine carboxy-lyase) activity in mouse epidermal and epidermal-dermal preparations. No change in the basal level of 3':5'-cyclic AMP occurred in epidermal-dermal preparations within 30 min of TPA application. Intraperitoneal injection of the beta-agonist isoproterenol resulted in a dose-dependent accumulation of 3':5'-cyclic AMP occurred in epidermal-dermal preparations within 30 min of TPA application. Intraperitoneal injection of the beta-agonist isoproterenol resulted in a dose-dependent accumulation of 3':5'-cyclic AMP 10 min after injection, but caused no induction of ornithine decarboxylase. When isoproterenol was injected 10 min prior to an application of either 1.7 or 17 nmol of TPA, the magnitude of the ornithine decarboxylase induction was the same as induction with TPA alone. Topical application of 17 nmol of TPA caused no increase in the level of 3':5'-cyclic GMP present in the mouse epidermal-dermal preparations 2-20 min after application. Intraperitoneal injection of 1.75 mumol of dibutyryl 3':5'-cyclic GMP and/or butyryl derivatives of cyclic GMP caused a 6-fold increase in the level of cyclic GMP and/or butyryl derivatives of cyclic GMP in epidermal-dermal preparations within 5 min of injection, and the level remained elevated for at least 20-30 min. This dose of dibutyryl 3':5'-cyclic GMP was incapable of inducing ornithine decarboxylase. Injection of dibutyryl 3':5'-cyclic GMP 5 min before application of 1.7 nmol of TPA or 30 min before application of 17 nmol of TPA did not alter the magnitude of the ornithine decarboxylase induction produced by TPA alone. These results suggest that early increases in the total intracellular levels of either 3':5'-cyclic AMP or 3':5'-cyclic GMP are not part of the mechanism by which TPA induces ornithine decarboxylase in the epidermis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach U. Cyclic AMP-mediated induction of ornithine decarboxylase of glioma and neuroblastoma cells. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3087–3091. doi: 10.1073/pnas.72.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird W. M., Sedgwick J. A., Boutwell R. K. Effects of phorbol and four diesters of phorbol on the incorporation of tritiated precursors into DNA, RNA, and protein in mouse epidermis. Cancer Res. 1971 Oct;31(10):1434–1439. [PubMed] [Google Scholar]
- Belman S., Troll W. Phorbol-12-myristate-13-acetate effect on cyclic adenosine 3',5'-monophosphate levels in mouse skin and inhibition of phorbol-myristate-acetate-promoted tumorigenesis by theophylline. Cancer Res. 1974 Dec;34(12):3446–3455. [PubMed] [Google Scholar]
- Bertsch S., Marks F. Lack of an effect of tumor-promoting phorbol esters and of epidermal G1 chalone or DNA synthesis in the epidermis of newborn mice. Cancer Res. 1974 Dec;34(12):3283–3288. [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byus C. V., Russell D. H. Ornithine decarboxylase activity: control by cyclic nucleotides. Science. 1975 Feb 21;187(4177):650–652. doi: 10.1126/science.163486. [DOI] [PubMed] [Google Scholar]
- Cailla H. L., Racine-Weisbuch M. S., Delaage M. A. Adenosine 3',5' cyclic monophosphate assay at 10-15 mole level. Anal Biochem. 1973 Dec;56(2):394–407. doi: 10.1016/0003-2697(73)90205-4. [DOI] [PubMed] [Google Scholar]
- Derubertis F. R., Zenser T. Activation of murine lymphocytes by cyclic guanosine 3',5'-monophosphate: specificity and role in mitogen activity. Biochim Biophys Acta. 1976 Mar 25;428(1):91–103. doi: 10.1016/0304-4165(76)90111-2. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause E. G., Halle W., Wollenberger A. Effect of dibutyryl cyclic GMP on cultured beating rat heart cells. Adv Cyclic Nucleotide Res. 1972;1:301–305. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marks F., Grimm W. Diurnal fluctuation and -adrenergic elevation of cyclic AMP in mouse epidermis in vivo. Nat New Biol. 1972 Dec 6;240(101):178–179. doi: 10.1038/newbio240178a0. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Greengard P. Adenosine 3',5'-monophosphate: electrophysiological evidence for a role in synaptic transmission. Science. 1972 Oct;178(58):310–312. doi: 10.1126/science.178.4058.310. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y., Otani S., Matsui I., Morisawa S. Control of ornithine decarboxylase activity by cyclic nucleotides in the phytohemagglutinin induced lymphocyte transformation. Biochem Biophys Res Commun. 1975 Sep 2;66(1):328–335. doi: 10.1016/s0006-291x(75)80332-9. [DOI] [PubMed] [Google Scholar]
- Murad F., Manganiello V., Vaughan M. A simple, sensitive protein-binding assay for guanosine 3':5'-monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):736–739. doi: 10.1073/pnas.68.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. G., Simsiman R. C., Boutwell R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis and their specificity for tumor promotion. Cancer Res. 1975 Sep;35(9):2426–2433. [PubMed] [Google Scholar]
- O'Brien T. G., Simsiman R. C., Boutwell R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975 Jul;35(7):1662–1670. [PubMed] [Google Scholar]
- Raineri R., Simsiman R. C., Boutwell R. K. Stimulation of the phosphorylation of mouse epidermal histones by tumor-promoting agents. Cancer Res. 1973 Jan;33(1):134–139. [PubMed] [Google Scholar]
- Richman R., Park S., Akbar M., Yu S., Burke G. Regulation of thyroid ornithine ornithine decarboxylase (ODC) by thyrotropin. I. The rat. Endocrinology. 1975 Jun;96(6):1403–1412. doi: 10.1210/endo-96-6-1403. [DOI] [PubMed] [Google Scholar]
- Rohrsehneider L. R., Boutwell R. K. Phorbol esters, fatty acids and tumour promotion. Nat New Biol. 1973 Jun 13;243(128):212–213. doi: 10.1038/newbio243212a0. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Stambrook P. J. Cell cycle specific fluctuations in adenosine 3':5'-cyclic monophosphate and polyamines of Chinese hamster cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1482–1486. doi: 10.1073/pnas.72.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm D. E., Morris H. P., Webb T. E. Early biochemical changes in phytohemagglutinin-stimulated peripheral blood lymphocytes from normal and tumor-bearing rats. Eur J Cancer. 1974 Feb;10(2):107–113. doi: 10.1016/0014-2964(74)90061-9. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Verma A. K., Dixon K. E., Froscio M., Murray A. W. Localization of adenosine o',5'-monophosphate in mouse epidermis by immunofluorescence. J Invest Dermatol. 1976 Apr;66(4):239–241. doi: 10.1111/1523-1747.ep12482157. [DOI] [PubMed] [Google Scholar]
- Weber T. H., Goldberg M. L. Effect of leukoagglutinating phytohemagglutinin on cAMP and cGMP levels in lymphocytes. Exp Cell Res. 1976 Feb;97(2):432–435. doi: 10.1016/0014-4827(76)90637-6. [DOI] [PubMed] [Google Scholar]
- Wedner H. J., Dankner R., Parker C. W. Cyclic GMP and lectin-induced lymphocyte activation. J Immunol. 1975 Dec;115(6):1682–1687. [PubMed] [Google Scholar]
- Wells J. N., Wu Y. J., Baird C. E., Hardman J. G. Phosphodiesterases from porcine coronary arteries: inhibition of separated forms by xanthines, papaverine, and cyclic nucleotides. Mol Pharmacol. 1975 Nov;11(6):775–783. [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]


