Abstract
We tested how to eradicate long-established immunogenic tumors that were resistant to the monoclonal antibody-mediated blockade of PD-L1 (PD-1 ligand 1) and cytotoxic T lymphocyte-associated protein 4 (CTLA4). Bacterial vaccination with a tumor-specific peptide exhibiting a high affinity for its respective MHC molecule consistently eradicated tumors when combined with a PD-L1 blocking antibody. This approach can be translated to the clinic by combining cancer cell whole-exome sequencing with algorithms to identify mutant peptides with high peptide-MHC binding affinity.
Keywords: vaccination, Salmonella, PD-1, CTLA-4, rescue, T cell
Many clinical studies are focusing on how to rescue the function of T cells against immunogenic tumors. Monoclonal antibodies that block immunosuppressive T-cell receptors such as programmed cell death 1 (PDCD1, best known as PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA4) elicit strong therapeutic responses in some patients;1-3 an effect that seems to be durable with anti-PD-1 since many tumors that responded to therapy did not relapse within the 1st year after treatment initiation.2,3 However, the majority of cancer patients, including individuals with signs of a pre-existing T-cell response, do not respond to these antibodies.2,4
The current clinical challenge is therefore to develop a strategy to rescue T-cell responses in patients that are resistant to immunostimulatory antibodies. To address this challenge, we have recently characterized a murine model of melanoma that, when well established, is resistant to monoclonal antibodies blocking CTLA-4 and PD-L1 (PD-1 ligand 1), despite expressing a strong tumor-specific antigen (ovalbumin) and being heavily infiltrated by tumor-specific CD8+ T cells.5 These long-established tumors (at least 14 d old and exceeding 100 mm3 in size) were rejected when mice were treated with anti-PD-L1 in combination with intravenous Salmonella typhimurium A1-R (A1-R) expressing ovalbumin. Antigen-expressing A1-R rescued the proliferation of endogenous tumor-specific CD8+ T cells in the lymphoid organs and cytokine production within neoplastic lesions. Anti-PD-L1 antibodies amplified the peripheral T-cell response generated by antigen-expressing A1-R and apparently prevented PD-1-expressing TILs from losing effector functions within the tumor.5
Our vaccination approach targeted the ovalbumin-derived peptide SIINFEKL as a model mutant tumor-specific antigen.5 This peptide exhibits a high affinity for H-2Kb (IC50 = 0.9 nM).6 Recent studies have highlighted the importance of targeting tumor-specific peptides with high binding affinity for MHC molecules. Engels et al. demonstrated that adoptively-transferred T cells can eradicate established tumors when targeting peptides with high, but not low, affinity for MHC molecules.6 Indeed, the T cell-mediated lysis of cancer cells in vitro does not depend on high-affinity peptide-MHC binding, but the production of cytokines by T cells upon recognition of cross-presented cancer cell-derived peptides in vivo requires a high affinity interaction. In support of this notion, Robbins and van Rooij showed that tumor-infiltrating lymphocytes from patients that had objective responses following adoptive T cell transfer or the administration of anti-CTLA4 antibodies recognized high affinity mutant tumor-specific peptides.7,8 In contrast, T-cell responses to shared melanoma-associated antigens such as Melan-A (best known as MART-1) and premelanosome protein (PMEL, best known as gp100) do not correlate with favorable clinical outcome.9 These data demonstrate that the efficacy of T cell-based immunotherapy seems to rely on targeting tumor-specific peptide with high affinity for MHC molecules.
It is probable from the above that the success of our vaccination approach relied on bacteria delivering exogenous tumor-specific peptides with high MHC-binding affinity. Robbins et al. demonstrated that high-affinity mutant peptides can be identified by (i) the whole-exome sequencing of malignant vs. matched normal cells (to identify somatic mutations), followed by (ii) the algorithmic evaluation of the affinity of mutant peptides for MHC molecules.7 We propose that this approach should be used to identify mutant peptides that can be introduced into bacteria for therapeutic anticancer vaccination (Fig. 1). Delivering multiple CD8+ T-cell epitopes will likely prevent the relapse of tumors as antigen-loss variants 10 The ease whereby bacteria can be genetically modified to express different peptides makes this approach feasible.
