Abstract
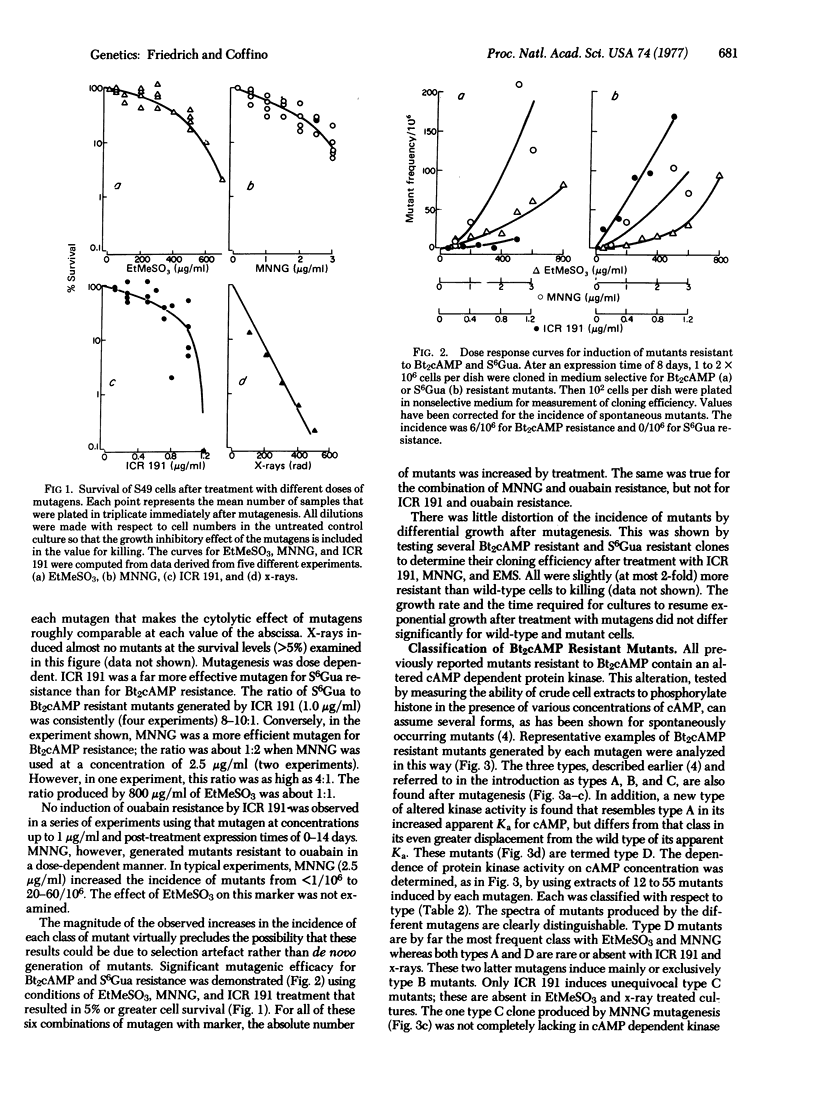
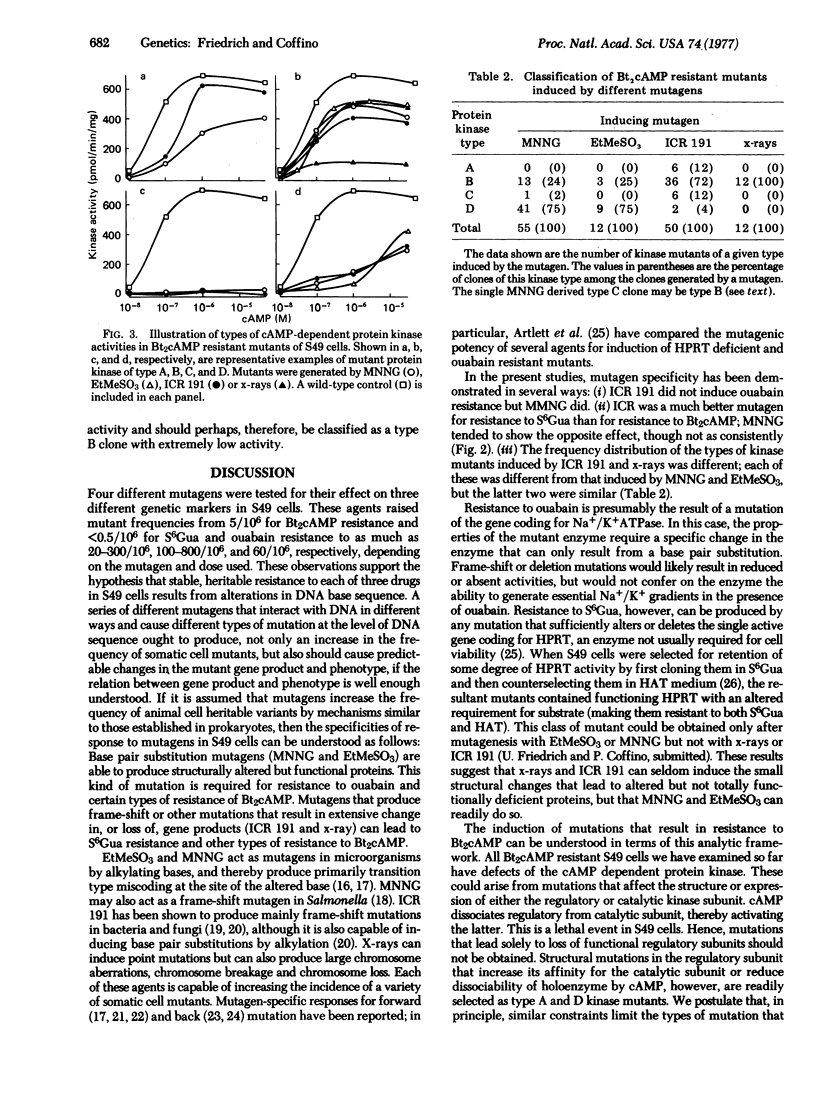
The effects of mutagens on three genetic markers--resistance to ouabain, 6-thioguanine, and dibutyryl cyclic AMP (Bt2cAMP), were investigated in a mouse lymphoma cell line, S49. Nitrosoguanidine, ethyl methanesulfonate, ICR 191, and x-rays were used. Mutagen-specific responses were seen. Ouabain resistance was induced by nitrosoguanidine, but not by ICR 191. ICR 191 induced resistance to 6-thioguanine more efficiently than did nitrosoguanidine; the converse was true of resistance to Bt2cAMP. The relative frequency of biochemically distinguishable subtypes of mutants resistant to Bt2cAMP was characteristic of the mutagen used to generate them. The results can be interpreted as follows: nitrosoguanidine and ethyl methanesulfonate frequently, but ICR 191 and x-rays rarely, give rise to DNA base sequence changes that result in structurally altered but functional proteins. This type of change is required for induction of mutants resistant to ouabain and of certain classes of mutants resistant to Bt2cAMP. Resistance to 6-thioguanine and other classes of mutants resistant to Bt2cAMP can result from DNA base sequence changes that lead to extensive alteration of protein structure or expression; these changes are induced by ICR 191 or x-rays.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Whitfield H. J., Jr Frameshift mutagenesis in Salmonella. Cold Spring Harb Symp Quant Biol. 1966;31:221–225. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- Arlett C. F., Turnbull D., Harcourt S. A., Lehmann A. R., Colella C. M. A comparison of the 8-azaguanine and ouabain-resistance systems for the selection of induced mutant Chinese hamster cells. Mutat Res. 1975 Dec;33(2-3):261–278. doi: 10.1016/0027-5107(75)90202-x. [DOI] [PubMed] [Google Scholar]