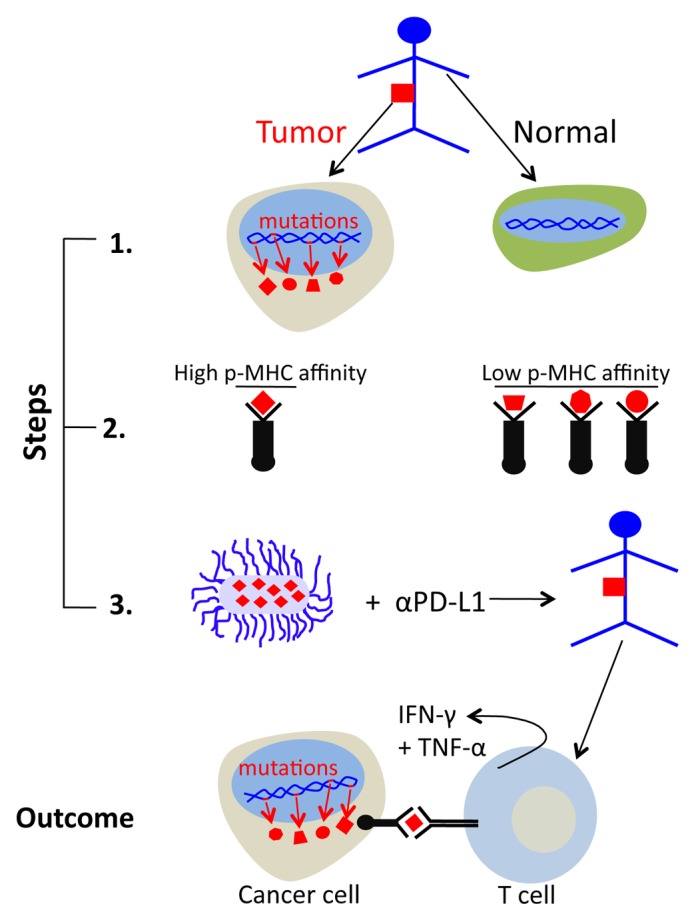
Figure 1. Therapeutic vaccination approach based on exome sequencing and peptide affinity prediction. Exome sequencing should be applied to malignant vs. matched non-transformed cells to identify tumor-specific somatic mutations. (1) The binding affinity of tumor-specific peptides for MHC molecules should be predicted with a dedicated algorithm. (2) Salmonella typhimurium A1-R should be engineered to express high-affinity peptide/s and used for anticancer vaccination (3). This approach has potential to rescue cytokine production by tumor-specific CD8+ T cells within neoplastic lesions, leading to the regression of established tumors.
In summary, we identified a therapeutic vaccination approach that synergizes with anti-PD-L1 to eradicate tumors that are resistant to PD-L1 and CTLA-4 blocking antibodies alone. Translating our approach to the clinic may be achieved by using genomic sequencing combined with a peptide-MHC binding affinity algorithm to identify tumor-specific peptides that can be expressed by bacteria. While it would be ideal to use a non-personalized approach to rescue dysfunctional T cells (for instance Toll-like receptor agonists), untargeted strategies have not demonstrated the capacity to overcome the resistance of some tumors to PD-1 and CTLA-4 blockade. The high throughput nature of current genomics, affinity-predicting algorithms, and bacterial engineering make our approach clinically realistic, despite it being personalized to each patient.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by the National Institutes of Health grants R01-CA22677 R01-CA37156 and P01-CA97296, to H.S. and the Graduate Training in Growth and Development grant T32 HD009007 to DB.
Citation: Binder DC, Schreiber H. High-affinity peptide-based anticancer vaccination to overcome resistance to immunostimulatory antibodies. OncoImmunology 2013; 2:e26704; 10.4161/onci.26704
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26704
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder DC, Engels B, Arina A, Yu P, Slauch JM, Fu YX, et al. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol Res. 2013;1:123–33. doi: 10.1158/2326-6066.CIR-13-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, Kranz DM, Meredith SC, Rowley DA, Schreiber H. Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell. 2013;23:516–26. doi: 10.1016/j.ccr.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–52. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, et al. Tumor Exome Analysis Reveals Neoantigen-Specific T-Cell Reactivity in an Ipilimumab-Responsive Melanoma. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.47.7521. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kvistborg P, Shu CJ, Heemskerk B, Fankhauser M, Thrue CA, Toebes M, van Rooij N, Linnemann C, van Buuren MM, Urbanus JH, et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology. 2012;1:409–18. doi: 10.4161/onci.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wortzel RD, Philipps C, Schreiber H. Multiple tumour-specific antigens expressed on a single tumour cell. Nature. 1983;304:165–7. doi: 10.1038/304165a0. [DOI] [PubMed] [Google Scholar]