- Baumal R., Birshtein B. K., Coffino P., Scharff M. D. Mutations in immunoglobulin-producing mouse myeloma cells. Science. 1973 Oct 12;182(4108):164–166. doi: 10.1126/science.182.4108.164. [DOI] [PubMed] [Google Scholar]
- Chu E. H. Mammalian cell genetics. 3. Characterization of x-ray-induced forward mutations in Chinese hamster cell cultures. Mutat Res. 1971 Jan;11(1):23–34. doi: 10.1016/0027-5107(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- Cole J., Arlett C. F. Ethyl methanesulphonate mutagenesis with L5178Y mouse lymphoma cells: a comparison of ouabain, thioguanine and excess thymidine resistance. Mutat Res. 1976 Mar;34(3):507–525. doi: 10.1016/0027-5107(76)90226-8. [DOI] [PubMed] [Google Scholar]
- Creech H. J., Preston R. K., Peck R. M., O'Connell A. P. Antitumor and mutagenic properties of a variety of heterocyclic nitrogen and sulfur mustards. J Med Chem. 1972 Jul;15(7):739–746. doi: 10.1021/jm00277a011. [DOI] [PubMed] [Google Scholar]
- Daniel V., Litwack G., Tomkins G. M. Induction of cytolysis of cultured lymphoma cells by adenosine 3':5'-cyclic monophosphate and the isolation of resistant variants. Proc Natl Acad Sci U S A. 1973 Jan;70(1):76–79. doi: 10.1073/pnas.70.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Caskey C. T. Mutant chinese hamster cells with a thermosensitive hypoxanthine-guanine phosphoribosyltransferase. Cell. 1975 Jun;5(2):115–122. doi: 10.1016/0092-8674(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Hochman J., Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. A structural gene mutation affecting the regulatory subunit of cyclic AMP-dependent protein kinase in mouse lymphoma cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5051–5055. doi: 10.1073/pnas.72.12.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science. 1975 Nov 28;190(4217):896–898. doi: 10.1126/science.171770. [DOI] [PubMed] [Google Scholar]
- Isono K., Yourno J. Chemical carcinogens as frameshift mutagens: Salmonella DNA sequence sensitive to mutagenesis by polycyclic carcinogens. Proc Natl Acad Sci U S A. 1974 May;71(5):1612–1617. doi: 10.1073/pnas.71.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IX. Quantitation of mutagenesis by physical and chemical agents. J Cell Physiol. 1969 Dec;74(3):245–258. doi: 10.1002/jcp.1040740305. [DOI] [PubMed] [Google Scholar]
- Knaap A. G., Simons J. W. A mutational assay system for L5178Y mouse lymphoma cells, using hypoxanthine-guanine-phosphoribosyl-transferase (HGPRT) -deficiency as marker. The occurrence of a long expression time for mutations induced by X-rays and EMS. Mutat Res. 1975 Oct;30(1):97–110. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THE INOSINIC ACID PYROPHOSPHORYLASE ACTIVITY OF MOUSE FIBROBLASTS PARTIALLY RESISTANT TO 8-AZAGUANINE. Proc Natl Acad Sci U S A. 1963 Sep;50:568–573. doi: 10.1073/pnas.50.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawley P. D. Some chemical aspects of dose-response relationships in alkylation mutagenesis. Mutat Res. 1974 Jun;23(3):283–295. doi: 10.1016/0027-5107(74)90102-x. [DOI] [PubMed] [Google Scholar]
- Mankovitz R., Buchwald M., Baker R. M. Isolation of ouabain-resistant human diploid fibroblasts. Cell. 1974 Nov;3(3):221–226. doi: 10.1016/0092-8674(74)90135-4. [DOI] [PubMed] [Google Scholar]
- Morrow J., Prickett M. S., Fritz S., Vernick D., Deen D. Mutagenesis studies on cultured mammalian cells. The sensitivity of the asparagine-requiring phenotype to several chemical agents. Mutat Res. 1976 Mar;34(3):481–488. doi: 10.1016/0027-5107(76)90224-4. [DOI] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]


